Abstract
Atherosclerotic lesions are infiltrated by macrophages and T lymphocytes, potentially reactive to pathogens. We studied in vivo activated T lymphocytes that infiltrate atherosclerotic plaques of Helicobacter pylori-infected patients with or without anti-Chlamydia pneumoniae antibodies. In all atherosclerotic lesions, T helper type 1 (Th1) cells were predominant. C. pneumoniae-specific T cells were detected only in the plaques of anti-C. pneumoniae seropositive patients, whereas H. pylori-specific T cells were found in the gastric mucosa but not in the plaques of the same patients. Plaque-derived Th1 cells expressed cytotoxicity, proapoptotic activity, and help for monocyte tissue factor production. Although multifactorial, atherosclerosis can be regarded as a Th1-driven immunopathological condition.
Observations in humans and animals led to the hypothesis that atherosclerotic plaques derive from specific cellular and molecular mechanisms that can be ascribed to an inflammatory disease of the arterial wall, whose lesions invariably consist of monocyte-derived macrophages and T lymphocytes (1–3). Activated macrophages and T cells would be responsible for in situ production of enzymes, growth factors, cytokines, and chemokines that further expand the process. If inflammation continues unabated, it results in an increased number of plaque-infiltrating macrophages and T cells, both of which emigrate from the blood and proliferate within the lesions, resulting in a remodeling of the arterial wall (4).
A pathogenetic role for infections in atherosclerosis is suggested by the detection of pathogens in the arterial vessels and by more or less strong association between atherosclerosis and serological responses to pathogens, such as cytomegalovirus, herpes simplex virus, Haemophilus influenzae, Chlamydia pneumoniae, or Helicobacter pylori, or between the extent of atherosclerosis and the infectious burden (2, 5–13). However, the role of cell-mediated immunity and the functional status of pathogen-specific T cells within atherosclerotic lesions remain poorly characterized.
We have studied the antigen specificity and functional profile of in vivo activated T lymphocytes that infiltrate atherosclerotic plaques. In the lymphocytic infiltrates of human atherosclerotic lesions, we show predominance of T cells producing T helper type (Th)1 cytokines. We detected C. pneumoniae DNA and C. pneumoniae-specific T cells but not H. pylori-specific T cells in atherosclerotic plaques of anti-C. pneumoniae seropositive patients infected by H. pylori, whereas we isolated H. pylori-specific T cells in the gastric mucosa of the same patients. Plaque-derived T cell clones either specific for C. pneumoniae or with unknown specificity exhibited Th1 effector functions, including helper function for tissue factor (TF) production by monocytes, proapoptotic activity, and perforin-mediated cytotoxicity against autologous antigen-presenting cells (APCs).
Methods
Patients. Carotid plaques were obtained by endoarterectomy from 10 patients (eight males and two females, mean age 68; range 61–72 years) with atherosclerotic arteriopathy. Patients were selected on the basis of positive 13C-urea breath test, assessing H. pylori infection, and serology (HELICOBLOT 2.0; Genelabs Diagnostic, Geneva). Eight patients suffered mild to moderate dyspepsia, and six of them accepted gastroscopy. Five patients [anti-C. pneumoniae seropositive patients (Cp-pos)] had detectable serum levels of anti-C. pneumoniae antibodies (Eurospital, Trieste, Italy), whereas the other five patients were seronegative [anti-C. pneumoniae seronegative patients (Cpneg)]. Anti-C. pneumoniae serology was confirmed by standard microimmunofluorescence assay (cut-off value = 32).
Detection of C. pneumoniae in Atherosclerotic Plaques. The presence of C. pneumoniae was investigated by nested PCR, as reported (14). DNA was extracted from fragments of all of the endoarterectomy and gastric specimens by QIAamp DNA kit (Qiagen, Hilden, Germany). Nested PCR consisted of two rounds of amplification using two sets of primers, each in a 50-μl volume. On completion of primary PCR (37 cycles), 2 μl of the PCR product was added into fresh reaction mix containing the second set of primers and amplified for 25 cycles. The amplified DNA products were analyzed by electrophoresis in 1.5% agarose gel, stained with ethidium bromide, and hybridized as reported (14). The nested PCR for C. pneumoniae included an outer primer pair (HL-1, HR-1) and an inner pair (HM-1, HR-2) that generated a product of 204 bp. The details of primers and probe are as follows: HL-1, 5′-GTTGTTCATGAAGGCCTACT-3′-end; HR-1, 5′-TGCATAACCTACGGTGTGTT-3′-end; HM-1, 5′-GTGTCATTCGCCAAGGTTAA-3′-end; HR-2, 5′-ACCTGTCCAAGGTTCATCCT-3′-end; and DNA probe, 5′-GTGTCATTCGCCAAGGTTAAAGTCTACGTT-3′-end.
