Abstract
Immunization of mice with two myasthenogenic peptides, p195–212 and p259–271, which are sequences of the human acetylcholine receptor, resulted in myasthenia gravis (MG)-associated immune responses. A dual altered peptide ligand (APL) composed of the two APLs of the myasthenogenic peptides inhibited, in vitro and in vivo, those responses. The aims of this study were to further elucidate the mechanism/s by which the dual APL down-regulates MG-associated responses in vivo and characterize the cell population/s involved in this immunomodulatory suppressive effect. We have shown here that s.c. administration of the dual APL activates CD4CD25-expressing cells in lymph nodes (LN) of SJL mice. Furthermore, depletion of these cells diminished significantly the inhibitory effect of the APL on p195–212-specific proliferative responses. Depletion of the CD4+CD25+ cells was accompanied with a decrease in the secretion of the immunosuppressive cytokine, transforming growth factor (TGF)-β. Administration of the dual APL resulted also in the up-regulation of the expression of cytotoxic T lymphocyte antigen (CTLA)-4 and in a down-regulated expression of CD28 on LN cells. Blockade of the CTLA-4 function, in vitro, abrogated the inhibitory effect of the dual APL on the proliferative responses specific to p195–212. Thus, our results suggest that the active suppression exerted by the dual APL is mediated by the CD4+CD25+ immunoregulatory cell population, either directly through the CTLA-4 molecule expressed on these cells, and/or indirectly by causing the differentiation of other regulatory T cell population/s that secrete immunosuppressive cytokines.
Myasthenia gravis (MG) is a T cell-regulated, antibody-mediated autoimmune disease. Impaired neuromuscular transmission characteristic of MG results from antibodies against the nicotinic acetylcholine receptor (AChR) of skeletal muscle (1, 2). Nevertheless, a role for T cells in MG and experimental autoimmune MG (EAMG) was shown in several studies (3–7). Previous work performed in our laboratory demonstrated that two peptides representing sequences of the human AChR α-subunit, p195–212 and p259–271, were able to stimulate peripheral blood lymphocytes of patients with MG, and to serve as immunodominant T cell epitopes of SJL and BALB/c mice respectively (8, 9). Altered myasthenogenic peptides, which are single amino acid-substituted analogs of p195–212 (207Ala) and p259–271 (262Lys), as well as a dual altered peptide ligand (APL) composed of the tandemly arranged two single analogs (Lys-262-Ala-207), were synthesized and were shown to inhibit the proliferative responses of both p195–212- and p259–271-specific T cell lines in vitro as well as the in vivo priming to the myasthenogenic peptides (10–12). In addition, the single and the dual APL were also found to be capable of inhibiting the proliferative responses of peripheral blood lymphocytes of MG patients to both myasthenogenic peptides p195–212 and p259–271 (13). The dual APL could reverse myasthenogenic manifestations in mice with EAMG induced either by pathogenic T cell lines or by the Torpedo AChR (10, 14). In an attempt to elucidate the mechanism/s by which the dual APL down-regulates EAMG-associated responses, we demonstrated that the dual APL acts by actively suppressing myasthenogenic T cell responses in a specific manner. The active suppression is mediated, at least partially, by the up-regulation of the secretion of transforming growth factor (TGF)-β [T helper (Th) 3–type cytokine], which was accompanied by down-regulation of IFN-γ and IL-2 (Th 1-type cytokines) secretion (15). Furthermore, the inhibitory effect of the dual APL could be adoptively transferred to p195–212 or Torpedo AChR-immunized mice (15). Nevertheless, the mechanisms by which the dual APL exerts its effect in vivo have not been completely elucidated yet. Hence, the purpose of this study has been to attempt a better insight into these in vivo mechanisms.
The term “regulatory T cell” describes a variety of T cells that display suppressive functions in vitro or in vivo. One of the best characterized subsets is defined by a constitutive expression of the IL-2 receptor α-chain (CD25) (16). These CD4+CD25+ regulatory T cells (Treg) play a central role in the maintenance of peripheral tolerance. Depletion of these cells in murine adoptive transfer models leads to various autoimmune diseases (17). The suppressive function of CD25+ Treg cells and the suppressive capacity depend on direct cell–cell contact. However, once activated, the suppression is antigen nonspecific and independent of inhibitory cytokines (17, 18).
CD28 and its homologue, cytotoxic T lymphocyte antigen (CTLA)-4, are the primary regulatory molecules that enhance or inhibit T cell activation, respectively. CD4+CD25+ T cells are the only CD4+ cells in the naive mouse that express the CTLA-4 antigen (19–21). This provocative finding raised the possibility that CTLA-4 plays a critical role in the induction of suppression mediated by the CD4+CD25+ T cell population.
