Abstract
Proliferation of pluripotent, bone marrow stem cells, which develop to lymphoid and myeloid progenitors, is negatively regulated by estrogen. Although in estrogen deficiency and in estrogen receptor knockout mice there is significant alteration in bone marrow hematopoiesis, the effects of aging on estrogen receptor deficiencies in mice have not been investigated yet. In this study we show that by 1.5 years of age, estrogen receptor β knockout (ERβ–/–) mice develop pronounced splenomegaly that is much more severe in females than in males. Further characterization of these mice revealed myelogenous hyperplasia in bone marrow, an increase in the number of granulocytes and B lymphocytes in blood, lymphadenopathy, and infiltration of leukocytes in the liver and lung. Analysis by flow cytometry of the bone marrow cells revealed that the percentage and total number of Gr-1hi/Mac-1hi-positive granulocytes were increased by 15–30% and 100%, respectively. The numbers of B cells in the bone marrow and spleen were significantly higher in ERβ–/– mice than in WT littermates. Some of the ERβ–/– mice also had a severe lymphoproliferative phenotype. Thus the absence of ERβ results in a myeloproliferative disease resembling human chronic myeloid leukemia with lymphoid blast crisis. Our results indicate a previously unknown role for ERβ in regulating the differentiation of pluripotent hematopoietic progenitor cells and suggest that the ERβ–/– mouse is a potential model for myeloid and lymphoid leukemia. Furthermore, we suggest that ERβ agonists might have clinical value in the treatment of leukemia.
Over 100 years ago, it was noted that women were more affected by systemic lupus erythematosus than men. In fact, the incidence of many autoimmune diseases is higher in women (1). For this reason it has been speculated for a long time that estrogen plays important roles in the immune system. Loss of estrogen in ovariectomized mice results in splenomegaly (2) and increased production of colony-forming units-granulocyte/erythroid/macrophage/megakaryocytes, burst-forming units-erythroid cells (3, 4, 5), and B lymphocytes in mouse bone marrow (6). Conversely, pregnancy or administration of exogenous estrogen decreases bone marrow B lymphocyte population in mice (7, 8). Recently, it was shown that estrogen represses the differentiation of multipotent hematopoietic stem cells into both lymphoid and myeloid cells (9, 10). Thus, estrogen is directly implicated in the proliferation and differentiation of various cell lineages in normal hematopoietic tissue.
Estrogen exerts its effects through two distinct receptors, estrogen receptor (ER)α and ERβ. ERα has been detected in nonhematopoietic cells in bone marrow and in B lymphocyte precursors in mouse (8). ERβ has also been found in nonhematopoietic cells in mouse bone marrow (11) and human spleen (12). Recently, the roles of ER in lymphopoiesis have been investigated in ERα–/– and ERβ–/– mice. The hematopoietic progenitor profiles in ERα–/– mice were similar to those of WT mice, but there were fewer bone marrow B lymphocyte subpopulations (13). Administration of estrogen to ERα–/– mice caused a reduction in B lymphopoiesis (13). In contrast to the situation in estrogen deficiency, where there is splenomegaly (2), ERα–/– mice showed a slightly reduced spleen weight (14). These results seemed to indicate that negative effects of estrogen on the immune system might be mediated by ERβ.
We have examined the long-term effects of ERβ loss on the immune and hematological systems. We found that there is pronounced splenomegaly in 1.5-year-old ERβ–/– mice as well as myeloproliferative disease, which resembles human chronic myeloid leukemia (CML).
Materials and Methods
Mouse Strains and Housing. ERβ–/– mice on a C57BL/6J/129 background were kept in the animal facility at Huddinge University Hospital (Huddinge, Sweden) under specific pathogen-free conditions and fed standard laboratory chow.
Peripheral Blood Analysis. The mice were anaesthetized with Hypnorm (Janssen) by i.p. injections. The chest cavity was opened, and 500 μl of blood was withdrawn from the heart with a 22-gauge needle. The syringes were coated with 0.5 M EDTA to prevent coagulation. The blood was placed immediately into EDTA-coated tubes and mixed thoroughly. Nucleated cells were counted by using a 1:50 dilution of peripheral blood in Tuerk solution (Fluka). RBCs were counted by using a 1:5,000 dilution of peripheral blood in PBS. The blood smears were prepared and stained with May–Grünewald solution (Fluka).
Histological Methods. Tissues were fixed in 4% paraformaldehyde/PBS, paraffin-embedded, sectioned, and stained with hematoxylin/eosin. The bones were placed in 4% paraformaldehyde/PBS overnight and decalcified in 5.5% EDTA/10% formalin solution for 1 week. The tissues then were embedded in paraffin and cut into 4-μm sections. Chloroacetate esterase (Sigma) and myeloperoxidase (DAKO) staining were performed according to manufacturer instructions.