Generation of T Cell Clones from Atherosclerotic Plaques and Gastric Mucosa. Fragments of plaques were cultured for 7 days in RPMI medium 1640 supplemented with IL-2 (50 units/ml; Eurocetus, Milan) to expand in vivo-activated T cells. Specimens were then disrupted, and single T cells were cloned under limiting dilution, as described (15–18). Clones were screened for responsiveness to C. pneumoniae and H. pylori antigens by measuring [3H]thymidine uptake after 60 h of coculture with irradiated autologous mononuclear cells in the presence of medium, C. pneumoniae sonicated elementary bodies (EB) [104 inclusion forming units (IFU)/ml], recombinant heat-shock protein (HSP)-60, HSP-10 and the outer membrane protein (OMP)-2 (10 μg/ml), all prepared as endotoxin-free materials, as reported elsewhere (19). All clones were also assessed for responsiveness to H. pylori lysate (NCTC11637 strain, 10 μg/ml) (16). At 16 h before harvesting, 0.5 μCi of [3H]dT (Amersham Pharmacia Biotech) were added, and radionuclide uptake was measured in a β counter. The mitogenic index (MI) was calculated as the ratio between mean values of cpm obtained in stimulated cultures and those obtained in the presence of medium alone. MI >5 was considered as positive. Biopsy specimens of gastric antral mucosa were cultured for 7 days in IL-2-conditioned medium, and single T cell blasts were cloned and screened for responsiveness to C. pneumoniae and H. pylori antigens, as described (16).
Assessment of the Cytokine Profile of T Cell Clones. To assess their cytokine production, T cell blasts (106 cells per ml) of each clone were stimulated for 36 h with phorbol-12-myristate 13-acetate (10 ng/ml) in wells coated with anti-CD3 mAb, as reported (20). To assess the cytokine production of C. pneumoniae-specific clones on antigen stimulation, 5 × 105 T cell blasts of each clone were cocultured for 48 h in 0.5 ml of medium with 5 × 105 irradiated autologous peripheral blood mononuclear cells in the absence or presence of C. pneumoniae EB (104 IFU/ml). At the end of culture period, duplicate samples of each supernatant were assayed for IFN-γ, tumor necrosis factor (TNF)-α, IL-4, and IL-5 (BioSource International, Camarillo, CA) (20).
Perforin-Mediated Cytotoxicity and Fas–Fas Ligand-Mediated Proapoptotic Activity. Perforin-mediated cytolytic activity of T cell clones was assessed as reported (20). T cell blasts of C. pneumoniae-specific clones were incubated at ratios of 10, 5, and 2.5 to 1 with 51Cr-labeled autologous Epstein–Barr virus transformed (EBV)-B cells preincubated with C. pneumoniae EB (104 IFU/ml) or H. pylori lysate (10 μg/ml). After centrifugation, microplates were incubated for 8 h at 37°C, and 0.1 ml of supernatant was removed for measurement of 51Cr release, as reported (20). The ability of C. pneumoniae-specific T cell clones to induce Fas–Fas ligand-mediated apoptosis was assessed using Fas+ Jurkat cells as target (21). T cell blasts from each clone were cocultured with 51Cr-labeled Jurkat cells at an effector/target ratio of 10, 5, and 2.5 to 1 for 18 h in the presence of phorbol-12-myristate-13-acetate (10 ng/ml) and ionomycin (1 mmol/l), as reported (18). To block Fas–Fas ligand interaction, the anti-Fas antagonistic monoclonal antibody M3 (Immunex) was used at a 5 μg/ml final concentration in a 30-min pretreatment of 51Cr-labeled Jurkat cells, as reported (22).
Assay for T Cell Clone Helper Function for Monocyte TF Production. T cell blasts of C. pneumoniae-specific clones (8 × 105 per ml) were cocultured for 16 h with autologous monocytes (4 × 105 per ml) in the presence of medium or C. pneumoniae antigen (104 IFU per ml). Plaque-infiltrating T cell clones with unknown specificity from Cp-neg patients were cocultured for 16 h with autologous monocytes in the absence or presence of phytohemagglutinin (1% vol/vol). At the end of the culture period, TF protein was quantitated by a specific ELISA (American Diagnostica, Greenwich, CT) in duplicate samples of the supernatants obtained from cell suspensions after solubilization of membrane proteins with Triton X-100 and ultracentrifugation, as reported (23).
Results
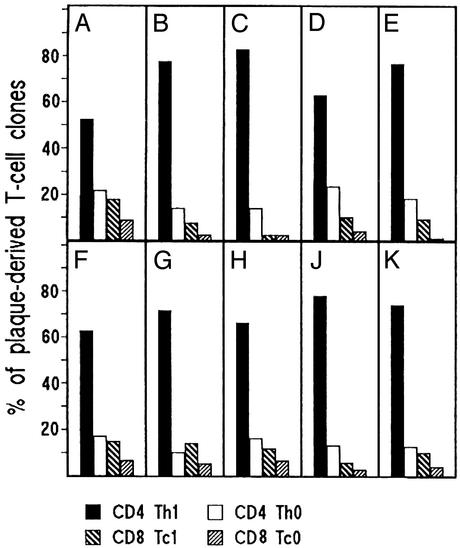
Predominance of Th1 Lymphocytes in Atherosclerotic Lesions. Among patients undergoing carotid endarterectomy, we selected 10 H. pylori-infected individuals, five of whom were also seropositive for anti-C. pneumoniae antibodies, and five were seronegative. Fragments of carotid plaques of all patients were cultured in IL-2-conditioned medium to allow the preferential in vitro expansion of in vivo activated T cells resident in the plaques. Single T cell blasts were then cloned by a cloning procedure that has proved useful and accurate for studies of tissue-infiltrating T cells in various diseases (15, 16, 17, 18, 24). A total number of 206 CD4+ and 31 CD8+ T cell clones were obtained from the plaques of the Cp-pos patients (Fig. 1 A–E), whereas 215 CD4+ and 41 CD8+ were the T cell clones derived from the plaques of the Cp-neg patients (Fig. 1 F–K). All plaque-derived clones were assessed for their cytokine profile by measuring mitogen-induced production of IFN-γ, TNF-α, IL-4, and IL-5. In both Cp-pos and Cp-neg patients, the majority (mean ± SD 70 ± 12% and 70 ± 6%, respectively) of plaque-derived clones were CD4 able to secrete IFN-γ and TNF-α but not IL-4, thus showing a polarized Th1 profile (Fig. 1). CD4 clones able to secrete both IFN-γ and IL-4 (type 0 profile or Th0) accounted for only 17 ± 5% and 14 ± 3% of clones from Cp-pos and Cp-neg patients, respectively. Likewise, in both series of patients, the proportions of cytotoxic CD8 clones producing type 1 cytokines (Tc1 clones) (9 ± 5% vs. 4 ± 3% and 11 ± 4% vs. 5 ± 2%, respectively) were relatively higher than those of CD8 clones producing both type 1 and 2 cytokines (Tc0 clones) (Fig. 1).
