Abstract
Type 1 diabetes occurs as a result of an autoimmune attack on the insulin-producing beta cells. Although CD8 T cells have been implicated both early and late in this process, the requirement for direct interaction between these cells and MHC class I on the beta cells has not been demonstrated. By using nonobese diabetic mice lacking beta cell class I expression, we show that both initiation and progression of insulitis proceeds unperturbed. However, without beta cell class I expression, the vast majority of these mice do not develop hyperglycemia. These findings demonstrate that a direct interaction between CD8 T cells and beta cells is not required for initiation or early disease progression. The requirement for class I on beta cells is a relatively late checkpoint in the development of diabetes.
Keywords: T lymphocytes, nonobese diabetic, severe combined immunodeficient
Type I diabetes (T1D) is a disease in which the insulin-producing beta cells are specifically killed by infiltrating mononuclear cells. The mononuclear infiltrate, termed insulitis, contains a heterogeneous population of cells, including macrophages, CD8 T cells, CD4 T cells, and B cells (1–3). It has been shown that all nonobese diabetic (NOD) mice develop insulitis; however, in many cases this inflammation is “benign” in that it does not result in the destruction of the insulin-producing beta cells, causing hyperglycemia (4–6). Only in a proportion of mice does the inflammatory process become “malignant” and result in death of the beta cells and consequent hyperglycemia. The events that precipitate this switch from benign to malignant inflammation are not well understood.
A large body of evidence using histological analysis and cell transfers suggests that both CD8 and CD4 T cells are absolutely required for insulitis and diabetes development in NOD mice (7, 8); the presence of beta cells and their antigens is also required, because a diabetogenic splenocyte population did not develop in NOD mice that had undergone early ablatement of beta cells (9, 10). Notably, it is not known whether, and if so when, T cells require cognate interaction with the islet beta cells, or whether disease initiation and progression to destructive insulitis can occur as a result of T cell interactions with other local MHC-positive cells. Our understanding of this process is critical to the design of a strategy to regulate the disease in predisposed individuals, and in particular, a mechanistic understanding of the cellular events that cause the final killing of beta cells is crucial to prevention of disease recurrence in patients undergoing islet transplantation.
Consistent with the essential role of T cells in disease is the involvement of the MHC in disease susceptibility (11). Although at least 15 susceptibility regions that contribute to disease may exist, the strongest susceptibility region in both the human disease and the NOD mouse model of diabetes is the MHC region (11 12). Identification of the individual genes within the MHC has proven problematic because of strong linkage disequilibrium; however, both the MHC class I and MHC class II genes have been implicated in susceptibility to diabetes in both humans and mice (11–14).
If a direct interaction between T cells and islet beta cells is required for the initiation of inflammation or for progression of the inflammatory lesion to become destructive of beta cells, it is likely to be by way of CD8 T cells rather than CD4 T cells. Islet beta cells express MHC class I, which is up-regulated during the inflammatory response due to the effect of cytokines such as γIFN (15, 16). Expression of MHC class II by islet beta cells in NOD mice has not been detected and it is therefore thought that the role of CD4 T cells in disease is more likely to be interaction with local class II-positive antigen-presenting cells (APCs) (17). It has been shown that when splenic T cells are transferred from young prediabetic NOD donors, both CD8 T cells and CD4 T cells are required to efficiently transfer diabetes to naive NOD scid recipients (8). It has also been shown that depletion of CD8 T cells by treatment with monoclonal antibody prevents insulitis (18) and diabetes development in cyclophosphamide-treated NOD mice (19). In addition, β2-microglobulin (β2M)–/– NOD mice, which lack MHC class I expression and CD8 T cells, do not develop insulitis (20–23). However, despite numerous studies, the precise role of CD8 T cells in the disease process has remained unclear and confusing. In particular, although evidence exists that beta cell-specific CD8 T cells are recruited into the islets, no evidence demonstrates an essential role for the direct interaction between CD8 T cells and islet beta cells for the initiation or progression of disease. Furthermore, evidence implicating CD8 T cells as the final effector of beta cell destruction is limited and conflicting (24–28).
In this article we describe NOD mice that have been genetically modified to lack class I expression on their islet beta cells, but express class I normally in all other tissues. Lymphocyte development occurs normally in these mice producing an unmanipulated repertoire. These mice have been compared with their littermates to examine the role of beta cell class I expression in the development of insulitis and for the switch from benign to malignant insulitis, which results in hyperglycemia.
Experimental Procedures
Mice. All mice were housed under specific pathogen-free conditions at the John Curtin School of Medical Research. NOD/LtJcs mice were obtained from the Animal Breeding Establishment, Australian National University. NOD-β2M–/– mice were a gift from T. Kay (St. Vincent's Institute of Medical Research, Melbourne; ref. 29) and were used at the 11th backcross onto the NOD background.