Bone Marrow Cells and Splenocytes. The femurs and tibiae were surgically removed from the animals. The bones were cut, and marrow was flushed out with 10 ml of PBS containing 1 mM EDTA. The spleen was minced and suspended in PBS. Bone marrow cells and splenocytes were centrifuged at 515 × g for 5 min. Pelleted spleen cells were resuspended in PBS. After washing in PBS the total number of leukocytes from the organs was calculated by using a hematocytometer.
Flow-Cytometric Analysis and Cell Sorting. FITC-conjugated antibodies (anti-Gr-1 and anti-IgM) and phycoerythrin-conjugated antibodies (anti-Mac-1 and anti-B220) were purchased from PharMingen. Single-cell suspensions were prepared from bone marrow and spleens, and 1 × 106 cells were incubated with 10% rat serum (Sigma) for 30 min at 4°C and stained with the monoclonal antibodies described above. After a 30-min incubation on ice, cells were washed twice and resuspended in PBS with 1% paraformaldehyde. The analyses were carried out by using a FACSCalibur (Becton Dickinson) and FACSVantage (Becton Dickinson) for cell sorting.
Western Blot Analysis. Sorted cells (10 × 106 cells) were washed in PBS buffer and homogenized in 1 ml of 0.5 M Tris·HCl buffer (pH 6.8) containing 0.4% SDS. Supernatant was recovered after centrifugation at 17,310 × g for 10 min, and 500 μl of 40% trichloroacetic acid was added. The precipitated proteins were separated by SDS/PAGE with 10% polyacrylamide gels and transferred to poly(vinylidene difluoride) membranes. Immunoblotting was performed after blocking the membranes with 10% fat-free powdered milk in PBS containing 0.1% Nonidet P-40. The blots were incubated overnight at 4°C with rabbit IgG raised against the ligand-binding domain of human ERβ. Signals were detected by using a peroxidase-conjugated goat anti-rabbit secondary antibody and ECL Plus (Amersham Pharmacia).
Results
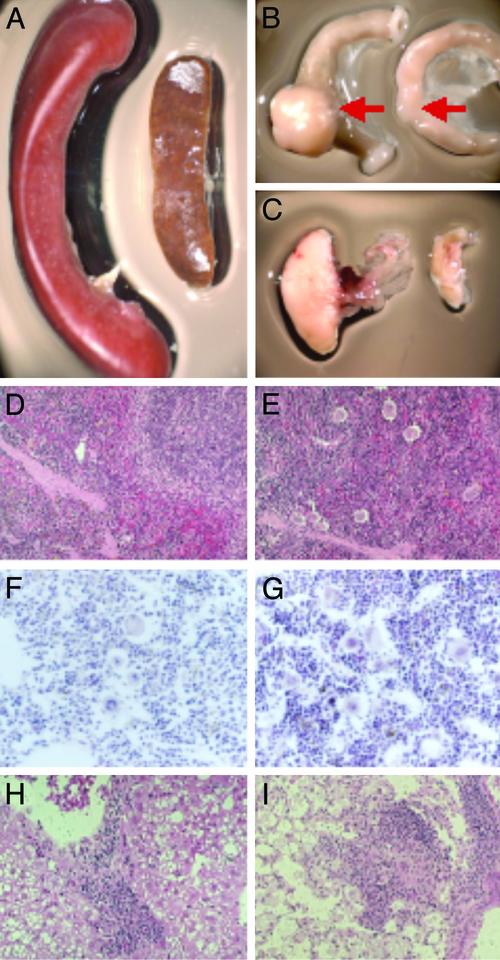
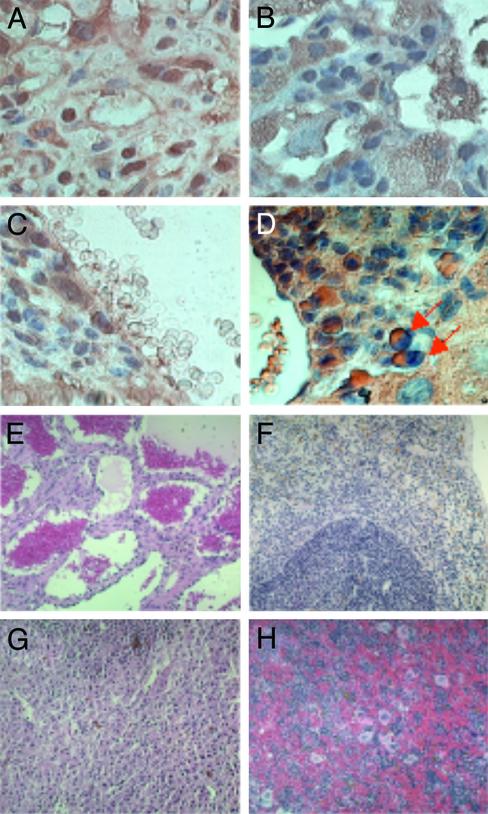
Aged ERβ–/– Mice Show Pronounced Splenomegaly and Leukocyte Infiltration in Nonhematopoietic Organs. We studied long-term effects of ERβ deficiency using aging ERβ–/– mice. All female and male ERβ–/– mice by 1.5 years of age developed a profound splenomegaly (Fig. 1A) and lymphadenopathy (Fig. 1 B and C), although the degree of enlargement varied among mice. Spleen (Fig. 1 D and E) and bone marrow (Fig. 1 F and G) histology in ERβ–/– mice revealed a significant increase in the number of megakaryocytes and in total cellularity.
