Abstract
The induction of an acute inflammatory response followed by the release of polypeptide cytokines and growth factors from peripheral blood monocytes has been implicated in mediating the response to vascular injury. Because the Cu2+-binding proteins IL-1α and fibroblast growth factor 1 are exported into the extracellular compartment in a stress-dependent manner by using intracellular Cu2+ to facilitate the formation of S100A13 heterotetrameric complexes and these signal peptideless polypeptides have been implicated as regulators of vascular injury in vivo, we examined the ability of Cu2+ chelation to repress neointimal thickening in response to injury. We observed that the oral administration of the Cu2+ chelator tetrathiomolybdate was able to reduce neointimal thickening after balloon injury in the rat. Interestingly, although immunohistochemical analysis of control neointimal sections exhibited prominent staining for MAC1, IL-1α, S100A13, and the acidic phospholipid phosphatidylserine, similar sections obtained from tetrathiomolybdate-treated animals did not. Further, adenoviral gene transfer of the IL-1 receptor antagonist during vascular injury also significantly reduced the area of neointimal thickening. Our data suggest that intracellular copper may be involved in mediating the response to injury in vivo by its ability to regulate the stress-induced release of IL-1α by using the nonclassical export mechanism employed by human peripheral blood mononuclear cells in vitro.
Keywords: phosphatidylserine, interleukin 1, restenosis, tetrathiomolybdate, fibroblast growth factor
The elucidation of the pathways involved in the regulation of the vascular response to injury is critical for the management of human diseases in which the pathology may be regulated by stress-induced endothelial and vascular smooth muscle cell responses. Although a variety of polypeptide growth factors and cytokines have been implicated as mediators of the vascular response to injury (1–5), our current understanding involves a fundamental role for the migration of peripheral blood mononuclear (PBM) cells into sites of injury as a delivery system for these biological response modifiers as regulators of both the inflammatory and angiogenic responses (6, 7).
Prior studies have suggested that members of the IL-1 and fibroblast growth factor (FGF) (8, 9) gene families may significantly contribute to vessel-wall pathology in response to injury. The IL-1 and FGF gene families are composed of at least 10 and 23 members, respectively (10–13), and membership is defined by structural homology with the prototype members of each gene family, namely IL-1α/IL-1β and FGF1/FGF2. Although the IL-1 and FGF prototypes exhibit limited primary sequence homology (14), these prototype polypeptides are viewed as crystallographic homologs (15). Furthermore, the IL-1 and FGF prototypes do not contain classical signal peptide sequences to direct their export through the endoplasmic reticulum-Golgi apparatus (16), and although only 3 of the 23 FGF genes lack this feature, 8 of the 10 IL-1 genes lack a classical signal peptide sequence. Because it is well established that the IL-1 and FGF prototypes function in the extracellular compartment as ligands for high-affinity cell-surface receptors (11, 13), the identification of the mechanism(s) used by the IL-1 and FGF prototypes for nonclassical release could potentially yield new insight into proinflammatory and angiogenic disorders.
The appearance of IL-1α and FGF1 in the extracellular compartment is regulated by convergent yet distinct nonclassical export pathways induced by cellular stress (17, 18). Both IL-1α (19) and FGF1 (20) use intracellular Cu2+ to force the assembly of a multiprotein complex near the inner surface of the plasma membrane (19, 20). Although both IL-1α and FGF1 form Cu2+-dependent heterotetrameric complexes with S100A13 to facilitate their release, the FGF1 release pathway also requires the function of the extravesicular domain of synaptotagmin (Syt)1 for export (21). Interestingly, the overexpression of S100A13 overcomes the requirement for stress-induced transcription for export of IL-1α (19) and FGF1 (22), and it is known that both FGF1 (23) and IL-1α (19) exhibit molten globule character, which may facilitate the ability of both IL-1α and FGF1 to associate with and traverse the plasma membrane. It is also interesting to note that IL-1α, FGF1, Syt1, and S100A13 are Cu2+-binding proteins (20) and are able to associate with phosphatidylserine (pS), an acidic phospholipid that is known to translocate from the inner to the outer surface of the plasma membrane (24). Further, studies in both murine NIH 3T3 cells and human U937 cells have demonstrated that the stress-induced IL-1α (19) and FGF1 (20) release pathways are sensitive to inhibition by the Cu2+ chelator tetrathiomolybdate (TTM), suggesting that intracellular Cu2+ may play an important role in the regulation of these pathways in vivo.