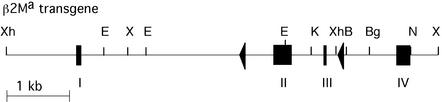
Generation of a NOD-β2Ma Transgenic Founder. To investigate the effect of deletion of the β2M gene from islet beta cells on diabetes development in the NOD mouse, a β2Ma transgene flanked by dormant loxP sites was made (Fig. 1). The β2Ma gene construct was made and injected into NOD zygotes to create β2Ma transgenic NOD founder mice. One founder was subsequently crossed onto the NOD-β2M–/– background as described (14). Wild-type β2M, transgenic β2M, and β2M– genes were distinguished by PCR as described (14). The incidence of diabetes in β2Maβ2M–/– NOD mice was not significantly different from control β2M+/– NOD mice (27%) (14).
Fig. 1.
The β2Ma transgene was constructed as described (14). In brief, exons II and III of the β2Ma gene were flanked by 150-bp loxP sites inserted into the NdeI site of intron A and the BamHI site of intron C. Thin lines, introns and flanking DNA; boxes, exons; triangles, loxP sites; B, BamHI; Bg, BglI; E, EcoRI; N, NdeI; X, XbaI; and Xh, XhoI.
Generation of a Line of Transgenic β2Maβ2M–/– HIP-Cre NOD Transgenic Mice. The HIP-Cre NOD transgenic mice used in this study contain the gene for the enzyme Cre under the control of the human insulin promoter (HIP). The HIP-Cre construct was made by joining the 1.9-kb 5′ region of the human insulin promoter flanked by BamHI and NcoI sites (gift from Genentech; ref. 30), to the 1.2-kb Cre MluI fragment excised from the plasmid pMC-Cre (gift from DNAX; ref. 31). This construct was injected into NOD zygotes to create transgenic NOD founder mice. Of the three lines generated, one was found to express Cre in a tissue-specific manner. This line was subsequently crossed onto the NOD-β2M–/– background. The HIP-Cre transgene was identified by a PCR-based strategy with the primers GTCGATGCAACGAGTGATGAGGTT and TGCTAACCAGCGTTTTCGTTCTGCC specific for the Cre gene. The incidence of diabetes in the HIP-Cre NOD transgenic mice was identical to the background incidence occurring in NOD mice in the colony (data not shown). HIP-cre NODβ2M–/– mice were then crossed to β2Maβ2M–/–NOD mice to generate HIP-creNODβ2Maβ2M–/– mice.
Detection of Cre-Mediated Recombination. DNA was purified from spleen, thymus, mesenteric lymph nodes, and isolated islets by standard methods. DNA was purified from peripheral blood by using the QIAamp blood kit (Qiagen, Valencia, CA). Recombination between the loxP sites of the β2M gene was detected by PCR with the primers CGAACATTACATTATTTACATTCCAGAC and GCAGGCGTATGTATCAGTCTCAGT.
Monitoring of Hyperglycemia. Mice were monitored regularly for glycosuria by using TesTape (Eli Lilly). Once glycosuria was present, hyperglycemia was confirmed by measurement with a Medisense (Waltham, MA) companion 2 m and scored as diabetic after two positive readings above 15 mmol/liter.
Cyclophosphamide Treatment. Diabetes was accelerated by i.p. injection of 300 mg/kg cyclophosphamide (cycloblastin; Pharmacia), dissolved in PBS. Mice were then monitored for 17 days for the onset of diabetes.
Histology. The degree of insulitis was scored by blind histological analysis of hematoxylin/eosin-stained sections taken from 250- to 300-day-old β2Maβ2M–/– Cre+, β2Maβ2M–/– Cre-, β2M–/–, and β2M+/– male and female mice. Paraffin-embedded sections were cut at three levels, and between 10 and 100 islets were scored from each pancreas. The proportion of islets exhibiting each level (grades 0–4) of insulitis was determined and expressed as a percentage of the total number of islets scored. Grade 0 represents no infiltrate, grade 1 represents periductal accumulation of mononuclear cells, grade 2 represents circumferential accumulation of mononuclear cells, grade 3 represents intraislet infiltration, and grade 4 represents severe structural derangement and complete loss of beta cells. The phenotype of the insulitis lesion was also examined by immunohistochemical staining of frozen Tissue-Tek OCT compound (Bayer, Elkhart, IN)-embedded and acetone-fixed sections taken from 250-day-old female β2Maβ2M–/– Cre+, β2Maβ2M–/– Cre–, and β2M+/– NOD mice. Primary antibodies for CD4+ T cells (GK1.5), CD8+ T cells (53.6), and macrophages (F4/80) were used as indicated and followed by horseradish peroxidase-conjugated anti-rat Ig (DAKO). Color was developed with diaminobenzidine tetrahydrochloride and sections counterstained with hematoxylin. Insulin was stained for using anti-swine insulin (DAKO) and peroxidase–antiperoxidase methodology (DAKO).