Fig. 1.
Aged ERβ–/– mice develop myeloproliferative disease. Massive splenomegaly (A) and enlargement of Peyer's patches (B) and mesenteric lymph nodes (C) from 18-month-old ERβ–/– mice (Left) compared with an age-matched WT littermate (Right) are shown. (D–G) Spleen histology of WT (D) and ERβ–/– (E) mice and bone marrow histology of WT (F) and ERβ–/– (G) mice. The femurs were surgically removed and fixed in 4% paraformaldehyde-buffered PBS and decalcified in 5.5% EDTA/10% formalin for 7 days. Nonhematopoietic organs such as liver (H) and lung (I) show a massive infiltration of myeloid cells.
We kept seven female and six male ERβ–/– mice for >1.5 years. Two of the female mice and one male ERβ–/– mouse died with pronounced hepatosplenomegaly with leukocyte infiltration. All the age-matched WT litter mates survived and appeared normal. In nonhematopoietic organs such as liver and lung there was a significant infiltration mainly of granulocytes with some B lymphocytes (Fig. 1 H and I). In some ERβ–/– mice there was infiltration of macrophages and B lymphocytes in the lung, and in other ERβ–/– mice there was eosinophilia in the lung. Although there were variations in the types of cells infiltrating the various tissues, all the ERβ–/– mice but none of the WT littermates showed a myeloproliferative-like syndrome. Histological analysis of various tissues including liver, kidney, and heart did not show any sign of infection (data not shown).
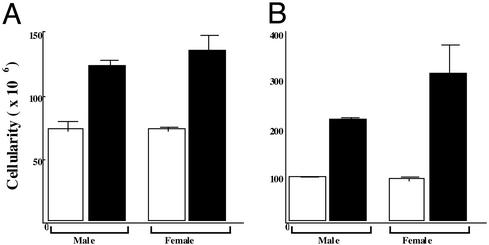
Hypercellularity in ERβ–/– Mice. The cellularity of bone marrow from the femurs, tibiae (Fig. 2A), and spleen (Fig. 2B) was determined by using a hematocytometer. The total cellularity was higher in ERβ–/– mice than WT mice (Fig. 2). As early as 1 month of age, at a time when the spleens were of normal size, the total number of WBCs was normal, and there was no leukocyte infiltration in tissues; however, there was a significant hypercellularity of bone marrow (data not shown). This finding indicates that the cause of the splenomegaly is a primary hypercellularity in the bone marrow.
Fig. 2.
Mice lacking ERβ have a striking increase in cellularity in bone marrow (A) and spleen (B). ERβ–/– (black column) and age-matched WT male and female (white column) 1.5-year-old mice were killed, and from each mouse one femur and one tibia were flushed with PBS. The cells were washed and resuspended in 10 ml of PBS. The total cells per leg were calculated based on hematocytometer counts [data are shown as mean (n = 5 per group)].
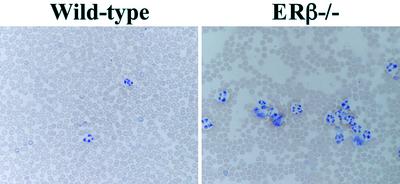
Blood Profiling of Aged ERβ–/– Mice. Analysis of peripheral blood smears from 1.5-year-old ERβ–/– and WT mice revealed significant increases in numbers of neutrophils and monocytes (Fig. 3). Lymphoid blast crisis was evident in some of the 1.5-year-old ERβ–/– mice (Table 1). The number of leukocytes in the peripheral blood was increased 3- to 4-fold in 1.5-year-old mice (Table 1). Differential blood counts in most ERβ–/– mice indicated elevated numbers of mature neutrophilic granulocytes, eosinophils, and monocytes. In none of the 1-year-old mice analyzed was there evidence of lymphoid blast crisis (data not shown). These observations demonstrate that loss of the ERβ gene results in myeloid cell hyperplasia with an overall hematological profile very much like the clinical picture of CML.
Fig. 3.
Blood smears of WT and ERβ–/– mice. Blood was withdrawn from the mice by cardiac puncture and smeared on microscope slides. The slides were stained with May–Grünewald Giemsa solution.