Fig. 1.
Cytokine profile of the T cell clones derived from atherosclerotic plaques. Plaque-derived T cell clones were obtained from 10 H. pylori-infected patients, five of whom (A–E) were seropositive for anti-C. pneumoniae antibodies, whereas the other five (F–K) were seronegative. Duplicate samples of supernatants of mitogen-stimulated T cell clones were assayed for cytokine production. CD4+ and CD8+ clones able to produce IFN-γ, but not IL-4, were categorized as Thl and Tc1, whereas CD4+ and CD8+ clones producing both IFN-γ and IL-4 were coded as Th0 and Tc0, respectively.
C. pneumoniae DNA and C. pneumoniae-Specific T Cells in Atherosclerotic Plaques. Nested PCR on endarterectomy specimens showed C. pneumoniae genomic material in each of the plaques obtained from the 5 Cp-pos patients but not in the plaques from the five Cp-neg patients. Likewise, PCR for C. pneumoniae DNA was negative in the samples of gastric mucosa (data not shown). Plaque-derived T cell clones from Cp-pos and Cp-neg patients were assayed for proliferation in response to C. pneumoniae EB and to H. pylori lysate. None of the 72 CD8+ clones derived from the plaques of either Cp-pos or Cp-neg patients showed proliferation to those antigens. In contrast, 46 (22%) of the 206 CD4+ T cell clones generated from plaque-infiltrating T cells of Cp-pos patients proliferated significantly to C. pneumoniae EB but not to the H. pylori lysate. Under the same conditions, none of the 215 CD4+ clones from the plaques of Cp-neg patients showed significant proliferation to the same antigens (Table 1).
Table 1. Antigen specificity of T cell clones isolated from atherosclerotic plaques.
| No. of clones reactive to: (%)
|
|||
|---|---|---|---|
| Patients and source of T cells | Total no. of CD4+ T cell clones obtained | C. pneumoniae | H. pylori |
| C. pneumoniae DNA-positive plaques from Cp-pos patients | |||
| A | 34 | 9 (26) | 0 |
| B | 40 | 8 (20) | 0 |
| C | 42 | 12 (29) | 0 |
| D | 59 | 8 (14) | 0 |
| E | 31 | 9 (29) | 0 |
| C. pneumoniae DNA-negative plaques from Cp-neg patients | |||
| F | 37 | 0 | 0 |
| G | 34 | 0 | 0 |
| H | 55 | 0 | 0 |
| J | 46 | 0 | 0 |
| K | 43 | 0 | 0 |
| Gastric antral mucosa | |||
| A | 42 | 0 | 10 (24) |
| C | 51 | 0 | 9 (18) |
| D | 46 | 0 | 13 (28) |
| G | 39 | 0 | 6 (15) |
| H | 33 | 0 | 10 (30) |
| J | 47 | 0 | 11 (23) |
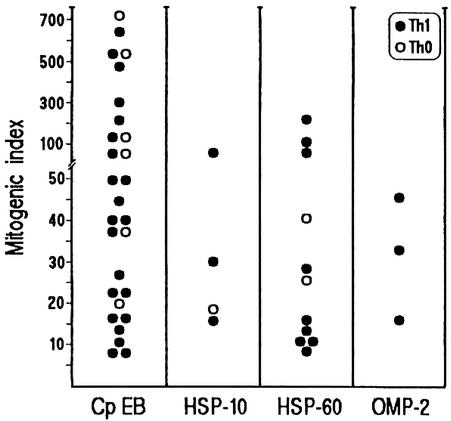
Among the 46 plaque-infiltrating CD4+ clones that reacted to C. pneumoniae EB, 11 clones were specific for recombinant C. pneumoniae HSP-60, four to HSP-10, and three to the OMP-2 protein, whereas the other 28 proliferated only to C. pneumoniae EB (Fig. 2).
Fig. 2.
Antigen repertoire of plaque-infiltrating T cell clones reactive to C. pneumoniae. Th1 and Th0 clones were tested for proliferation to C. pneumoniae EB (Cp EB), recombinant C. pneumoniae HSP-60, HSP-10, and OMP-2 in the presence of irradiated autologous APCs.