Preparation of Isolated Islet Cell Suspensions. Pancreatic islets were isolated by collagenase digestion and hand picking as described (6). If cells were to be examined for MHC class I expression whole islets were first cultured for 18 h in RPMI 1640 culture medium containing 100 units of IFNγ (gift from A. Hapel, John Curtin School of Medical Research), 10% FCS and penicillin, streptomycin, gentamicin, and 4 mM L-glutamine. Single cell islet suspensions were made by digestion for 10 min with trypsin-EDTA (GIBCO/BRL) at 37°C followed by passage through a blunted 20-gauge needle five times.
Fluorescence-Activated Cell Sorting (FACS). The content of the insulitis lesion was quantified by FACS staining of islet cell suspensions taken from 250-day-old female β2Maβ2M–/– Cre+, β2Maβ2M–/– Cre–, and β2M+/– mice. Cells were stained with anti-CD4 (GK1.5-biotin), anti-CD8 (53.6-FITC), anti-CD45R (B220-PE), anti-H-2Kd (SF-1.1-biotin), anti-H-2Db (28-14-8-biotin; PharMingen), and anti-CD3 biotin (Exalpha, Boston), followed by streptavidin-PE (Serotec). T cells specific for an insulin peptide (amino acids 15–23) in the context of H-2Kd were stained for using the tetramer G9, and the Listeria monocyto-genes-specific tetramer LLO was used as a control (32). Analysis was then performed with a FACScan (BD Biosciences). Expression of H-2Kd on islet beta cells was determined by using the biotinylated antibody SF-1.1 (PharMingen) followed by streptavidin-APC (Biomedia, Foster City, CA). H-2Kd-positive and -negative cells were sorted by using a FACStar (BD Biosciences) into eight-well chamber slides before acetone fixation and staining for insulin. Dead cells were identified and excluded by using propidium iodide.
Statistics. Diabetes incidence was compared by using the log-rank test. Two-tailed P values were calculated by using an unpaired Student's t test for column comparisons, and Fisher's exact test was used for contingency table comparison.
Results
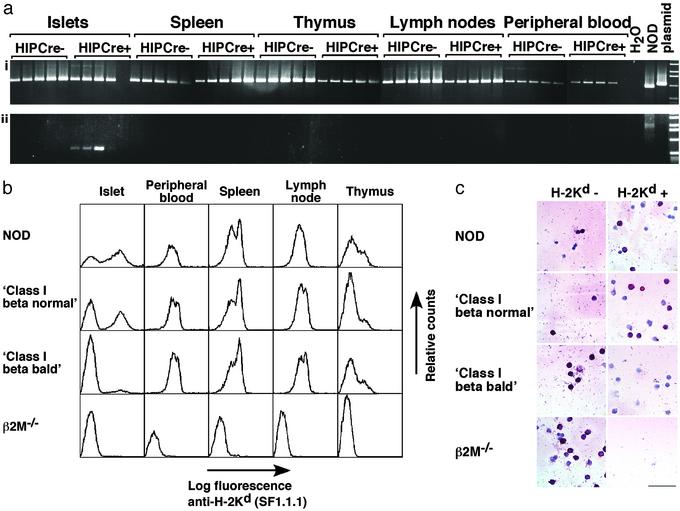
Cre-Mediated Disruption of the β2M Gene Occurs Specifically in the Islet. The β2Ma transgene contains dormant loxP sites in introns A and C (Fig. 1; ref. 14). Cre-mediated recombination of these loxP sites was predicted to cause excision of exons II and III, removing the coding regions for amino acids 4–99 of the 99-aa β2M protein. The NOD β2Maβ2M–/– transgenic mice were crossed with NOD HIP-Cre β2M–/– mice and examined to determine whether Cre-mediated recombination was taking place in the islet beta cells. To test whether the enzyme Cre was being expressed in the pattern expected and was mediating recombination in vivo, genomic DNA was prepared from the islets, spleen, thymus, mesenteric lymph nodes, and peripheral blood of Cre-positive and Cre-negative littermate β2Maβ2M–/– mice. Analysis by PCR determined that the recombined form of the β2M gene was present in the islets of β2Ma HIP-Cre double-positive mice (Fig. 2a). It was detected by using as little as 6.25 ng of template DNA and was not seen in other tissues even by using concentrations of template DNA that were 10 times higher. It was therefore concluded that Cre expression was able to mediate precise recombination of the β2Ma transgene in vivo and that recombination was occurring in an islet-specific manner.