Table 1. Hematological parameters of 1.5-year-old mice.
| Genotype | Sex | WBCs, 1010 per liter | Lymphocytes,* % | Neutrophils,* % | Eosinophils,* % | Monocytes,* % | RBCs, × 1012 per liter |
|---|---|---|---|---|---|---|---|
| WT | M | 0.5 | 66 | 29 | 1 | 4 | 7.8 |
| M | 0.42 | 62 | 33 | 1 | 4 | 7.9 | |
| M | 0.51 | 68 | 30 | — | 2 | 9.2 | |
| M | 0.54 | 65 | 27 | — | 8 | 7.1 | |
| M | 0.5 | 52 | 33 | 1 | 4 | 7.6 | |
| F | 0.52 | 64 | 32 | 1 | 3 | 8.9 | |
| F | 0.43 | 62 | 36 | — | 2 | 7.1 | |
| F | 0.48 | 63 | 30 | 2 | 5 | 7.4 | |
| F | 0.53 | 64 | 31 | 1 | 4 | 8.0 | |
| F | 0.55 | 65 | 32 | — | 3 | 7.1 | |
| F | 0.57 | 67 | 31 | — | 2 | 6.9 | |
| ERβ-/- | M | 1.9 | 27 | 44 | 20 | 9 | 7.8 |
| M | 1.8 | 18 | 57 | 17 | 8 | 7.4 | |
| M | 1.7 | 32 | 58 | 2 | 8 | 7.6 | |
| M | 1.9 | 79 | 16 | 1 | 4 | 4.8 | |
| M | 1.8 | 83 | 12 | 1 | 4 | 5.6 | |
| F | 1.6 | 27 | 62 | 5 | 6 | 7.8 | |
| F | 2.1 | 17 | 77 | 8 | 8 | 7.2 | |
| F | 2.2 | 33 | 35 | 4 | 28 | 7.6 | |
| F | 1.9 | 81 | 16 | 1 | 2 | 4.6 | |
| F | 1.8 | 86 | 12 | 1 | 1 | 4.2 |
Nucleated cells (leukocytes) were counted by using 1:20 dilutions of peripheral blood in Teurk solution. Red blood cells were counted by using 1:5,000 dilutions of peripheral blood in PBS buffer.
Differential cell counts were performed by identifying at least 300 cells per May—Grünwald Giemsa-stained peripheral blood smear.
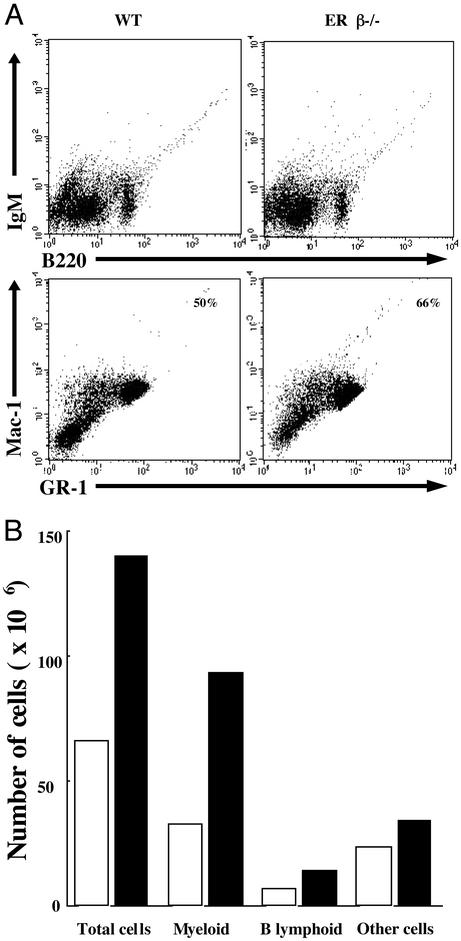
Granulopoiesis Is Enhanced in ERβ–/– Mice. Flow-cytometric analysis confirmed the age-dependent 15–30% increase in Gr-1hi/Mac-1hi granulocyte population in bone marrow (Fig. 4A) and that there was a 2-fold increase in the absolute number of granulocytes in this tissue (Fig. 4B). Although the total number of pro/pre-B lymphocytes (B220+/IgM–) in ERβ–/– mice was higher than in WT mice (Fig. 4B), there was a relative decrease in the percentage of B lymphocytes in bone marrow of ERβ–/– mice because of the overall hypercellularity (Fig. 4A). Together, these results indicate that ERβ–/– mice develop a progressive myeloid hyperplasia with overproduction of mature granulocytes and bone marrow B cells. Because the population of B lymphocytes is smaller than that of myeloid cells in normal bone marrow, the leukocytosis is manifested as granulocytosis in peripheral blood.
Fig. 4.