Lack of H. pylori-Specific T Cells in Atherosclerotic Plaques. Although in vivo activated T cells with a predominant type 1 profile were detected within the atherosclerotic plaques of Cp-neg individuals, no T cell reactivity to C. pneumoniae antigens was evident in vitro. These data, together with the failure to detect C. pneumoniae DNA in such plaques, suggest that the antigens involved in induction and maintenance of T cell responses in the plaques of Cp-neg patients are different and may be related to other pathogens. A possible candidate could be H. pylori (11). However, no T cell reactivity against H. pylori antigens could be found in the plaques of either Cp-pos or Cp-neg patients, despite the fact that all these patients harbored that pathogen in their stomach and were seropositive for anti-H. pylori antibodies. To further investigate this issue, biopsies of the gastric antral mucosa were obtained from three Cp-pos and three Cp-neg patients, who also suffered from persistent dyspepsia. This allowed both histological confirmation of H. pylori-associated chronic gastritis and generation of 261 CD4+ T cell clones from the gastric biopsies by the same culture and cloning protocol used for atherosclerotic plaques. None of the gastric T cell clones recognized C. pneumoniae EB, whereas a mean of 23% proliferated to H. pylori lysate (Table 1), confirming that, in the same H. pylori-infected individuals, T cells reactive to H. pylori infiltrated the gastric mucosa but had not colonized the atherosclerotic plaques in the carotids. By contrast, T cells reactive to C. pneumoniae selectively infiltrated the atherosclerotic plaques in Cp-pos individuals but were not detected in another site of inflammation, their gastric antrum.
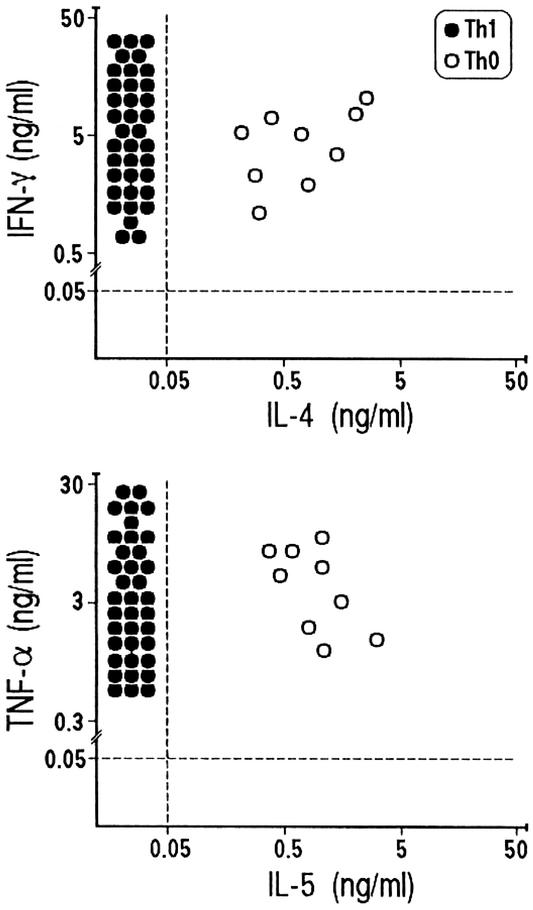
Th1 Effector Functions of Plaque-Derived C. pneumoniae-Specific T Cells. We then assessed the cytokine profile induced in C. pneumoniae-specific plaque-derived T cell clones by stimulation with the specific antigen in the presence of autologous APCs. All clones showed the same Th1 or Th0 profile that was initially assessed by mitogen stimulation. In particular, two of the 11 clones specific for HSP-60, one of the three clones specific for HSP-10, and six clones reactive to C. pneumoniae EB were confirmed in their ability to produce both IFN-γ and IL-4/IL-5 (Th0 profile), whereas the other C. pneumoniae-specific clones expressed a polarized Th1 profile (Fig. 3). Interestingly, stimulation with the appropriate antigen induced all clones to produce remarkable amounts of TNF-α (Fig. 3), comparable to those obtained in the same clones on mitogen stimulation in the absence of APC (data not shown).
Fig. 3.
C. pneumoniae antigen-induced cytokine production by plaque-infiltrating T cell clones. C. pneumoniae-specific Th1 or Th0 clones were stimulated with the appropriate antigen, and IFN-γ, TNF-α, IL-4, and IL-5 production was measured in culture supernatants. In unstimulated control cultures, levels of IFN-γ, TNF-α, IL-4, and IL-5 were consistently <0.05 ng/ml.
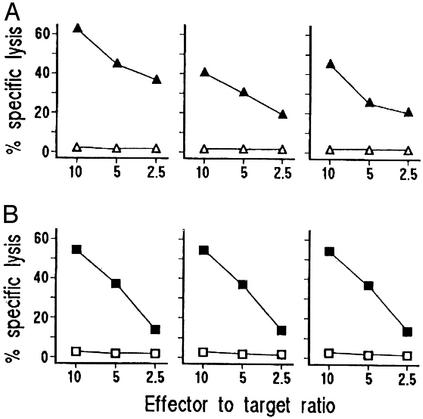
Because most antigen-activated Th1 and Th0 clones express perforin-mediated cytotoxicity against autologous APC (e.g., antigen-pulsed B cells) (20), we assessed the cytolytic potential of C. pneumoniae EB-specific T cell clones by using antigen-pulsed 51Cr-labeled autologous EBV-B cells as targets. At an effector/target ratio of 10:1, all 37 Th1 and six of nine Th0 clones lysed C. pneumoniae EB-presenting autologous EBV-B cells (Fig. 4A), whereas autologous EBV-B cells pulsed with H. pylori antigen and cocultured with the same clones were not lysed. Because activated effector T cells can also kill their targets by inducing apoptosis through Fas–Fas ligand interaction (21, 25, 26), we evaluated the ability of activated C. pneumoniae-specific clones to induce 51Cr release by Fas+ Jurkat cells undergoing apoptosis. On mitogen activation, 36 of 37 Th1 (97%) and five of nine Th0 clones were able to induce apoptosis in target cells (Fig. 4B). The role of Fas–Fas ligand interaction in this 51Cr release was confirmed by its inhibition (range 39.6–59.8%) by a blocking anti-Fas antibody.