Fig. 2.
(a) HIP-Cre-mediated recombination occurs specifically in islets. DNA (6.25, 12.5, 25, 50, and 100 ng) was prepared from islets, spleen, thymus, mesenteric lymph nodes, and peripheral blood of male 100-day-old β2Maβ2M–/– HIP-Cre– and HIP-Cre+ NOD mice. (i) Control PCR, which amplifies a 1.1-kbp product from the β2Ma transgene, a 950-bp product from the endogenous β2Ma gene, and a 2.5-kbp product from the β2M–/– gene (not seen in all samples). (ii) PCR to detect the recombined β2Ma transgene, which amplifies an 800-bp product from the HIP-Cre-recombined gene and a 2.5-kbp product from the unrecombined gene (seen in the NOD control only). (b) Expression of H-2Kd is normal in all class I beta normal and class I beta bald NOD transgenic mice except the islets of class I beta bald transgenic mice, where expression is lost from the surface of most islet cells. Single-cell suspensions were made from islets, peripheral blood, spleen, mesenteric lymph nodes, and thymus of NOD class I beta normal, class I beta bald, and β2M–/– sex-matched 70- to 160-day-old NOD mice. Cells were stained with biotinylated SF1.1.1 and streptavidin APC and analyzed by FACS. (c) HIP-cre-mediated recombination occurs specifically in islet beta cells. Islet cells were isolated from mouse pancreas and stained with SF1.1.1 (H-2Kd specific), then FACS-sorted into H-2Kd-positive and H-2Kd-negative pools (excluding dead cells). The sorted cells were then stained for insulin (red cells). Cells from NOD, class I beta normal, class I beta bald, and β2M–/– NOD mice are shown. (Bar = 100 μm.)
Specific Loss of MHC Class I Expression from the Islet Cells of β2Maβ2M–/–Cre+ Mice. FACS analysis of NOD β2Maβ2M–/– Cre+ and Cre– littermates confirmed that β2M-dependent expression of H-2Kd (Fig. 2b) and H-2Db (data not shown) was normal in all tissues except islets. The population of class I-negative islet cells was much larger in β2Maβ2M–/– Cre+ mice. To ascertain the percentage of islet beta cells that had lost class I expression, single-cell-suspended islet cells from NOD β2Maβ2M–/– Cre+ and Cre– littermates were FACS sorted according to H-2Kd expression and then stained for insulin. Analysis of islet cells from β2Maβ2M–/– Cre+ mice by this method determined that the mean proportion of MHC class I-negative islet beta cells was not significantly different from that seen in β2M–/– mice; and Cre– littermates expressed normal levels of class I on islet beta cells (Fig. 2c). β2Maβ2M–/– Cre+ NOD mice were then referred to as “class I beta bald” mice and β2Maβ2M–/– Cre– mice as “class I beta normal” mice. To ascertain the percentage of islet non-beta cells that had lost class I expression, the same method was used and insulin-negative islet cells were counted. No significant differences were found in the percentage of class I-negative, insulin-negative islet cells in class I beta bald NOD mice and class I beta normal NOD mice.
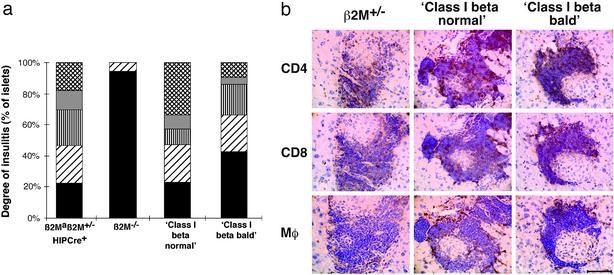
Initiation and Early Progression of Insulitis Is Independent of Islet Beta Cell Class I Expression. The class I beta bald mice were used to address the question of whether expression of MHC class I on islet beta cells is necessary for initiation and progression of insulitis. The degree of insulitis was not significantly different between class I beta normal, class I beta bald, and β2M+/– NOD mice (P < 0.05; Fig. 3a). The phenotype of the insulitis lesion was also examined by immunohistochemical staining of frozen sections taken from 250-day-old female class I beta bald, class I beta normal, and β2M+/– NOD mice for CD4+ T cells, CD8+ T cells, and macrophages. No qualitative differences in the insulitis lesion between class I beta bald, class I beta normal, and β2M+/– NOD mice were observed (Fig. 3b). The content of the insulitis lesion was quantified by FACS staining of islet cell suspensions taken from 250-day-old female class I beta bald, class I beta normal, and β2M+/– NOD mice. No differences were seen in the proportions of CD4+ T cells, CD8+ T cells, CD8+ anti-insulin/H-2Kd clonotype (G9 tetramer), CD3+ T cells, or B cells found in the lymphoid population of the islet cell suspension (Table 1).