In the present study, we wanted to test the possibility that the inhibitory effect of the dual APL is mediated at least partially by a CD4+CD25+ immunoregulatory T cell population. We demonstrate here that s.c. administration of the dual APL increases the size of this T cell population in lymph node (LN) of SJL mice. Furthermore, depletion of the CD4+CD25+ T cells diminished significantly the inhibitory effect of the dual APL on proliferative responses specific to the myasthenogenic peptide p195–212. The depletion of CD4+CD25+ T cells decreased TGF-β secretion of LN cells of treated mice, which was accompanied by an elevation in IFN-γ secretion. Administration of the dual APL caused also an elevation in CTLA-4 expression on LN cells of treated mice, accompanied by a decrease in CD28 expression. Coculturing of LN cells of the treated mice with anti-CTLA-4 neutralizing antibodies abolished significantly the inhibition of the proliferative responses of LN cells, exerted by the dual APL.
Materials and Methods
Mice. Female mice of the inbred strain SJL (The Jackson Laboratory) were used at the age of 8–12 wk.
Synthetic Peptides and Peptide Analog. Peptide p195–212 (DTPYLDITYHFVMQRLPL) and the dual APL Lys-262-Ala-207 (VIVKLIPSTSSAVDTPYLDITYHFVAQRLPL), which is composed of the single analogs of p195–212 (Ala-207 in which methionine was substituted by alanine) and of another myasthenogenic peptide p259–271 (VIVELIPSTSSAV) denoted Lys-262 (in which glutamic acid was substituted by arginine), were synthesized and characterized as described (10). A batch of the dual APL Lys-262-Ala-207 synthesized (97% purity) by UCB-Bioproducts was also used in the present study.
Pretreatment with the Dual APL. SJL mice were injected s.c. with the dual APL (100–500 μg per mouse in PBS) or with PBS three times at 2-day intervals. Splenocytes obtained from these mice were cultured in enriched medium for 24 h and 44 h and stained with anti-CD4-phycoerythrin (PE), anti-CD25-FITC Abs and their matched isotype controls.
Administration of the Dual APL to SJL Mice Immunized with p195–212. SJL mice were administered s.c. with the dual APL (200 μg per mouse in PBS) either concomitant with the immunization with 10 μg per mouse p195–212 in complete Freund's adjuvant (CFA; Difco) or 7 days after immunization with the myasthenogenic peptide. In part of the experiments, the percentage of CD4+CD25+ T cells in the LN was monitored daily by using a fluorescence-activated cell sorter (FACS; Becton Dickinson) analysis, and in others a proliferation assay was performed (15) either after CD25+ T cell depletion or in the presence of anti-CTLA-4-neutralizing antibodies.
Fluorescence Staining of LN Cells. LN cells were stained with anti-CD4-PE antibody (clone GK1.5), anti-CD25-FITC antibody (clone 7D4), anti-CD28-FITC antibody (clone 37.51), and anti-CTLA-4-PE antibody (clone 1B8) and their matched isotype controls (Southern Biotechnology Associates). Briefly, LN cells were washed with 10% FCS/PBS, incubated with the relevant antibody, washed again, and analyzed with FACS. For CTLA-4 staining, the cells were first incubated with a fixation solution (Serotec), washed, and resuspended in permeabilization solution (Serotec) in the presence of the anti-CTLA-4-PE antibody.
Depletion of CD4+CD25+ T Cells. The depletion of the CD25+ T cells was performed as follows by using the StemSep system (StemCell Technologies, Vancouver). LN cells obtained from the dual APL-treated mice were incubated with an anti-CD25-biotinylated antibody (clone 7D4; Southern Biotechnology Associates), washed, and incubated with 10% normal mouse serum for 1 h. The cells were further incubated with an anti-biotin tetrameric complex (StemCell Technologies), washed again, and incubated with magnetic beads (StemCell Technologies). The cells were run through a column (StemCell Technologies), and the cell suspension coming out of the column was collected and used for proliferation assay, cytokine secretion assay, and FACS analysis.
Proliferation of LN Cells in the Presence of Anti-CTLA-4-Neutralizing Antibodies. SJL mice were immunized with 10 μg per mouse p195–212 in CFA, and 7 days later part of them were administered s.c. with the dual APL (200 μg per mouse in PBS). Ten days after immunization with p195–212, a proliferation assay was performed by using popliteal LN cells obtained from immunized mice (15) with or without an anti-CTLA-4-neutralizing antibody (MR1 clone; Southern Biotechnology Associates) or its matched isotype control (Jackson ImmunoResearch).
Secretion and Detection of Cytokines. LN cells (5 × 106 per ml) of the tested mice were stimulated with p195–212 for 48 h-72 h. Supernatants were collected and analyzed for cytokine content by ELISA, using the relevant standard capture and detecting antibodies (from PharMingen and R & D Systems).