It is well established that Cu2+ is not only a potent inducer of angiogenesis in vivo (25), but studies with arterial Cu2+ cuffs and stents have also implicated Cu2+ as a promoter of the arterial response to injury (26). Because (i) TTM has been suggested to be efficacious in the clinical management of human cancer (27), (ii) TTM is able to significantly inhibit NF-κB activity as a component of its antiangiogenic activity in vivo (28), and (iii) IL-1 is an important regulator of NF-κB activity (29), we questioned whether TTM could be used to limit the recruitment of mononuclear cells in response to vascular injury in vivo by restricting the export of IL-1α. We report that the induction of moderate copper deficiency by the oral administration of TTM impairs neointimal thickening and significantly reduces the expression of IL-1α and S100A13. In addition, we used a monoclonal antibody that recognizes the acidic phospholipid pS and demonstrated that TTM is also able to significantly limit the appearance of pS after vascular injury in vivo. Last, the somatic gene transfer of the IL-1 receptor antagonist protein (IRAP) also significantly reduces the response to vascular injury. These data suggest that members of the IL-1 gene family may play a prominent role in restenosis in vivo.
Methods
Cell Culture and Immunoblot Analysis. Human PBM cells were isolated by Fico/Lite density gradient centrifugation from heparinized blood of healthy donors. Mononuclear cells were further isolated by adherence on tissue-culture dishes for 2 h and maintained in RPMI medium 1640 (Sigma) containing 10% FBS. Mononuclear cells were activated with 10 ng/ml of phorbol 12-myristate 13-acetate (PMA; Sigma) for 24 h before each experiment. Before heat shock (2 h at 42°C), the mononuclear cells were plated on fibronectin (10 mg/cm3)-coated cell-culture dishes. Briefly, IL-1α from the conditioned medium was resolved by Cu2+-affinity chromatography (Hi Trap, Amersham Pharmacia), eluted with 60 mM imidazole, resolved by 15% acrylamide SDS/PAGE, and evaluated by IL-1α immunoblot analysis (17). TTM was purchased from Sigma–Aldrich and used as described (19, 20). Briefly, cells were incubated for 18 h at 37°C in either the absence or presence of 250 nM TTM and subjected to heat shock as described (19, 20).
Ceruloplasmin Levels, Surgical Procedures, Tissue Preparation, and Adenoviral Gene Transfer. Serum ceruloplasmin was used as a surrogate marker of copper status (27, 28, 30). The level of serum ceruloplasmin was quantitated before the administration of TTM as well as on the day of the injury and on the final day of TTM delivery as described (30).
Sprague–Dawley male rats (Charles River Breeding Laboratories) weighing 350–450 g at 12–16 weeks of age (n = 72) were anesthetized with an i.p. injection of ketamine (100 mg/kg) and xylazine (6 mg/kg), and balloon injury of the carotid artery was performed by using a Fogarty 2F embolectomy catheter as described (31). The animals were killed 4, 7, or 14 days postinjury, the entire left carotid arteries were harvested and immersed in either 4% formaldehyde or acetone/ethanol (1:1), and the injured carotid arteries were excised and sliced in three sections representing the proximal, middle, and distal part of the vessel. The specimens were dehydrated and embedded in paraffin for sectioning, and the left carotid artery segments were used for histological, morphometric, and immunohistochemical studies. Evan's blue staining of the denuded arteries was performed as described (32).