Fig. 3.
(a) Degree of insulitis was not reduced in class I beta bald mice by histological analysis. Insulitis is scored in the following way: 0, no insulitis; 1, periductal insulitis; 2, circumferential insulitis; 3, intraislet infiltration; and 4, severe structural derangement. The mean scores of female β2Maβ2m+/– HIP-Cre+ (n = 7), β2M–/– (n = 7), class I beta normal (n = 5), and class I beta bald (n = 13) NOD mice are shown. Black bars, 0; diagonal lines, 1; vertical lines, 2; gray, 3; and cross-hatched lines, 4. The proportion of islets exhibiting each level of insulitis severity was not significantly different between class I beta normal and class I beta bald NOD mice (P > 0.05). (b) Content of lymphocytic infiltrate of grade 3 islets from class I beta bald and class I beta normal NOD mice. Consecutive frozen sections taken from 250-day-old female mice were stained with the antibodies 53.6 (CD8 specific), GK1.5 (CD4 specific), and F4/80 (macrophage specific). Sections from β2M+/–, class I beta normal, and class I beta bald NOD mice are shown. (Bar = 10 mm.)
Table 1. Proportions of lymphocyte cell subsets (%) in the islet infiltrate of class I beta normal and class I beta bald NOD mice as determined by FACS analysis.
| Mice* | B220+ | CD4+ | CD8+ |
|---|---|---|---|
| NOD-β2m+/- | 22.3 | 27.97 | 4.5 |
| Class I beta normal | 27.3 | 30.7 | 6.7 |
| Class I beta bald | 22.3 | 24.1 | 4.9 |
Pooled results from two female mice aged >5 months.
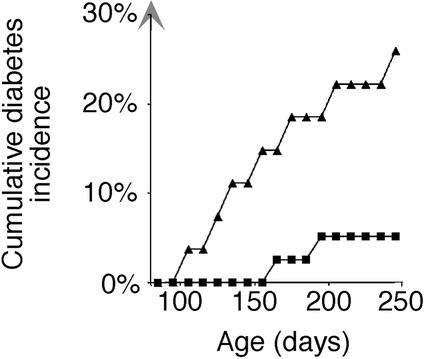
The Switch from Benign to Malignant Insulitis Resulting in Hyperglycemia Depends on Islet Beta Cell Class I Expression. The class I beta bald mice were used to investigate whether expression of MHC class I on islet beta cells is necessary for the switch from benign insulitis to “destructive” insulitis resulting in the development of hyperglycemia. A significant reduction occurred in the incidence of hyperglycemia in the class I beta bald mice that lacked beta cell class I expression compared with class I beta normal mice (Fig. 4). Whereas only 2 of 39 class I beta bald NOD mice developed hyperglycemia by 250 days, 7 of 27 class I beta normal littermates developed diabetes by the same age (P < 0.02, log-rank test). Disease onset was later in class I beta bald mice; between 170 and 250 days, compared with between 110 and 250 in class I beta normal mice.
Fig. 4.
Incidence of diabetes was significantly reduced in female class I beta bald NOD mice. Class I beta normal (▴, n = 27), and class I beta bald (▪, n = 39) NOD mice were followed for 250 days for development of diabetes. The incidence in class I beta bald NOD mice was significantly lower than littermate class I beta normal NOD mice (P < 0.020).
Cyclophosphamide has been shown to accelerate disease in NOD mice with established insulitis (32). Recent studies provide evidence that this is due to nonspecific activation of inflammatory pathways (33, 34). To determine whether the benign insulitis found in class I beta bald NOD mice could be converted to a destructive form, 250-day-old class I beta bald, NOD β2M+/–, and NOD mice were injected with cyclophosphamide. Cyclophosphamide injection of class I beta bald mice did not significantly increase the incidence of diabetes (1 of 8, 13%) above that seen spontaneously in these mice (2 of 39, 5%; P = 0.436). In contrast, disease was accelerated in NOD (5 of 6, 83%) and NOD β2M+/– (5 of 9, 56%) mice.
Discussion
Understanding the initiating events and processes of beta cell destruction are of enormous importance for understanding how to regulate T1D. Although this disease has generally been described as an autoimmune response directed specifically at the insulin-producing beta cells, it has not been demonstrated that the insulitis lesion that precedes beta cell destruction is in fact beta cell-specific. Although the disease is thought to be CD8 T cell-mediated, it is possible that the role of CD8 T cells in the initiation phase is not beta cell-specific. In this article the role of beta cell MHC class I in T1D was assessed in terms of the initiation and progression of disease in the NOD mouse model, yielding new perspectives on the disease process.