Results
Treatment with the Dual APL Up-Regulates CD4+CD25+ T Cells in Spleens of Treated Mice. We wanted to find out whether the CD4+CD25+ T cell population has a role in the down-regulating effects of the dual APL. Therefore, SJL mice were pretreated s.c. with the dual APL or PBS. Splenocytes obtained from these mice were cultured in vitro for 24 h and 44 h and stained for CD4 and CD25. After both periods of in vitro culturing, a similar trend was observed, namely, that the s.c. pretreatment with the dual APL increased the size of the CD4+CD25+ cell population in spleens of SJL mice. The most significant results were obtained after 44 h of in vitro culturing as can be seen in Fig. 1. It can be seen that the dose of 300 μg per mouse dual APL had the most prominent effect on the CD4+CD25+ T cell population (PBS pretreatment, 4.38%; 300 μg per mouse, 7.27%). The dual APL did not affect the CD4+CD25+ T cell population, when the cells were nonspecifically stimulated in vitro with an anti-CD3 antibody. These representative results repeated themselves in three different experiments.
Fig. 1.
Pretreatment with the dual APL increases the size of the CD4+CD25+ T cells in spleens of SJL mice. SJL mice were injected s.c. with the dual APL (100–500 μg per mouse in PBS) or with PBS three times at 2-day intervals. Splenocytes obtained from these mice were cultured in enriched medium for 44 h and stained with anti-CD4-PE, anti-CD25-FITC Abs, and their matched isotype controls. Results are expressed as the percentage of CD4+CD25+ T cells, and they represent one experiment of three performed.
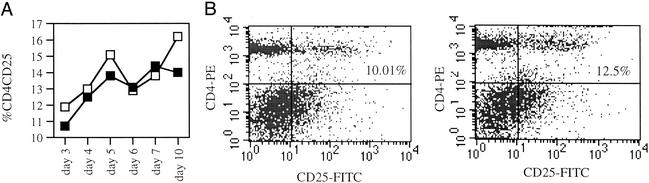
Administration of the Dual APL to Mice Immunized with p195–212 Increases CD4CD25-Expressing Cells in LN of SJL Mice. We wanted to find out whether the dual APL will also affect the CD4+CD25+ T cell population when administered concomitant with the myasthenogenic peptide p195–212. To this end, SJL mice were either administered s.c. with the dual APL concomitant with p195–212 immunization, or immunized with the myasthenogenic peptide p195–212 alone. Because most of the in vivo priming inhibition assays were performed 10 days after p195–212 immunization/dual APL administration, a time point at which the inhibitory effect of the dual APL can be demonstrated very clearly, we followed the kinetics of CD4+CD25+ T cell population expansion in the LN of the treated mice during this period. Fig. 2A shows two peaks (at days 4–5 and 10) in the expansion of CD4+CD25+ LN-derived T cells of mice administered s.c. with the dual APL, in comparison with mice immunized with the myasthenogenic peptide p195–212 alone. The results shown in Fig. 2 represent three independent experiments. Fig. 2B is a representative FACS analysis that demonstrates the elevation in the percentage of CD4+CD25+ T cells in the LN of dual APL-treated mice (12.5%), in comparison with mice immunized with p195–212 alone (10.01%) as observed 10 days after the concomitant immunization with p195–212 and administration of the dual APL.
Fig. 2.
The effect of dual APL administration on CD4+CD25+ T cell population in LN of SJL mice immunized with the myasthenogenic peptide p195–212. (A) SJL mice were injected intradermally in the hind footpads with 10 μg per mouse p195–212 in CFA (▪) and administered s.c. with the dual APL (200 μg per mouse in PBS; □). The percentage of CD4+CD25+ T cells was monitored daily by using FACS analysis. These results represent one experiment of three experiments performed. (B) SJL mice were treated as mentioned above. Ten days after the immunization with p195–212, LN cells were stained for CD4 and CD25 and analyzed by using FACS. The results presented are after reduction of the background staining obtained with the matched isotype controls, and they represent one experiment of three performed.
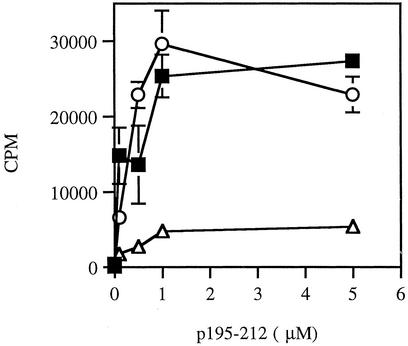
Depletion of CD4+CD25+ T Cells Abrogates the Inhibitory Effects of the Dual APL in SJL Mice. To determine the functional involvement of CD4+CD25+ cells in the suppressive action of the dual APL, we tested the effect of depletion of this cell population on the proliferative responses of LN cells of treated SJL mice. SJL mice were either administered s.c. with the dual APL concomitant with p195–212 immunization or immunized with the myasthenogenic peptide alone. Ten days after the immunization, we tested the proliferative responses of LN cells of treated mice that were depleted of CD25+ cells, in comparison with LN cells that did not undergo any manipulation. Fig. 3 demonstrates representative results of five experiments. As can be seen in Fig. 3, depletion of the CD4+CD25+ T cell population abrogated the inhibition of proliferation exerted by the dual APL. It is noteworthy that administration of the dual APL did not affect the proliferative response of the LN cells to Con A and that the depletion of CD4+CD25+ T cells increased slightly the latter, probably due to the removal of suppressive cells.