Fig. 4.
Cytotoxic and proapoptotic activity of C. pneumoniae-specific plaque-infiltrating T cells. (A) To assess their perforin-mediated cytotoxicity, C. pneumoniae-specific T cell clones were cocultured at different effector-to-target ratios with 51Cr-labeled autologous EBV-B cells pulsed with C. pneumoniae EB (▴) or H. pylori lysate (▵), and 51Cr release was measured as index of specific target cell lysis. (B) To assess their ability to induce apoptosis in target cells, C. pneumoniae-specific T cell clones stimulated with mitogen (▪) or medium alone (□) were cocultured with 51Cr-labeled Fas+ Jurkat cells, and 51Cr release was measured as index of apoptotic target cell death. Data of three representative clones are reported.
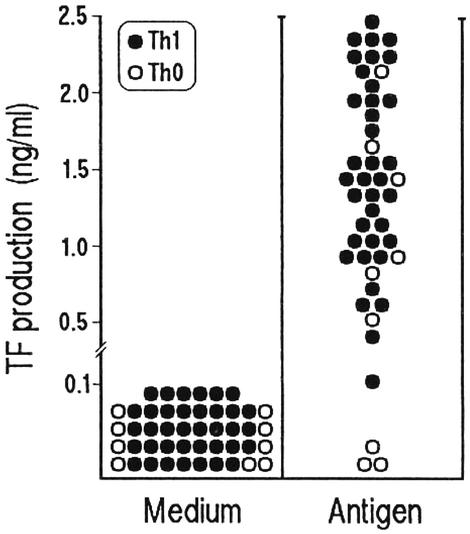
Plaque-Infiltrating T Cells Help Monocyte TF Production. Because plaque rupture and thrombosis are notable complications of atherosclerosis, we asked whether plaque-infiltrating T cells had the potential to express helper function for TF production by monocytes. Antigen-stimulated C. pneumoniae-specific T cell clones from the plaques of Cp-pos patients and mitogen-stimulated T cell clones from the plaques of Cp-neg patients were cocultured with autologous monocytes, and TF protein was measured. Apart from three Th0 and one Th1 clone that provided poor or no helper function, in 42 C. pneumoniae-specific clones, antigen stimulation resulted in the expression of substantial help for TF production by monocytes (Fig. 5). Likewise, in the series of plaque-infiltrating T cell clones with unknown specificity from Cp-neg patients, mitogen stimulation enabled 170 of 179 (95%) Th1 and 28 of 36 (78%) Th0 clones to induce TF production by monocytes (data not shown). These data are in agreement with the notion that activated Th1 cells and type 1 cytokines induce TF production by monocytes, whereas culture supernatants of activated Th2 cells or recombinant IL-4, IL-13, and IL-10 inhibit the Th1-induced TF production by monocytes in a dose-dependent fashion (23, 27).
Fig. 5.
C. pneumoniae-specific T cells induce TF production by monocytes. To assess their ability to induce TF production by monocytes, C. pneumoniae-specific Th1 and Th0 clones were cocultured with autologous monocytes in the presence of medium or C. pneumoniae EB, and TF production by monocytes was assessed by an appropriate ELISA.
Discussion
We have demonstrated that, in the clonal progeny of in vivo-activated plaque-infiltrating T cells, the ability to secrete IFN-γ and TNF-α is predominant, whereas production of Th2 cytokines is limited to a few clones. Such a cytokine pattern is present in the plaques of both anti-C. pneumoniae seropositive and seronegative individuals. The possibility that this outcome does not reflect the real attitude of plaque-infiltrating T cells in vivo but is the result of in vitro artifacts due to the culture in IL-2 and the cloning protocol was considered. However, using the same culture and cloning procedure, T cell clones with predominant Th2 profile were obtained from the bronchial mucosa or the conjunctiva of atopic patients (15, 28), as well as from the skin of patients with systemic sclerosis (29) or HIV-1 infection (30). Our in vitro findings are in agreement with the ex vivo data indicating a preferential expression of IFN-γ and IFN-γ-inducible CXC chemokines within atherosclerotic plaques, particularly in unstable angina (31, 32, 33, 34, 35). Because CXC chemokines attract cells equipped with CXCR3 receptor, a membrane molecule preferentially expressed by type 1 T cells (36), the in situ release of those chemokines may explain why the Th1 is the predominant functional profile of plaque-infiltrating T cells.