The role of CD8 T cells in the etiology of T1D has presented a confusing picture in the past, perhaps because the role of this population of cells changes as the inflammatory response surrounding the islets progresses from a non-beta cell-specific insulitis, to a beta cell-destructive process. The fact that β2M–/– NOD mice, which lack CD8 T cells, were found not to develop insulitis implicated CD8 T cells as having a crucial role in initiation of disease (20–23). However, from studies in these mice it was not known whether the requirement for CD8 T cells was only with APCs cross-presenting islet cell antigens, or whether CD8 T cells also had an essential interaction directly with class I on the beta cells. The class I beta bald NOD mice described in this article lack class I expression on their beta cells and, unlike β2M–/– NOD mice, express class I normally on all other cells, including APCs. Class I beta bald mice develop insulitis that is qualitatively and quantitatively indistinguishable from that in class I beta normal littermates. This finding demonstrates that the drive for the chronic inflammation of islet tissue is independent of class I expression on beta cells during the early benign phase of disease. It is therefore concluded that CD8 T cell activation and function during this phase of disease is not primarily directed at the beta cell. Rather, CD8 T cells are probably interacting with local APCs that are cross-presenting islet antigen on MHC class I (35).
Our finding that direct contact between CD8 T cells and beta cell MHC class I was not required to initiate insulitis is supported by early histological studies of the insulitis lesion and studies in perforin-deficient NOD mice (25, 27, 36, 37). The apparent contradiction between the finding that class I beta bald mice develop insulitis and the results of Serreze and colleagues, in which prediabetic NOD splenocytes did not transfer insulitis into NOD severe combined immunodeficient (SCID) β2M–/– recipients unless they were grafted with MHC class I+ islets (26), is consistent with the hypothesis that the initiation of insulitis depends not on beta cell class I expression but rather on expression of MHC class I by other islet intrinsic cell populations (38).
Although disease initiation was unperturbed in class I beta bald mice, these mice did have a significantly reduced incidence of hyperglycemia, indicating that expression of MHC class I on beta cells is important for the progression of disease. These findings suggest that the class I-dependent cytotoxicity of beta cells is a relatively late event in disease and represents a crucial checkpoint in the switch from benign to malignant inflammation resulting in hyperglycemia. The inability of most class I beta bald mice to progress from insulitis to beta cell destruction and hyperglycemia is most readily explained by a requirement for direct CD8 T cell interaction with class I on beta cells for this switch to occur. Because perforin-deficient NOD mice also develop an insulitis lesion that is quantitatively and qualitatively indistinguishable from wild-type NOD mice, and also develop diabetes at a dramatically reduced incidence and a later time of onset, it is likely that the predominant mechanism by which CD8 T cells mediate the switch from benign to malignant hyperglycemia is by the perforin/granzyme cytotoxicity pathway (25, 27).
Although the insulitis lesion in the majority of class I beta bald and perforin-deficient knockout NOD mice does not progress to hyperglycemia, it is important to emphasize that, in a small percentage of these mice, disease does progress to overt diabetes, albeit with delayed kinetics. This fact raises the question of which beta cell class I-independent mechanisms are driving the insulitis lesion that occasionally progresses to complete beta cell destruction. It may be that beta cells are initially killed through bystander damage resulting from cytokine or free radical production by inflammatory cells (39, 40). Alternatively, although by no means mutually exclusively, it may be that beta cells are killed by CD4 T cells by Fas-mediated killing (41). The selective sensitivity of islet beta cells to such damage may result in the liberation and cross-presentation of a critical threshold of beta cell antigens to CD8 T cells (39, 42). CD8 T cells, including cells specific for H-2Kd/insulin peptide, were found infiltrating the islets of the class I beta bald mice. These cells have presumably been activated by cross-presentation of insulin peptide by professional APCs but are unable to mediate direct beta cell killing in the absence of class I on beta cells. This model predicts that once a critical threshold of beta cell antigen has been released by nonspecific bystander killing and islet beta cell-specific CD8 T cells have been recruited and activated, the presence of MHC class I on islet beta cells will influence the efficiency of beta cell destruction.