Fig. 3.
Depletion of CD4+CD25+ T cells abrogates the inhibitory effect of the dual APL on the proliferative responses of LN cells of SJL mice. SJL mice were either administered s.c. with the dual APL (200 μg per mouse in PBS) concomitant with immunization with p195–212 (10 μg per mouse in CFA) or immunized with the myasthenogenic peptide alone. Ten days after immunization with p195–212, popliteal LN cells obtained from p195–212-immunized mice (▪) and dual APL-treated mice, before (▵) and after (○) CD25+ T cell depletion, were cultured with different concentrations of p195–212, and a proliferation assay was performed as described in Materials and Methods. Results are expressed as mean cpm of triplicates ± SD values, and they represent five experiments performed.
The depletion of the CD4+CD25+ T cell population also had an effect on cytokine secretion by the LN cells. Thus, whereas nondepleted LN cells of dual APL-treated mice secreted higher levels of TGF-β in comparison with mice immunized with p195–212 alone, CD25+-depleted LN cells of treated mice secreted reduced levels of TGF-β similar to those secreted by LN cells of p195–212-immunized mice (Fig. 4). Fig. 4 depicts also the effect of the depletion on the secretion of IFN-γ, which is considered to be a pathogenic cytokine in MG. An opposite trend has been observed, namely, LN cells of dual APL-treated mice secreted significantly lower levels of IFN-γ, in comparison with cells of p195–212-immunized mice, whereas the levels of IFN-γ secreted by the CD25+-depleted LN cells were similar to those produced by the LN cells of mice that were not treated with the dual APL.
Fig. 4.
The effect of CD4+CD25+ T cell depletion from LN cells of dual APL-treated mice on the secretion of TGF-β and IFN-γ. LN cells of the tested mice (5 × 106 cells per ml) were stimulated with p195–212 (5 μM) for 48–72 h. Supernatants were collected, and the concentrations of TGF-β (A) and IFN-γ (B) were determined by ELISA.
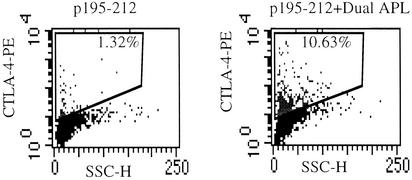
Anti-CTLA-4 Antibodies Abrogate the Inhibitory Effect of the Dual APL on the Proliferative Responses of LN Cells of SJL Mice. Because the inhibitory CTLA-4 costimulation molecule has been reported to be expressed on naturally occurring regulatory CD4+CD25+ T cells, we were interested in determining whether administration of the dual APL affects CTLA-4 expression on LN cells. For that purpose, SJL mice were either administered s.c. with the dual APL concomitant with the immunization with p195–212 or immunized with the myasthenogenic peptide alone. We followed CTLA-4 expression on the LN cells of the mice up to 4 days after dual APL administration. CTLA-4 expression reached a peak 72 h after dual APL administration. Thus, 0.12% vs. 1.18%, 1.32% vs. 10.63%, and 0% vs. 0.27% CTLA-4+ cells were determined in LN cells of mice immunized with p195–212 vs. LN cells of mice immunized with p195–212 and treated with the dual APL, 48 h, 72 h, and 96 h after the injections. Fig. 5 demonstrates the significant increase in CTLA-4 expression observed 72 h after APL administration, in LN cells of APL-treated mice in comparison with nontreated mice. The tendency shown in these representative results repeated itself in all five experiments performed. It is noteworthy that the dual APL did not have the same effect on CTLA-4 expression when the mice were immunized with ovalbumin and administered s.c. with the APL (data not shown).
Fig. 5.
The effect of dual APL administration on CTLA-4 expression on LN cells. SJL mice were either administered s.c. with the dual APL (200 μg per mouse in PBS) concomitant with immunization with p195–212 (10 μg per mouse in CFA) or immunized with the myasthenogenic peptide alone. LN cells were harvested 72 h after APL administration, and CTLA-4 expression was determined by using fluorescence staining.