A number of reports demonstrated that C. pneumoniae infection has atherogenic effects in mice (37, 38, 39), although its specific contribution to atherogenesis has recently been questioned (40). We have observed that subjects who came into contact with C. pneumoniae have not only humoral but also T cell-mediated responses to that pathogen. It is of note that C. pneumoniae-specific T cells colonize atherosclerotic plaques, where they reside in activation state near the source of their specific antigen(s), allowing them the opportunity for replication and expression of their effector functions. Our experimental protocol allowed us to clonally expand and to study in vivo activated T cells resident in the plaques. With a similar approach, Stemme et al. (41) succeeded in isolating a plaque-derived CD4+ clones specific to oxidized low-density lipoprotein. In contrast, other investigators used repeated stimulation with C. pneumoniae antigens and mitogens to expand plaque-infiltrating T cells, increasing the risk of in vitro artifacts in their analysis (42, 43). Our data on the fine specificity of plaque-derived C. pneumoniae-specific clones showed a precise antigenic role for chlamydial OMP-2, HSP-10, and HSP-60. This should encourage further studies on possible crossreactions between epitopes of C. pneumoniae antigens and epitopes of self proteins, such as that highlighted between the heart-muscle-specific α myosin heavy chain and the 60-kDa chlamydial OMP, which allowed the induction of autoimmune heart disease in mice (44). On the other hand, chlamydial HSP-60 expression characterizes ongoing inflammatory response in atherosclerosis, and it colocalizes with human HSP-60 within plaque-infiltrating macrophages (45), making possible a mechanism of molecular mimicry resulting in autoimmunity, as recently suggested (46).
The lack of responsiveness to a H. pylori lysate by plaque-infiltrating T cells and the concomitant demonstration of H. pylori-specific T cells in the gastric mucosa, but not in the carotid plaques, led us to favor the concept that H. pylori is not a major inflammatory factor associated with atherosclerosis (47).
It is possible to speculate that antigen-presenting macrophages within the plaque become targets of cytotoxic and proapoptotic activity by activated Th1 cells and are involved in the necrotic cores characteristic of complicated atherosclerotic lesions. A linkage has been suggested between the degree of macrophage apoptosis and plaque rupture, to which apoptotic death of smooth muscle cells may also contribute (48, 49, 50). Further studies are required to investigate whether the cytokine milieu generated within the plaque by activated T cells may enable “nonprofessional” APCs, such as smooth muscle or endothelial cells, to present antigens available in the plaque to infiltrating T cells and becoming a target of their cytolytic and proapoptotic activity.
We have highlighted a possible role for activated Th1 cells and their cytokines in driving the up-regulation of TF production by monocytes within atherosclerotic plaques, thus contributing to the thrombogenicity of lesions (51). The Th1 polarization of T cell responses and the poor production of Th2 cytokines occurring within the plaque may represent local risk factors of thrombosis, which associate with platelet adhesion to dysfunctional endothelium. Besides prostaglandins and leukotrienes, activated platelets release cytokines and growth factors that contribute to migration and growth of smooth muscle cells and monocytes (52), which can amplify the inflammatory response and contribute to the remodeling of vessels.
Overall, our findings support the hypothesis that a crucial component of atherosclerosis is represented by T cell-mediated immune responses that are inappropriate in terms of time of onset, intensity, and target. Particularly, we suggest that atherosclerosis is a Th1-driven immunopathological condition, part of which can result from chronic immune response to C. pneumoniae antigens. On the other hand, the most direct evidence for the critical role for Th1 cells, IFN-γ, and IFN-γ-driven molecules in atherosclerosis is provided by mice with combined deficiencies of apolipoprotein E (apoE) and the IFN-γ receptor, in which the development of atheromata is significantly reduced in comparison to mice with only apoE deficiency, whereas exogenous IFN-γ enhances atherosclerosis (53, 54).
Acknowledgments
We thank Professors S. M. Hedrick and C. A. Janeway, Jr., for critical reading of the manuscript. This work was supported by grants from the Italian Ministry of Health, the Ministry of University and Research, the Istituto Superiore di Sanitá, and the Associazione Italiana per la Ricerca sul Cancro.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Th, T helper type; TF, tissue factor; APC, antigen-presenting cell; Cp-pos, anti-Chlamydia pneumoniae seropositive patients; Cp-neg, anti-C. pneumoniae seronegative patients; EB, elementary bodies; HSP, heat-shock protein; TNF, tumor necrosis factor; EBV, Epstein–Barr virus transformed; IFU, inclusion forming unit; OMP, outer membrane protein.