The data presented in this article test the predictions of this model and give us a clear understanding of the role of beta cell class I expression in the development of insulitis and hyperglycemia. NOD mice that lack class I expression on their beta cells still develop insulitis in a manner that is not different from their unmanipulated littermates; however, the NOD mice that lack class I on beta cells do have a significantly reduced incidence of hyperglycemia. These studies confirm that cognate interaction with class I on the beta cells is not necessary for the initiation or early progression of insulitis, but is important for an efficient switch from benign to malignant insulitis. We hypothesize that malignant insulitis ensues only after a significant precursor frequency of cytotoxic CD8 T cells has been activated by APCs cross-presenting beta cell-specific antigen, which has been liberated as a result of bystander damage from the non-beta cell-specific infiltrate. Once sufficient beta cell-specific CD8 T cells have been activated, class I-positive beta cells are then rapidly destroyed by a direct interaction between cytotoxic CD8 T cells and the beta cells resulting in hyperglycemia. An inefficient beta cell class I-independent mechanism of beta cell killing is also present, and prevention of CD8 cytotoxicity will not prevent hyperglycemia in all animals.
Acknowledgments
We thank Linda Fitzgerald and Jennifer Kofler for excellent technical assistance. This work was supported by the Juvenile Diabetes Research Foundation.
Abbreviations: NOD, nonobese diabetic; T1D, type 1 diabetes; β2M, β2-microglobulin; APC, antigen-presenting cell; HIP, human insulin promoter; FACS, fluorescence-activated cell sorting.
References
- 1.Kanazawa, Y., Komeda, K., Sato, S., Mori, S., Akanuma, K. & Takaku, F. (1984) Diabetologia 27, 113–115. [DOI] [PubMed] [Google Scholar]
- 2.Bottazzo, G. F., Dean, B. M., McNally, J. M., MacKay, E. H., Swift, P. G. & Gamble, D. R. (1985) N. Engl. J. Med. 313, 353–360. [DOI] [PubMed] [Google Scholar]
- 3.Slattery, R. (1991) Baillieres Clin. Endocrinol. Metab. 5, 449–454. [DOI] [PubMed] [Google Scholar]
- 4.Charlton, B. & Mandel, T. E. (1989) Autoimmunity 4, 1–7. [DOI] [PubMed] [Google Scholar]
- 5.Andre, I., Gonzalez, A., Wang, B., Katz, J., Benoist, C. & Mathis, D. (1996) Proc. Natl. Acad. Sci. USA 93, 2260–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazda, L. S., Charlton, B. & Lafferty, K. J. (1997) J. Autoimmun. 10, 261–270. [DOI] [PubMed] [Google Scholar]
- 7.Yagi, H., Matsumoto, M., Kunimoto, K., Kawaguchi, J., Makino, S. & Harada, M. (1992) Eur. J. Immunol. 22, 2387–2393. [DOI] [PubMed] [Google Scholar]
- 8.Christianson, S. W., Shultz, L. D. & Leiter, E. H. (1993) Diabetes 42, 44–55. [DOI] [PubMed] [Google Scholar]
- 9.Larger, E., Becourt, C., Bach, J. F. & Boitard, C. (1995) J. Exp. Med. 181, 1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh, A. & Maki, T. (1996) Proc. Natl. Acad. Sci. USA 93, 11053–11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hattori, M., Buse, J. B., Jackson, R. A., Glimcher, L., Dorf, M. E., Minami, M., Makino, S., Moriwaki, K., Kuzuya, H., Imura, H., et al. (1986) Science 231, 733–735. [DOI] [PubMed] [Google Scholar]
- 12.Rotter, J. I., Anderson, C. E., Rubin, R., Congleton, J. E., Terasaki, P. I. & Rimoin, D. L. (1983) Diabetes 32, 169–174. [DOI] [PubMed] [Google Scholar]
- 13.Ikegami, H., Makino, S., Yamato, E., Kawaguchi, Y., Ueda, H., Sakamoto, T., Takekawa, K. & Ogihara, T. (1995) J. Clin. Invest. 96, 1936–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton Williams, E. E., Serreze, D. V., Charlton, B., Johnson, E. A., Marron, M. P., Mullbacher, A. & Slattery, R. M. (2001) Proc. Natl. Acad. Sci. USA 98, 11533–11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leiter, E. H., Christianson, G. J., Serreze, D. V., Ting, A. T. & Worthen, S. M. (1989) J. Exp. Med. 170, 1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas, H. E., Parker, J. L., Schreiber, R. D. & Kay, T. W. (1998) J. Clin. Invest. 102, 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McInerney, M. F., Rath, S. & Janeway, C. A., Jr. (1991) Diabetes 40, 648–651. [DOI] [PubMed] [Google Scholar]