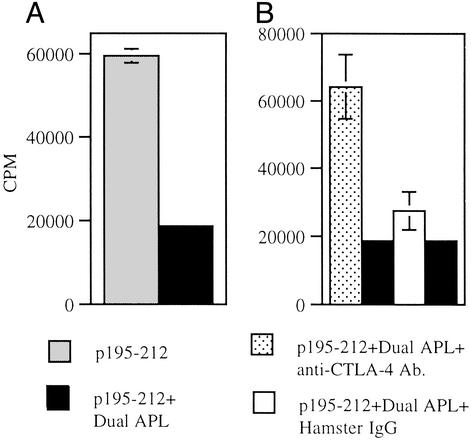
We wanted to determine whether the elevation in CTLA-4 expression on LN cells of treated mice observed after dual APL administration is involved in a functional manner in the diminished proliferative response of LN cells of these mice. Because we observed that the elevation in CTLA-4 expression peaks at 72 h after dual APL administration, SJL mice were immunized with the myasthenogenic peptide p195–212 and 7 days later administered s.c. with the dual APL, and their LN cells were harvested 10 days after p195–212 immunization. Fig. 6A shows that administration of the dual APL inhibited the proliferative responses of the LN cells whereas, in the presence of the anti-CTLA-4-neutralizing antibodies, the LN cells regained their capacity to proliferate in response to the priming antigen p195–212 (Fig. 6B). In contrast, the matched isotype control did not have the same effect on the proliferative responses of the LN cells of the dual APL-treated mice.
Fig. 6.
Anti-CTLA-4-neutralizing antibodies abrogate the inhibitory effect of the dual APL on the proliferative responses of LN cells of SJL mice. SJL mice were immunized with 10 μg per mouse p195–212 in CFA, and 7 days later part of them were administered s.c. with the dual APL (200 μg per mouse in PBS). Ten days after immunization with p195–212, a proliferation assay was performed as described in Materials and Methods. (A) Proliferation of LN cells cultured with different concentrations of p195–212. (B) Proliferation of LN cells in the presence of an anti-CTLA-4-neutralizing antibody or its matched isotype control. Results are expressed as mean cpm of triplicates ± SD values.
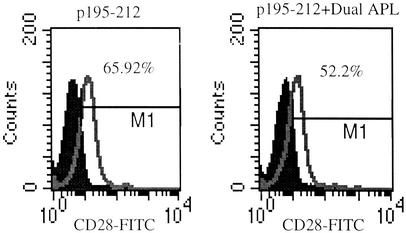
Administration of the Dual APL Down-Regulates the Expression of CD28 on LN Cells of Treated Mice. CD28 and CTLA-4 costimulation molecules were shown to have opposite effects on T cell activation, namely, CD28 being the one that supports T cell activation and CTLA-4 being the molecule that mediates the termination of the immune response. Unlike CTLA-4, which is induced 2–3 days after T cell activation and is not expressed constitutively on the cells, CD28 is expressed constitutively on T cells. Based on that, we wanted to test whether the dual APL has an effect on CD28 expression on LN-derived T cells of treated mice. SJL mice were either administered s.c. with the dual APL concomitant with p195–212 immunization, or immunized with p195–212 alone. LN-derived T cells were obtained from the mice at different time points after p195–212 immunization, and CD28 expression was determined by using FACS analysis. As can be seen in Fig. 7, a significant decrease (p195–212, 66.26%; p195–212+dual APL, 51.52%) was observed 24 h after dual APL administration. A similar effect was also observed 6 (p195–212, 66.34%; p195–212+dual APL, 60.18%) and 10 (p195–212, 66.7%; p195–212+dual APL, 59.26%) days after dual APL administration, but the differences between the nontreated and the treated mice were less profound. This effect was observed in three independent experiments performed.
Fig. 7.
The down-regulation of CD28 expression on LN-derived T cells of SJL mice after administration of the dual APL. SJL mice were either administered s.c. with the dual APL (200 μg per mouse in PBS) concomitant with immunization with p195–212 (10 μg per mouse in CFA) or immunized with the myasthenogenic peptide alone. LN-derived T cells were obtained by a procedure of panning and stained with anti-CD28-FITC antibody. Results are presented as percentage of CD28+ T cells found in LN 24 h after dual APL administration, and they represent one experiment of three experiments performed.
Discussion
The results presented in this study show that s.c. administration of the dual APL up-regulated CD4CD25-expressing cells in the spleens and LN of SJL mice. Furthermore, depletion of these cells diminished significantly the inhibitory effect of the dual APL on p195–212-specific proliferative responses and decreased TGF-β secretion. The dual APL up-regulated CTLA-4 expression whereas it down-regulated CD28 expression on LN cells. In vitro blockade of CTLA-4 function abrogated the inhibitory effect of the dual APL on the proliferative responses specific to p195–212. Our results suggest that the active suppression exerted by the dual APL is mediated by CD4+CD25+ cells via up-regulation of CTLA-4 expression and TGF-β secretion.