For the adenoviral studies, the full-length human IRAP cDNA with a Myc sequence at the 5′ end was inserted into the shuttle plasmid pADlox, containing the mouse cytomegalovirus promoter, by using standard PCR techniques. A viral stock of Ad-IRAP was plaque-purified, further propagated in 293 cells, and purified by CsCl2 centrifugation. The concentration of infectious viral particles was determined as described (33). The denuded carotid artery was incubated with 100 μl (2 × 109 plaque-forming units per ml) of Ad-IRAP (n = 5) or Ad-insertless (n = 2) for 20 min. The adenovirus was removed, the carotid artery section was flushed, and blood flow was restored. After a follow up period of 14 days, the animals were killed after anesthesia, and the carotid arteries were processed as described above.
Histomorphometric Studies and Statistical Analysis. Morphometric analysis of the arterial segment was performed in a blind manner on cross sections stained with hematoxylin/eosin and orcein. With a computerized digital microscopic planimetry algorithm (Optimas, version 5.22), the areas within the external elastic lamina (EEL) area, the internal elastic lamina (IEL) area, and the luminal area were measured. Other areas were calculated as: medial area = EEL area — IEL area; neointimal area = IEL area — luminal area; and neointima-to-medium ratio = neointimal area/medial area. All variables are expressed as the mean ± SEM, and the Student's t test was used to examine the differences between the experimental groups. A value of P ≤ 0.05 was considered significant.
Generation and Characterization of Murine Monoclonal Antibody 1H6. To generate monoclonal antibodies against pS, BALB/c mice were immunized with liposomes containing 70% pS and 30% phosphatidylglycerol. Liposomes were resuspended in ethanol and injected five times i.p. with an interval of 3 weeks between injections. Hybridoma selection was performed as described (34). Approximately 14 days postfusion, supernatants from wells with proliferating hybrid cells were collected and tested for the presence of anti-pS antibodies. The reactivity of the selected antibody was established by screening hybridoma supernatants on pS, cholesterol, phosphatidylethanolamine, and phosphatidylcholine (Avanti Polar Lipids) either immobilized on plastic for analysis by ELISA or dried on a nitrocellulose membrane for analysis by immunoblot methods.
The binding of anti-pS (monoclonal antibody 1H6) was assessed on freshly isolated human platelets. Platelet-rich plasma was obtained as described (35), and the platelets were isolated by centrifugation and divided in two samples. The “nonactive” platelets were treated with 10 μg/ml prostaglandin-1α (Sigma) to prevent spontaneous activation, and the “active” platelets were incubated with 10 nM PMA for 30 min at room temperature. Both samples were fixed with ice-cold methanol and stained with the anti-pS antibody 1H6. For control experiments, the 1H6 antibody was preabsorbed with a 100-fold excess of pS liposomes prepared as described (23). The labeling was visualized by an anti-mouse IgG antibody conjugated to Cy3 (Molecular Probes).
Immunohistochemical Analysis. To evaluate S100A13, Myc, pS, and IL-1α expression in balloon-injured arteries, paraffin-embedded specimens were sliced into 5-μm cross sections, the paraffin was removed with xylene washes, and the specimens were mounted on glass slides and incubated in 10% hydrogen peroxide for 90 min to block endogenous peroxidase activity. Nonspecific binding was minimized by preincubating the sections with 5% BSA (Sigma) in PBS. The sections were sequentially incubated with either a rabbit anti-S100A13, a monoclonal anti-Myc (Oncogene Science), a rabbit anti-IL-1α, a monoclonal anti-platelet-endothelial cell adhesion molecule (PECAM; DAKO), or the affinity-purified anti-pS monoclonal antibody. After two washes with PBS, the sections were incubated with either an anti-rabbit or an anti-mouse IgG-conjugated horseradish peroxidase antibody (Bio-Rad). Staining was visualized with chromogen (Bio-Rad). The presence of macrophages was evaluated by immunostaining with anti-CD11b (MAC1) by using acetone/ethanol-fixed sections.