(A) Single-cell suspensions from bone marrow of ERβ–/– mice and their WT littermates were stained with indicated combinations of antibodies for flow-cytometric studies. The numbers in the boxes indicate the percentage of myeloid cells. The data show one analysis from a single mouse, which is representative of the data obtained with six pairs of mice including males and females. In mice used in this analysis, total cellularity of ERβ–/– bone marrow was increased >2-fold over control, and there was splenomegaly with granulocytosis in the blood. (B) The estimated total number of myeloid and B lymphoid cells in bone marrow of ERβ–/– (black column) and WT (white column) mice is shown. The analysis shown is one of six and is from the mice shown in A.
Granulocytes in peripheral blood do not have potential to proliferate, but B lymphocytes have great ability to develop and proliferate in the secondary lymph organs. Because myeloid leukemia is a disease of multipotent stem cells, it is not surprising that B cell lymphoproliferative disease appears in the late stage of myeloid leukemia. In ERβ–/– mice, the percentage of granulocytes and B lymphocytes in spleen was not different than that of WT mice (data not shown), but the total number of each cell type was increased because splenic cellularity increased by 2- to 5-fold.
ERβ–/– Mice Develop a Syndrome Resembling CML. Although 1.5-year-old WT mice show normal blood profiles, ERβ–/– mice developed (with a 100% penetrance) signs of a hematological neoplasia resembling CML in humans. The progressive expansion of the myeloid and B lymphoid cell pools finally leads to lymphadenopathy and splenomegaly. In myeloproliferative disease, alterations in bone marrow and lymphoid tissue precede changes in the composition of blood leukocytes for a long time. Only when the spleen has tripled its normal size is there a significant shift toward the leukemic disease. Examination of blood smears of ERβ–/– mice with fully developed myeloproliferative disease, which is associated with moderately elevated numbers of leukocytes, revealed that there was granulocytosis. Morphologically, the WBCs at this disease stage appeared mostly normal. Transition of the chronic form to fatal blast crisis is a characteristic feature of human CML. Histological analysis of tissues from 2-year-old ERβ–/– mice revealed a massive blast cell infiltration of both hematopoietic and nonhematopoietic organs such as lung, liver, and spleen.
The cells positive for chloroacetate esterase staining and myeloperoxidase immunohistostaining only appeared as focal areas in infiltrated organs, and the majority of the infiltrated cells appeared to be nontransformed cells. Human CML often shows lymphoid blast crisis and anemia. At 2 years of age, surviving ERβ–/– mice showed various phenotypes in the spleen indicating myelofibrosis (Fig. 5 E and G), anemia (Fig. 2F), and even accumulation of RBCs (Fig. 5H). The results indicate that the myeloproliferative disease with various symptoms in ERβ–/– mice can progress to blast crisis in very old mice and thus has a natural course resembling that of human CML in which there is often lymphoid blast crisis.
Fig. 5.
Histological examination of spleen (A) and nonhematopoietic organs lung (B) and liver (C and D) indicates a massive myeloid and blast cell infiltration. The tissues of WT mice do not show any infiltration. Analysis by myeloperoxidase immunohistochemistry (A–C) and chloroacetate esterase staining (D) shows focal areas of blastic cells. (E–H) In the spleens of 2-year-old ERβ–/– mice various histological phenotypes are evident.
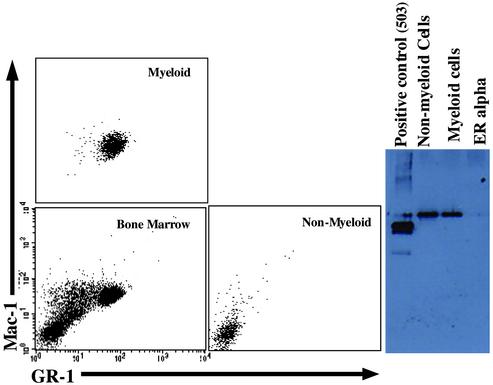
ERβ Is Expressed in Bone Marrow Granulocytes. ERβ has been detected in rat bone, in nonhematopoietic cells in mouse bone marrow, and in peripheral monocytes, but it has not been demonstrated in bone marrow myeloid cells. We sorted out Gr-1hi/Mac-1hi bone marrow myeloid cells by fluorescence-activated cell sorting and measured ERβ by Western blotting of extracts from these cells. The results clearly show that ERβ is expressed in highly purified Gr-1hi/Mac-1hi myeloid cells (Fig. 6).
Fig. 6.
Myeloid cells in the bone marrow of WT mice express ERβ protein. Bone marrow cells from 3-month-old female WT mice were separated by flow cytometry into Gr-1–/Mac-1– (nonmyeloid) and Gr-1hi/Mac-1hi (myeloid) cells and analyzed for the expression of ERβ by Western blotting.