The induction of tolerance is essential for the maintenance of immune homeostasis and for the prevention of autoimmune diseases. CD4+CD25+ T cells have been shown to inhibit autoimmune diabetes in mice and rats (20, 22), and depletion of these cells in murine adoptive transfer models was shown to lead to various autoimmune diseases (17). Furthermore, these cells were shown to prevent the expansion of other T cells in vivo (23) and inhibit T cell activation in vitro (24–25, 26). We show in the present study that, on APL administration, the CD4+CD25+ cells expanded and peaked, in comparison with nontreated mice, at day 5. This first peak is followed by a decrease in the size of this cell population and an additional increase toward day 10. Because the inhibitory effects (inhibition of proliferative responses and an increase in TGF-β secretion) of the dual APL were observed at day 10, we suggest that the peak in the size of the CD4+CD25+ cell population at this day is responsible for the observed suppressive effects. The reason for the decrease in the size of this population observed after day 5 is not clear, but this trend repeated itself in all of the experiments performed.
We have used in the present study the cell depletion technique to confirm the role of CD4+CD25+ cells in the inhibition of proliferation of LN cells of APL-treated mice. We show here that the depletion of this population of immunoregulatory cells abrogated the suppressive effect exerted by the dual APL on the proliferative responses of the LN cells, namely, these cells play a significant role in the active suppression of the myasthenogenic-associated T cell responses by the dual APL. We have previously demonstrated that the active suppression exerted by the APL is mediated, at least partially, by the up-regulation of the secretion of TGF-β, which was accompanied by down-regulation of IFN-γ and IL-2 secretion (15).
It has been reported that cytokines are not essential for the suppression mediated by the CD4+CD25+ cells in vitro. It has been hypothesized that cell–cell contact on the surface of APCs may lead to reduced IL-2 production and proliferation of CD4+CD25— T cells by CD4+CD25+ T cells. This notion was supported by the finding that exogenous IL-2 or stimulation with anti-CD28 monoclonal antibodies restored the anti-CD3–induced proliferation of CD4+CD25+ Treg cells and abrogated the suppression in a mixture of CD25— and CD25+ T cells (24, 27). It is noteworthy that we have previously shown that recombinant IL-2 could abrogate the inhibition of LN cell proliferative responses exerted by the dual APL (15). In contrast to in vitro findings, studies using regulatory T cells in vivo in different settings and different autoimmune models have clearly demonstrated the importance of both IL-10 and TGF-β for the function of regulatory T cells. Because we tested the involvement of CD4+CD25+ cells in an in vivo model, we suggest that in our case there is an important role for cytokines in the mechanism by which these immunoregulatory cells exert their inhibitory effect/s. Recently, it was proposed by Jonuleit et al. (28) that the CD4+CD25+ T cells may not represent the regulatory T cell population per se, but rather a population that is required for the differentiation and expansion of bona fide regulatory T cells that release inhibitory mediators such as biologically active TGF-β. This secondary systemic suppressive effect mediated by induced suppressor cells is cell contact independent (28). We show here that CD4+CD25+ cell depletion resulted in a decrease in TGF-β secretion, accompanied by an elevation in IFN-γ secretion. The effect of the depletion on TGF-β secretion was observed in most of the experiments, but not in all of them. It is possible that, in those cases where we could not observe the effect of the dual APL on TGF-β secretion, the relatively high number of immunoregulatory cells, induced by the dual APL, exerted their inhibitory effect through cell-to-cell contact and not through TGF-β secretion. On the other hand, in the experiments in which up-regulated secretion of TGF-β was observed, the number of immunoregulatory cells was lower, and a secondary systemic effect mediated by induced suppressor cells secreting TGF-β was required for an efficient inhibitory effect of the APL. It thus seems that TGF-β is involved in the inhibitory effect of the dual APL, but in certain circumstances this effect can be mediated without the presence of this cytokine.
To further elucidate the mechanism/s by which the dual APL suppresses myasthenogenic-associated T cell responses, we tested whether it has an effect on the expression of the costimulation molecules CD28 and CTLA-4. We show here that dual APL administration down-regulated CD28 expression on LN-derived T cells while up-regulating CTLA-4 expression. Furthermore, anti-CTLA-4-neutralizing antibodies abrogated the inhibitory effect of the dual APL on the LN cells' proliferative responses. It is noteworthy that the dual APL did not have the same effect on CTLA-4 expression when the mice were immunized with ovalbumin and administered s.c. with the dual APL (data not shown). Based on these results, it seems that, in this specific early stage of activation of the T cells, the dual APL increases CTLA-4 expression on a very small population of cells, namely cells that encountered p195–212, and has no effect on cells primed with another antigen.
It is currently thought that CD28 provides naive T cells with a costimulatory signal for promoting IL-2 formation and cell expansion as well as preventing induction of anergy and cell death. On the other hand, CTLA-4 that is expressed on T cell activation, apparently transduces a negative signal to activated T cells, thereby attenuating the T cell responses (29–31). Furthermore, it was previously demonstrated that CTLA-4 expression is up-regulated after T cell activation and reaches a maximum after 2–3 days (32), a range of time that correlates with the peak in CTLA-4 expression observed by us, on dual APL administration.