- 18.Wang, B., Gonzalez, A., Benoist, C. & Mathis, D. (1996) Eur. J. Immunol. 26, 1762–1769. [DOI] [PubMed] [Google Scholar]
- 19.Charlton, B., Bacelj, A. & Mandel, T. E. (1988) Diabetes 37, 930–935. [DOI] [PubMed] [Google Scholar]
- 20.Katz, J., Benoist, C. & Mathis, D. (1993) Eur. J. Immunol. 23, 3358–3360. [DOI] [PubMed] [Google Scholar]
- 21.Serreze, D. V., Leiter, E. H., Christianson, G. J., Greiner, D. & Roopenian, D. C. (1994) Diabetes 43, 505–509. [DOI] [PubMed] [Google Scholar]
- 22.Sumida, T., Furukawa, M., Sakamoto, A., Namekawa, T., Maeda, T., Zijlstra, M., Iwamoto, I., Koike, T., Yoshida, S., Tomioka, H., et al. (1994) Int. Immunol. 6, 1445–1449. [DOI] [PubMed] [Google Scholar]
- 23.Wicker, L. S., Leiter, E. H., Todd, J. A., Renjilian, R. J., Peterson, E., Fischer, P. A., Podolin, P. L., Zijlstra, M., Jaenisch, R. & Peterson, L. B. (1994) Diabetes 43, 500–504. [DOI] [PubMed] [Google Scholar]
- 24.Wang, Y., Pontesilli, O., Gill, R. G., La Rosa, F. G. & Lafferty, K. J. (1991) Proc. Natl. Acad. Sci. USA 88, 527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kagi, D., Odermatt, B., Seiler, P., Zinkernagel, R. M., Mak, T. W. & Hengartner, H. (1997) J. Exp. Med. 186, 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serreze, D. V., Chapman, H. D., Varnum, D. S., Gerling, I., Leiter, E. H. & Shultz, L. D. (1997) J. Immunol. 158, 3978–3986. [PubMed] [Google Scholar]
- 27.Amrani, A., Verdaguer, J., Anderson, B., Utsugi, T., Bou, S. & Santamaria, P. (1999) J. Clin. Invest. 103, 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prange, S., Zucker, P., Jevnikar, A. M. & Singh, B. (2001) Transplantation 71, 982–985. [DOI] [PubMed] [Google Scholar]
- 29.Kay, T. W., Parker, J. L., Stephens, L. A., Thomas, H. E. & Allison, J. (1996) J. Immunol. 157, 3688–3693. [PubMed] [Google Scholar]
- 30.Sarvetnick, N., Liggitt, D., Pitts, S. L., Hansen, S. E. & Stewart, T. A. (1988) Cell 52, 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu, H., Zou, Y. R. & Rajewsky, K. (1993) Cell 73, 1155–1164. [DOI] [PubMed] [Google Scholar]
- 32.Harada, M. & Makino, S. (1984) Diabetologia 27, 604–606. [DOI] [PubMed] [Google Scholar]
- 33.Andre Schmutz, I., Hindelang, C., Benoist, C. & Mathis, D. (1999) Eur. J. Immunol. 29, 245–255. [DOI] [PubMed] [Google Scholar]
- 34.Kanagawa, O., Militech, A. & Vaupel, B. A. (2002) J. Immunol. 168, 6159–6164. [DOI] [PubMed] [Google Scholar]
- 35.Kurts, C., Cannarile, M., Klebba, I. & Brocker, T. (2001) J. Immunol. 166, 1439–1442. [DOI] [PubMed] [Google Scholar]
- 36.Fujita, T., Yui, R., Kusumoto, Y., Serizawa, Y., Makino, S. & Tochino, Y. (1982) Biomed. Res. 3, 429–443. [Google Scholar]
- 37.Fujino Kurihara, H., Fujita, H., Hakura, A., Nonaka, K. & Tarui, S. (1985) Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 49, 107–120. [DOI] [PubMed] [Google Scholar]
- 38.Winer, S., Tsui, H., Lau, A., Song, A., Li, X., Cheung, R. K., Sampson, A., Afifiyan, F., Elford, A., Jackowski, G., et al. (2003) Nat. Med. 9, 198–205. [DOI] [PubMed] [Google Scholar]
- 39.Malaisse, W. J., Malaisse Lagae, F., Sener, A. & Pipeleers, D. G. (1982) Proc. Natl. Acad. Sci. USA 79, 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asayama, K., Kooy, N. W. & Burr, I. M. (1986) J. Lab. Clin. Med. 107, 459–464. [PubMed] [Google Scholar]
- 41.Amrani, A., Verdaguer, J., Thiessen, S., Bou, S. & Santamaria, P. (2000) J. Clin. Invest. 105, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurts, C., Miller, J. F., Subramaniam, R. M., Carbone, F. R. & Heath, W. R. (1998) J. Exp. Med. 188, 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]