Results
Although Cu2+-affinity chromatography has been used to adsorb IL-1α from medium conditioned by temperature stress (19), little is known about the ability of IL-1α to associate with Cu2+. As a result, cell lysates from PMA-treated human PBM cells were used as a source of the precursor and mature forms of IL-1α, and Cu2+-chelation affinity chromatography was used to evaluate the ability of IL-1α to associate with Cu2+. As shown in Fig. 1A, the precursor and mature forms of IL-1α were eluted at ≈35 mM imidazole, suggesting that both forms of IL-1α were able to bind Cu2+ and the mature form of IL-1α is likely to contain the domain responsible for Cu2+ affinity.
Fig. 1.
IL-1α is a Cu2+-binding protein that is released from human PBM cells in a stress- and Cu2+-dependent manner. (A) Cell lysates from PMA-activated human PBM cells (PBMC) were adsorbed to a Cu2+-affinity column and eluted with an imidazole gradient (0–100 mM) as described (20). The column was stripped with 50 mM EDTA, and all fractions including the flow were resolved by 15% acrylamide SDS/PAGE and evaluated by IL-1α immunoblot analysis. (B) Conditioned medium (CM) obtained from temperature-stressed (42°C, 2 h) PMA-activated human PBM cells was processed by Cu2+-affinity chromatography, and eluted fractions were analyzed as described for A.
Because the release of IL-1α (19) and FGF1 (20) use S100A13 to form a Cu2+-dependent heterotetramer and the Cu2+ chelator TTM is able to repress the release of FGF1 in response to cellular stress, we evaluated whether TTM was also able to inhibit the heat-shock-induced export of IL-1α from PMA-treated human PBM cells in vitro. As shown in Fig. 1B, human PBM cells are not only able to release both the precursor and mature forms of IL-1α in response to temperature stress, but TTM is also able to repress the release of both the precursor and mature forms of IL-1α. These data further suggest that both the precursor and mature forms of IL-1α are susceptible to nonclassical Cu2+-dependent and temperature stress-mediated export in vitro.
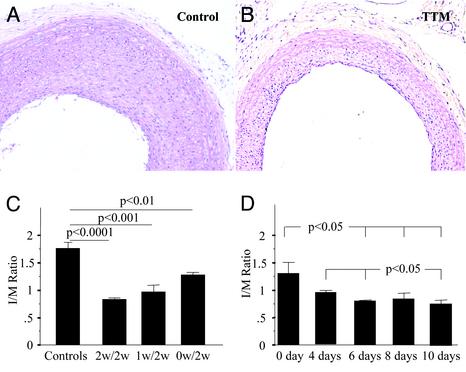
Because TTM is able to repress the stress-induced release of IL-1α in vitro, and the function of proinflammatory cytokines has been implicated as regulators of restenosis in vivo, we evaluated whether TTM administration would be able to modify the response to injury after balloon injury in the rat. Because TTM is clinically administered as an oral compound (27), rats were prefed TTM either 1 or 2 weeks before or on the day of balloon injury. Plasma ceruloplasmin levels were monitored, and the oral administration of TTM at a dosage of 10 mg/kg was able to rapidly reduce and sustain the plasma level of ceruloplasmin in all TTM-treated animals at a 50% level of reduction after 1 week and a 70–80% reduction after 2 weeks (data not shown). This result is consistent with data in mice to prevent tumor angiogenesis as a result of Cu2+ deficiency (28). The TTM-treated rats were subjected to balloon injury, and after 2 weeks of daily TTM administration postinjury the animals were killed, and the extent of neointimal thickening was evaluated. Animals not fed TTM served as additional controls. As shown in Fig. 2 A and B, TTM was able to repress neointimal thickening significantly when administered 2 weeks before injury. Indeed, this reduction was also evident, albeit to a lesser extent, in the rats fed oral TTM either 1 week before or on the day of balloon injury (Fig. 2C). The kinetics of the response of neointimal thickening to TTM withdrawal was also evaluated, and interestingly, we observed a plateau in the maximal response 6 days after the withdrawal of TTM (Fig. 2D).
Fig. 2.