It was previously demonstrated that a costimulatory signal through CTLA-4 is required for the activation of CD4+CD25+ Treg cells to mediate suppression (19–21). However, the up-regulation in CTLA-4 expression observed by us does not exclude the possibility that CTLA-4 is not the only accessory molecule required for the activation of the CD4+CD25+ cells. In contrast to CTLA-4, the CD4+CD25+ Treg cells apparently do not need CD28 for their activation (21, 24, 27). We cannot be certain that the changes in CD28/CTLA-4 expression on the dual APL administration occurred only on CD4+CD25+ cells. Nevertheless, the results mentioned above regarding these costimulatory molecules suggest that the dual APL induces the suppressive activity of the immunoregulatory cells by up-regulating the expression of the inhibitory costimulatory molecule (CTLA-4) while decreasing the expression of the activating costimulatory molecule (CD28). Alternatively, it is possible that the up-regulation of CTLA-4 expression occurred on CD4+CD25+ cells and the down-regulation of CD28 occurred on the pathogenic T cells. The up-regulation of CTLA-4 expression is in agreement with the up-regulation of TGF-β secretion observed on the administration of the dual APL. It has been reported that cross-linking of CTLA-4 may enhance production of TGF-β by activated T cells (33, 34). This finding raises the possibility that CTLA-4 does not directly inhibit T cell activation but does so by the active induction of this inhibitory cytokine.
Most of the reports on CD4+CD25+ Treg cells are of studies performed with naive animals. Nevertheless, recently it was shown that systemic administration (i.v. and oral) of ovalbumin induced peripheral tolerance by the generation of immunoregulatory CD4+CD25+ T cells (35, 36). The results presented in the present study demonstrate that the CD4+CD25+ cells are definitely involved in the active suppression exerted by the dual APL on the myasthenogenic-associated T cell responses. Although further studies will determine whether these CD4+CD25+ cells are identical to the naturally occurring CD4+CD25+ Treg cells, there are sufficient similarities in the phenotypes and function of these cells (e.g., CTLA-4 up-regulation, abrogation of their inhibitory effect on depletion and TGF-β secretion) to raise the possibility that they are derived from the naturally occurring CD4+CD25+ Treg cells. Although the full mechanism/s used by these immunoregulatory cells to mediate their suppressive effect is not completely elucidated yet, the results presented here indicate that the CD4+CD25+ cells act, at least partially, through an up-regulation of the inhibitory costimulating molecule CTLA-4. These cells may secrete TGF-β either by themselves (through CTLA-4 signaling) or in a non-direct manner that triggers other regulatory T cell populations to secrete this suppressive cytokine.
Acknowledgments
This research was supported by Peptor, Israel (M.S. and E.M.).
Abbreviations: APL, altered peptide ligand; CTLA, cytotoxic T lymphocyte antigen; LN, lymph node; MG, myasthenia gravis; Treg, T regulatory; AChR, acetylcholine receptor; TGF, transforming growth factor; CFA, complete Freund's adjuvant; FACS, fluorescence-activated cell sorter; PE, phycoerythrin.
References
- 1.Drachman, D. B. (1994) N. Engl. J. Med. 330, 1797–1810. [DOI] [PubMed] [Google Scholar]
- 2.Lindstrom, J., Shelton, D. & Fuji, Y. (1994) Adv. Immunol. 42, 233–284. [DOI] [PubMed] [Google Scholar]
- 3.Shi, F. D., Li, H., Wang, H., Bai, X., van der Meide, P. H., Link, H. & Ljunggren, H. G. (1999) J. Immunol. 162, 5757–5763. [PubMed] [Google Scholar]
- 4.Karachunski, P. I., Ostlie, N. S., Okita, D. K. & Conti-Fine, B. M. (1997) J. Clin. Invest. 100, 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang, G. X., Navikas, V. & Link, H. (1997) Muscle Nerve 20, 543–551. [DOI] [PubMed] [Google Scholar]