Discussion
By 1.5 years of age, mice in which the ERβ gene is disrupted developed myelogenous hyperplasia in bone marrow and granulocytosis in blood. Histological analysis of liver and lung demonstrated massive infiltration of leukocytes, mainly granulocytes with a minor fraction of B lymphocytes. Additional analysis of the bone marrow cells by flow cytometry revealed that the percentage and total number of Gr-1hi/Mac-1hi granulocytes were increased by 15–30% and 100%, respectively. Thus, as ERβ–/– mice age, they develop a myeloproliferative disease that resembles human CML with lymphoid crisis. These results indicate a previously unknown role of ERβ in regulating proliferation and differentiation of pluripotent hematopoietic stem cells. Interestingly, 1.5-year-old mice heterozygous for loss of ERβ (ERβ+/–) also showed signs of myeloproliferative disease, indicating that a full dose of ERβ is necessary for normal hematopoiesis.
The effect of estrogen on multipotent hematopoietic stem cells has been well demonstrated in WT and ERα–/– mice (9, 13). Estrogen treatment in vivo decreases the percentage of c-Kit+/Sca-1+ cells, i.e., early hematopoietic stem cells that can differentiate into myeloid and lymphoid lineages (13). Estrogen can still partially decrease the percentage of c-Kit+/Sca-1+ cells in the absence of ERα (in ERα–/– mice) (13), suggesting that both ERα and ERβ are mediators of estrogenic control of c-Kit+/ Sca-1+ cells. This negative function of estrogen in c-Kit+/Sca-1+ cells may inf luence colony-forming units-granulocyte/erythroid/macrophage/megakaryocyte (CFU-GEMM) myeloid stem cells. It has been shown that loss of estrogen results in increased production of CFU-GEMM (3, 4). Our results suggest that loss of ERβ but not ERα mimics the effect of loss of estrogen on myeloid proliferation.
After ovariectomy in mice, there is a significant increase in the number of pro/pre-B lymphocytes (B220low/IgM–) in bone marrow (15). Interestingly, in ERα–/– mice, there is a slight increase in cellularity of bone marrow even in young mice (4-month-old) but a decrease in the number of pro/pre-B lymphocytes (B220low/IgM–) and mature B lymphocytes (B220high/IgM+) in bone marrow (13). In contrast, in ERβ–/– mice there is an increase in the number of pro/pre-B lymphocytes. Thus when only ERβ is present, as in the case of ERα–/– mice, the differentiation of pro/pre-B lymphocytes is inhibited; and when only ERα is present, as in the case of ERβ–/– mice, there is an increase in the total number of pro/pre-B lymphocytes. These results show that with respect to B lymphocytes in mice, loss of ERβ mimics loss of estrogen.
One other characteristic of estrogen deficiency is splenomegaly that is caused by enhanced B lymphopoiesis and myelopoiesis (2). Interestingly, it has been reported that the size and cellularity of spleen is reduced significantly in 4-month-old ERα–/– mice (14). We have studied the size of the spleen in 1-year-old ERα–/– mice and found that all ERα–/– mice have smaller spleens than do their WT littermates (data not shown). In the immune system, loss of ERα does not result in a phenotype typical of classical estrogen deficiency. Splenomegaly in ERβ–/– mice suggests that ERβ plays a critical role in hematopoiesis in bone marrow.
Possible Antagonism Between ERα and ERβ We also investigated hematological properties of aged ERα–/– mice and found no bone marrow hyperplasia; instead there is granulocytopenia in peripheral blood (unpublished data). These results suggest that ERβ is a critical ER in the mouse bone marrow and that a balance between ERβ and ERα is important in overall hematopoiesis in bone marrow. Under several circumstances, ERβ may function as an ERα antagonist (16–18).
Effect of Estrogen on Myeloid Cells. Studies of ovariectomized mice have shown that ovarian hormone deficiency promotes the expansion of a pool of bone marrow-derived progenitors that differentiate into myeloid and lymphoid cells. Normal peripheral monocytes originate from bone marrow myeloid cells, predominantly express ERβ protein, and undergo apoptosis after estrogen exposure (19). This finding indicates that monocytes are primarily regulated by ERβ protein. Unraveling the mechanism through which estrogen exerts its effects on myeloid cells could provide valuable information for understanding the pathogenesis of leukemic disease, because leukemia is a disease of transformed hematopoietic progenitors. The direct effects of estrogen in leukemia and leukemic cell lines have not been well studied. The presence of an estrogen-binding site in myeloid leukemia cells, named type II ER, has been reported (20), and ligands of this protein mediated inhibitory effects on leukemic cell proliferation (20–22).