References
- 1.Ross, R. (1999) N. Engl. J. Med. 340, 115–126. [DOI] [PubMed] [Google Scholar]
- 2.Epstein, S. E., Zhou, Y. F. & Zhu, J. (1999) Circulation 100, 20–28. [Google Scholar]
- 3.Jonasson, L., Holm, J., Skalli, O., Bondjers, G. & Hansson, G. K. (1986) Arteriosclerosis 6, 131–138. [DOI] [PubMed] [Google Scholar]
- 4.Hansson, G. K., Jonasson, L., Seifert, P. S. & Stemme, S. (1989) Arteriosclerosis 9, 567–578. [DOI] [PubMed] [Google Scholar]
- 5.Espinola-Klein, C., Rupprecht, H. J., Blankenberg, S., Bickel, C., Kopp, H., Rippin, G., Victor, A., Hafner, G., Schlumberger, W. & Meyer, J. (2002) Circulation 105, 15–21. [DOI] [PubMed] [Google Scholar]
- 6.Blankenberg, S., Rupprecht, H. J., Bickel, C., Espinola-Klein, C., Rippin, G., Hafner, G., Ossendorf, M., Steinhagen, K. & Meyer, J. (2001) Circulation 103, 2915–2921. [DOI] [PubMed] [Google Scholar]
- 7.Saikku, P., Leinone M., Tenkanen, L., Linnanmaki, E., Ekman, M. R., Manninen, V., Manttari, M., Frick, M. H. & Huttunen, J. K. (1992) Ann. Intern. Med. 116, 273–278. [DOI] [PubMed] [Google Scholar]
- 8.Rupprecht, H. J., Blankenberg, S., Bickel, C., Rippin, G., Hafner, G., Prellwitz, W., Schlumberge, W. & Meyer, J. (2001) Circulation 104, 25–31. [DOI] [PubMed] [Google Scholar]
- 9.Korner, I., Blatz, R., Wittig, I., Pfeiffer, D. & Ruhlmann, C. (1999) Vasa 28, 259–263. [DOI] [PubMed] [Google Scholar]
- 10.Chiu, B., Viira, E., Tucker, W. & Fong, I. W. (1997) Circulation 96, 2144–2148. [DOI] [PubMed] [Google Scholar]
- 11.Danesh, J., Wong, Y., Ward, M. & Muir, J. (1999) Heart 81, 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maass, M., Bartels, C., Engel, P. M., Mamat, U. & Sievers, H. H. (1998) J. Am. Coll. Cardiol. 31, 827–832. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi, K., Fujii, B., Kudo, S., Shirai, M., Yamashita, K., Gondo, T., Ishihara, T., Ito, H. & Nakazawa, T. (2000) J. Infect. Dis. 181 Suppl. 3, S441–S443. [DOI] [PubMed] [Google Scholar]
- 14.Khan, M. A. & Potter, C. W. (1996) J. Infect. 33, 173–175. [DOI] [PubMed] [Google Scholar]
- 15.Del Prete, G., De Carli, M., D'Elios, M. M., Maestrelli, P., Ricci, M., Fabbri, L. & Romagnani, S. (1993) Eur. J. Immunol. 23, 1445–1449. [DOI] [PubMed] [Google Scholar]
- 16.D'Elios, M. M., Manghetti, M., Almerigogna, F., Amedei, A., Costa, F., Burroni, D., Baldari, C. T., Romagnani, S., Telford, J. L. & Del Prete, G. (1997) Eur. J. Immunol. 27, 1751–1755. [DOI] [PubMed] [Google Scholar]
- 17.Bertoletti, A., D'Elios, M. M., Boni, C., De Carli, M., Zignego, A. L., Durazzo, M., Missale, G., Penna, A., Fiaccadori, F., Del Prete, G., et al. (1997) Gastroenterology 112, 193–199. [DOI] [PubMed] [Google Scholar]
- 18.D'Elios, M. M., Bergman, M. P., Azzurri, A., Amedei, A., Benagiano, M., De Pont, J. J., Cianchi, F., Vandenbroucke-Grauls, C. M., Romagnani, S., Appelmelk, B. J., et al. (2001) Gastroenterology 120, 377–386. [DOI] [PubMed] [Google Scholar]
- 19.Ciervo, A., Visca, P., Petrucca, A., Biasucci, L. M., Maseri, A. & Cassone, A. (2002) Clin. Diagn. Lab. Immunol. 9, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Prete, G., De Carli, M., Ricci, M. & Romagnani, S. (1991) J. Exp. Med. 174, 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vergelli, M., Hemmer, B., Muraro, P. A., Tranquilli, L., Biddison, W. E., Sarin, A., McFarland, H. F. & Martin, R. (1997) J. Immunol. 158, 2756–2761. [PubMed] [Google Scholar]
- 22.D'Elios, M. M., Amedei, A., Manghetti, M., Costa, M., Baldari, C. T., Quazi, A. S., Telford, J. L., Romagnani, S. & Del Prete, G. (1999) Gastroenterology 117, 1105–1112. [DOI] [PubMed] [Google Scholar]
- 23.Del Prete, G., De Carli, M., Lammel, R. M., D'Elios, M. M., Daniel, K. C., Giusti, B., Abbate, R. & Romagnani, S. (1995) Blood 86, 250–257. [PubMed] [Google Scholar]
- 24.Carter, L. L. & Swain, S. L. (1997) Curr. Opin. Immunol. 9, 177–182. [DOI] [PubMed] [Google Scholar]
- 25.Kagi, D., Vignaux, F., Ledermann, B., Burki, K., Depraetere, V., Nagata, S., Hengartner, H. & Golstein, P. (1994) Science 265, 528–530. [DOI] [PubMed] [Google Scholar]
- 26.Wang, J., Taniuchi, I., Maekawa, Y., Howard, M., Cooper, M. D. & Watanabe, T. (1996) Eur. J. Immunol. 26, 92–96. [DOI] [PubMed] [Google Scholar]
- 27.Fan, S. T., Glaserbrook, A. L. & Edgington, T. S. (1990) Cell. Immunol. 128, 52–62. [DOI] [PubMed] [Google Scholar]
- 28.Maggi, E., Biswas, P., Del Prete, G., Parronchi, P., Macchia, D., Simonelli, C., Emmi, L., De Carli, M., Tiri, A., Ricci, M., et al. (1991) J. Immunol. 146, 1169–1174. [PubMed] [Google Scholar]