- 6.Baggi, F., Andreetta, F., Caspani, E., Milani, M., Longhi, R., Mantegazza, R., Cornelio, F. & Antozzi, C. (1999) J. Clin. Invest. 104, 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang, G. X., Xia, B. G., Bakhiet, M., van der Meide, P. H., Wigzell, H., Link, H. & Olsson, T. (1996) J. Exp. Med. 184, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brocke, S., Brautbar, C., Steinman, L., Abramsky, O., Rothbard, J., Neumann, D., Fuchs, S. & Mozes, E. (1988) J. Clin. Invest. 82, 1894–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brocke, S., Dayan, M., Rothbard, J., Fuchs, S. & Mozes, E. (1990) Immunology 69, 495–500. [PMC free article] [PubMed] [Google Scholar]
- 10.Katz-Levy, Y., Paas-Rozner, M., Kirshner, S., Dayan, M., Zisman, E., Fridkin, M., Wirguin, I., Sela, M. & Mozes, E. (1997) Proc. Natl. Acad. Sci. USA 94, 3200–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz-Levy, Y., Dayan, M., Wirguin, I., Fridkin, M., Sela, M. & Mozes, E. (1998) J. Neuroimmunol. 85, 78–86. [DOI] [PubMed] [Google Scholar]
- 12.Kirshner, S. L., Zisman, E., Fridkin, M., Sela, M. & Mozes, E. (1996) Scand. J. Immunol. 44, 512–521. [DOI] [PubMed] [Google Scholar]
- 13.Zisman, E., Katz-Levy, Y., Dayan, M., Kirshner, S. L., Paas-Rozner, M., Karni, A., Abramsky, O., Brautbar, C., Fridkin, M., Sela, M. & Mozes, E. (1996) Proc. Natl. Acad. Sci. USA 93, 4492–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paas-Rozner, M., Dayan, M., Paas, Y., Changeux, J. P., Wirguin, Y., Sela, M. & Mozes, E. (2000) Proc. Natl. Acad. Sci. USA 97, 2168–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paas-Rozner, M., Sela, M. & Mozes, E. (2001) Proc. Natl. Acad. Sci. USA 98, 12642–12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155, 1151–1164. [PubMed] [Google Scholar]
- 17.Shevach, E. M. (2002) Nat. Rev. Immunol. 2, 389–400. [DOI] [PubMed] [Google Scholar]
- 18.Jonuleit, H. E., Schmitt, M., Stassen, A., Tuettenberg, J. & Enk, A. H. (2001) J. Exp. Med. 193, 1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Read, S., Malmstrom, V. & Powrie, F. (2000) J. Exp. Med. 192, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salomon, B., Lenscow, D. J., Rhee, L., Ashourian, N., Singh, B., Sharpe, A. & Bluestone, J. A. (2000) Immunity 12, 431–440. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi, T., Tagami, T., Yamazaki, S., Uede, T., Shimizu, J., Sakaguchi, N., Mak, T. W. & Sakaguchi, S. S. (2000) J. Exp. Med. 192, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens, L. A. & Mason, D. (2000) J. Immunol. 165, 3105–3110. [DOI] [PubMed] [Google Scholar]
- 23.Annacker, O., Pimenta-Araujo, R., Burlen-Defranoux, O., Barbosa, T. C., Cumano, A. & Bandeira, A. (2001) J. Immunol. 166, 3008–3018. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi, T., Kuniyasu, Y., Yoda, M., Sakaguchi, N., Itoh, M., Iwata, M., Shimizu, J. & Sakaguchi, S. (1998) Int. Immunol. 10, 1969–1980. [DOI] [PubMed] [Google Scholar]
- 25.Thornton, A. E. & Shevach, E. M. (1998) J. Exp. Med. 188, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thornton, A. M. & Shevach, E. M. (2000) J. Immunol. 164, 183–190. [DOI] [PubMed] [Google Scholar]
- 27.Itoh, M., Takahashi, T., Sakaguchi, N., Kuniyasu, Y., Shimizu, J., Otsuka, F. & Sakaguchi, S. (1999) J. Immunol. 162, 5317–5326. [PubMed] [Google Scholar]
- 28.Jonuleit, H., Schmitt, E., Kakirman, H., Stassen, M., Knop, J. & Enk, A. H. (2002) J. Exp. Med. 196, 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenschow, D. J., Walunas, T. L. & Bluestone, J. A. (1996) Annu. Rev. Immunol. 14, 233–258. [DOI] [PubMed] [Google Scholar]
- 30.Thompson, C. B. & Allison, J. P. (1997) Immunity 7, 445–450. [DOI] [PubMed] [Google Scholar]
- 31.Bluestone, J. A. (1997) J. Immunol. 158, 1989–1993. [PubMed] [Google Scholar]
- 32.Walunas, T. L., Lenschow, D. J., Bakker, C. Y., Linsley, P. S., Freeman, G. J., Green, J. M., Thompson, C. B. & Bluestone, J. A. (1994) Immunity 1, 405–413. [DOI] [PubMed] [Google Scholar]
- 33.Chen, W., Jin, W. & Wahl, S. M. (1998) J. Exp. Med. 188, 1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomes, N. A., Gattas, C. R., Barreto-de-Souza, V., Wilson, M. E. & DosReis G. A. (2000) J. Immunol. 164, 2001–2008. [DOI] [PubMed] [Google Scholar]
- 35.Thorstenson, K. M. & Khoruts, A. (2001) J. Immunol. 167, 188–195. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, X., Izikson, L., Liu, L. & Weiner, H. L. (2001) J. Immunol. 167, 4245–4253. [DOI] [PubMed] [Google Scholar]