Estrogen significantly inhibits cell growth, via cell-cycle arrest, of the ERα-negative human monoblastic leukemia cell line U937 (23). It is therefore interesting that normal human monocytes express ERβ and that certain flavonoids, which are ligands of ERβ, inhibit leukemic cell growth (22, 23). Furthermore, androgens have similar effects as estrogens on bone marrow (24); they also inhibit cell growth of the U937 cell line (23) and improve long-term survival in acute myeloid leukemia in humans (25).
Comparison of the ERβ–/– Phenotype with Other Mouse Models of CML. The disease we describe in ERβ–/– mice resembles CML, is polyclonal, and seems to originate from multipotent stem cells that can differentiate into both myeloid and B lymphoid lineages. Very similarly, in two different mouse models of CML, it has been demonstrated that the cells that initiate CML-like leukemia are early multipotent progenitors. Two typical chromosomal translocations are implicated in human CML, the t(9:22) Philadelphia chromosome and t(9:12) (26). As a result of chromosome translocations, two fusion proteins, BCR-ABL and Tel-Abl tyrosine kinase, are generated. Retroviral transduction of the BCR-ABL or TEL-ABL gene into mouse bone marrow cells directly induces CML-like myeloproliferative disease (26, 27). These murine CML-like diseases induced by human protein Tel-Abl or Bcr-Abl are characterized by massive polyclonal expansion of maturing myeloid cells, principally neutrophils. Although neutrophils constitute the predominant hematopoietic lineage in murine CML-like disease, the same spectrum of proviral clones was detected in macrophages, erythroid progenitors, and B lymphocytes (26, 27). In mice specifically lacking JunB in the myeloid lineage (28) and in mice in which the IFN consensus sequence-binding protein has been inactivated (29), the resulting CML-like syndrome is very similar to what we have described in ERβ–/– mice.
CML is a myeloproliferative disorder resulting from the neoplastic transformation of hematopoietic stem cells (30). It accounts for 15–20% of all human leukemias, and many patients of CML progress to acute myeloid leukemia, which accounts for >80% of all adult acute leukemias. The disease usually has a biphasic course. The initial, chronic phase is characterized by increased proliferation and maturation of myeloid cells, with granulocytes predominating. After 3–5 years the disease progresses to a blast crisis stage, characterized by accelerated accumulation of immature myeloid or lymphoid cells. Unfortunately, present clinical interventions rarely cure CML. Elucidation of the molecular mechanisms and the genes responsible for CML is critical for developing improved therapies for the disease.
Our results suggest a previously unknown role of ERβ in regulating the proliferation and differentiation of pluripotent hematopoietic progenitor cells. This study indicates that mutations in the ERβ gene may be responsible for myeloproliferative disease and constitute a genetic risk factor for developing acute myeloid leukemia. ERβ–/– mice may be a potential model for myeloid leukemia. Furthermore, it is possible that agonists against ERβ may have a value in chemotherapy of CML including Philadelphia chromosome disease.
Acknowledgments
We thank Drs. Yoko Omoto and Weihua Zhang for valuable discussions and Mrs. Christina Thulin-Andesrsson and Patricia Humire for excellent technical assistance. This study was supported by grants from the Swedish Cancer Fund and Karo Bio AB.
Abbreviations: ER, estrogen receptor; CML, chronic myeloid leukemia.
References
- 1.Whitacre, C. C. (2001) Nat. Immunol. 2, 777–780. [DOI] [PubMed] [Google Scholar]
- 2.Zhang, J., Pugh, T. D., Stebler, B., Ershler, W. B. & Keller, E. T. (1998) Calcif. Tissue Int. 62, 219–226. [DOI] [PubMed] [Google Scholar]
- 3.Erben, R. G., Raith, S., Eberle, J. & Stangassinger, M. (1998) Am. J. Physiol. 274, E476–E483. [DOI] [PubMed] [Google Scholar]
- 4.Jilka, R. L., Passeri, G., Girasole, G., Cooper, S., Abrams, J., Broxmeyer, H. & Manolagas, S. C. (1995) Exp. Hematol. (Charlottesville, Va) 23, 500–506. [PubMed] [Google Scholar]