- 29.Mavilia, C., Scaletti, C., Romagnani, P., Carossino, A. M., Pignone, A., Emmi, L., Pupilli, C., Pizzolo, G., Maggi, E. & Romagnani, S. (1997) Am. J. Pathol. 151, 1751–1758. [PMC free article] [PubMed] [Google Scholar]
- 30.Maggi, E., Giudizi, M. G., Biagiotti, R., Annunziato, F., Manetti, R., Piccinni, M. P., Parronchi, P., Zampognaro, S., Giannarini, L., Zuccati, G., et al. (1994) J. Exp. Med. 180, 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansson, G. K., Holm, J. & Jonasson, L. (1989) Am. J. Pathol. 135, 169–175. [PMC free article] [PubMed] [Google Scholar]
- 32.Liuzzo, G., Vallejo, A. N., Kopecky, S. L., Frye, R. L., Holmes, D. R., Goronzy, J. J. & Weyand, C. M. (2001) Circulation 103, 1509–1514. [DOI] [PubMed] [Google Scholar]
- 33.Mach, F., Sauty, A., Iarossi, A. S., Sukhova, G. K., Neote, K., Libby, P. & Luster, A. D. (1999) J. Clin. Invest. 104, 1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uyemura, K., Demer, L. L., Castle, S. C., Jullien, D., Berliner, J. A., Gately, M. K., Warrier, R. R., Pham, N., Fogelman, A. M. & Modlin, R. L. (1996) J. Clin. Invest. 97, 2130–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frostegard, J., Ulfgren, A. K., Nyberg, P., Hedin, U., Swedenborg, J., Andersson, U. & Hansson, G. K. (1999) Atherosclerosis 145, 33–43. [DOI] [PubMed] [Google Scholar]
- 36.Bonecchi, R., Bianchi, G., Bordignon, P. P., D'Ambrosio, D., Lang, R., Borsatti, A., Sozzani, S., Allavena, P., Gray, P. A., Mantovani, A., et al. (1998) J. Exp. Med. 187, 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu, H., Pierce, G. N. & Zhong, G. (1999) J. Clin. Invest. 103, 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moazed, T. C., Campbell, L. A., Rosenfeld, M. E., Grayston, J. T. & Kuo, C. C. (1999) J. Infect. Dis. 180, 238–241. [DOI] [PubMed] [Google Scholar]
- 39.Hansson, G. K. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1876–1890. [DOI] [PubMed] [Google Scholar]
- 40.Caligiuri, G., Rottenberg, M., Nicoletti, A., Wigzell, H. & Hansson, G. K. (2001) Circulation 103, 2834–2838. [DOI] [PubMed] [Google Scholar]
- 41.Stemme, S., Faber, B., Holm, J., Wiklund, O., Witztum, J. L. & Hansson, G. K. (1995) Proc. Natl. Acad. Sci. USA 92, 3893–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curry, A. J., Portig, I., Goodall, J. C., Kirkpatrick, P. J. & Gaston, J. S. H. (2000) Clin. Exp. Immunol. 121, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosorin, M., Surcel, H. M., Laurila, A., Lehtinen, M., Karttunen, R., Juvonen, J., Paavonen, J., Morrison, R. P., Saikku, P. & Juvonen, T. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1061–1067. [DOI] [PubMed] [Google Scholar]
- 44.Bachmaier, K., Neu, N., de la Maza, L. M., Pal, S., Hessel, A. & Penninger, J. M. (1999) Science 283, 1335–1339. [DOI] [PubMed] [Google Scholar]
- 45.Kol, A., Sukhova, G. K., Lichtman, A. H. & Libby, P. (1998) Circulation 98, 300–307. [DOI] [PubMed] [Google Scholar]
- 46.Wick, G., Perschinka, H. & Millonig, G. (2001) Trends Immunol. 22, 665–669. [DOI] [PubMed] [Google Scholar]
- 47.Koenig, W., Rothenbacher, D., Hoffmeister, A., Miller, M., Bode, G., Adler, G., Hombach, V., Marz, W., Pepys, M. B. & Brenner, H. (1999) Circulation 100, 2326–2331. [DOI] [PubMed] [Google Scholar]
- 48.Kolodgie, F. D., Narula, J., Burke, A. P., Haider, N., Farb, A., Hui-Liang, Y., Smialek, J. & Virmani, R. (2000) Am. J. Pathol. 157, 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geng, Y. J., Henderson, L. E., Levesque, E. B., Muszynski, M. & Libby, P. (1997) Arterioscler. Thromb. Vasc. Biol. 17, 2200–2208. [DOI] [PubMed] [Google Scholar]
- 50.Mallat, Z. & Tedgui, A. (2001) Circ. Res. 88, 998–1003. [DOI] [PubMed] [Google Scholar]
- 51.Toschi, V., Gallo, R., Lettino, M., Fallon, J. T., Gertz, S. D., Fernandez-Ortiz, A., Chesebro, J. H., Badimon, L., Nemerson, Y., Fuster, V., et al. (1997) Circulation 95, 594–599. [DOI] [PubMed] [Google Scholar]
- 52.Bombeli, T., Schwartz, B. R. & Harlan, J. M. (1998) J. Exp. Med. 187, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta, S., Pablo, A. M., Jiang, X., Wang, N., Tall, A. R. & Schindler, C. (1997) J. Clin. Invest. 99, 2752–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitman, S. C., Ravisankar, P., Elam, H. & Daugherty, A. (2000) Am. J. Pathol. 157, 1819–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]