- 5.Jilka, R. L., Hangoc, G., Girasole, G., Passeri, G., Williams, D. C., Abrams, J. S., Boyce, B., Boxmeyer, H. & Manolagas, S. C. (1992) Science 257, 88–91. [DOI] [PubMed] [Google Scholar]
- 6.Masuzawa, T., Miyaura, C., Onoe, Y., Kusano, K., Ohta, H., Nozawa, S. & Suda, T. (1994) J. Clin. Invest. 94, 9360–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina, K. L., Smithson, G. & Kincade, P. W. (1993) J. Exp. Med. 94, 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smithson, G., Medina, K., Ponting, I. & Kincade, P. W. (1995) J. Immunol. 155, 3409–3419. [PubMed] [Google Scholar]
- 9.Medina, K. L., Garret, K. P., Thompson, L. F., Rossi, M. I. D., Payne, K. J. & Kincade, P. W. (2001) Nat. Immunol. 2, 718–724. [DOI] [PubMed] [Google Scholar]
- 10.Kouro, T., Medina, K. L., Oritani, K. & Kincade, P. W. (2001) Blood 97, 2708–2715. [DOI] [PubMed] [Google Scholar]
- 11.Couse, J. F., Lindzey, J., Grandien, K., Gustafsson, J.-Å. & Korach, K. (1997) Endocrinology 138, 4613–4621. [DOI] [PubMed] [Google Scholar]
- 12.Kuiper, G. G. J. M., Enmark, E., Pelto-Huikko, M., Nilsson, S. & Gustafsson, J.-Å. (1996) Proc. Natl. Acad. Sci. USA 93, 5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurmond, T. S., Murante, F. G., Staples, J. E., Silverstone, A. E., Korach, S. K. & Gasiewicz, T. A. (2000) Endocrinology 141, 2309–2318. [DOI] [PubMed] [Google Scholar]
- 14.Erlandsson, M. C., Ohlsson, C., Gustafsson, J.-Å. & Carlsten, H. (2001) Immunology 103, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyaura, C., Onoe, Y., Masaki, Maki, K., Ikuta, K., Ito, M. & Suda, T. (1997) Proc. Natl. Acad. Sci. USA 94, 9360–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiper, G. G. J. M. & Gustafsson, J.-Å. (1997) FEBS Lett. 410, 87–90. [DOI] [PubMed] [Google Scholar]
- 17.Paech, K., Webb, P., Kuiper, G. G., Nilsson, S., Gustafsson, J.-Å., Kushner, P. J. & Scanlan, T. S. (1997) Science 277, 1508–1510. [DOI] [PubMed] [Google Scholar]
- 18.Vladusic, E. A., Hornby, A. E., Guerra-Vladusic, F. K. & Lupu, R. (1998) Cancer Res. 58, 210–214. [PubMed] [Google Scholar]
- 19.Mor, G., Sapi, E., Abrahams, V. M., Rutherford, T., Song, J., Hao, X. Y., Muzaffar, S. & Kohen, F. (2003) J. Immunol. 170, 114–122. [DOI] [PubMed] [Google Scholar]
- 20.Larocca, L. M., Piantelli, M., Leone, G., Sica, S., Thofill, L., Benedetti Panicini, P., Scambia, G., Mancuso, S., Capelli, A. & Raneletti, F. O. (1990) Br. J. Haematol. 75, 489–495. [DOI] [PubMed] [Google Scholar]
- 21.Teofili, L., Pierelli, L., Lovino, M. S., Leone, G., Scambia, G., De Vincenzo, R., Benedetti Panicini, P., Menichella, G., Macri, E., Piantelli, M., et al. (1992) Leuk. Res. 16, 497–503. [DOI] [PubMed] [Google Scholar]
- 22.Parker, B., Kaur, G., Nives-Niera, W., Tairni, M., Kohlgaden, G., Shimizu, T., Losiewicz, M., Pommier, Y., Sausville, E. & Senderowicz, A. (1998) Blood 91, 458–465. [PubMed] [Google Scholar]
- 23.Mossuz, P., Cousin, F., Castinel, A., Chauvet, M., Sotto, M. F., Polack, B., Sotto, J. J. & Kolodie, L. (1998) Leuk. Res. 22, 1063–1072. [DOI] [PubMed] [Google Scholar]
- 24.Smithson, G., Couse, J. F., Lubahn, D. B., Korach, K. S. & Kincade, P. W. (1998) J. Immunol. 161, 27–34. [PubMed] [Google Scholar]
- 25.Hollard, D., Sotto, J. J., Berhier, R., Leger, J. & Michallet, M. (1980) Cancer 45, 1540–1548. [DOI] [PubMed] [Google Scholar]
- 26.Li, S., Ilaria, R. L., Million, R. P., Daly, G. Q. & Van Etten, R. A. (1999) J. Exp. Med. 189, 1399–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Million, R. P., Aster, J., Gilliland, G. & Van Etten, R. A. (2002) Blood 99, 4568–4577. [DOI] [PubMed] [Google Scholar]
- 28.Passegue, E., Jochum, W., Schorpp Kistner, M., Mohle-Steinlein, U. & Wagner, E. R. (2001) Cell 104, 21–32. [DOI] [PubMed] [Google Scholar]
- 29.Holtschke, T., Lohler, J., Kannno, Y., Fehr, T., Giese, N., Rosenbauer, F., Lou, J., Knobeloch, K.-P., Gabriele, L., Waring, J. F., et al. (1996) Cell 87, 307–317. [DOI] [PubMed] [Google Scholar]
- 30.Jorgensen, H. G. & Holyoake, T. L. (2001) Oncology 19, 89–106. [DOI] [PubMed] [Google Scholar]