Copper chelation reduces the formation of neointima after balloon injury in the rat carotid artery. TTM was administered daily in a dose of 10 mg/kg dissolved into 45 ml of drinking water. TTM administration started 2 weeks before the day of the injury (n = 6), 1 week before the day of the injury (n = 6), and on the day of the injury (n = 5). All animals were treated with TTM for 2 weeks after balloon injury. The rats without TTM treatment served as a control (n = 5). (A) Representative cross section of the control group not treated with TTM; sections are stained with hematoxylin/eosin. (Magnification, ×10.) (B) TTM administration for 2 weeks before and 2 weeks after balloon injury. (Magnification, ×10.) (C) Bar graph showing the neointima/medium (I/M) ratio (mean ± SEM) in all four groups of rats. (D) Bar graph showing the neointima/ratio (mean ± SEM) in five groups of animals that were treated with TTM for 2 weeks before balloon injury followed by the withdrawal of TTM on the day of the injury or 4, 6, 8, and 10 days after balloon injury.
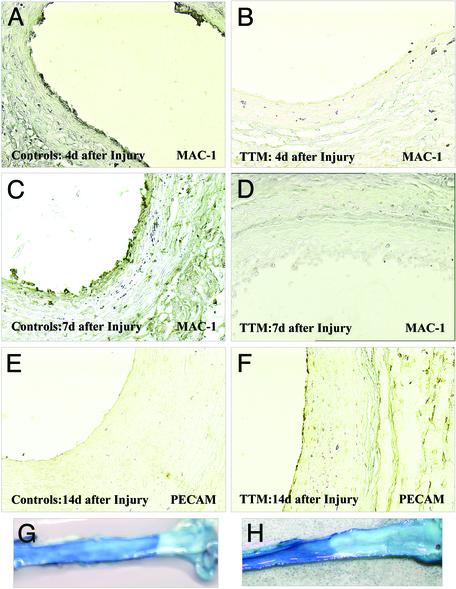
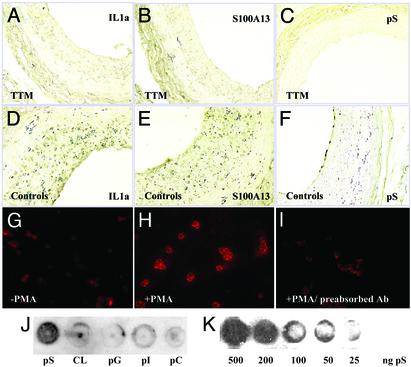
Because TTM was able to significantly impair neointimal thickening in vivo, we evaluated whether this response was due to its ability to inhibit the release of IL-1α in response to injury. Thus one would anticipate that a lack of extracellular IL-1α would impair mononuclear cell infiltration into the denuded area. Indeed, immunohistochemical analysis with the MAC1 antibody was able to resolve the presence of mononuclear cells 4 and 7 days postinjury in the controls (Fig. 3 A and C) but failed to report the presence of these cells at these time points in the arteries of animals pretreated with TTM for 2 weeks before injury (Fig. 3 B and D). Because TTM is also able to repress the stress-induced release of IL-1α (22) and S100A13 (19) in vitro, we examined these tissues for the expression of IL-1α and S100A13. As shown in Fig. 4, immunohistochemical analysis revealed the presence of IL-1α (Fig. 4 A and D) and S100A13 (Fig. 4 B and E) in control arteries 2 weeks after balloon injury but failed to resolve their presence in arteries at this time point from rats pretreated with TTM 2 weeks before injury. A similar response was also observed for FGF1 expression under similar conditions, and IL-1α and FGF1 immunoblot analysis revealed similar TTM-dependent changes (data not shown). These data suggest that the ability of TTM to reduce neointimal thickening may be the result of its ability to repress the expression and potentially the release of not only IL-1α but also S100A13, the chaperone that forms intracellular Cu2+-dependent heterotetrameric complexes with both IL-1α (19) and FGF1 (22).
Fig. 3.
TTM inhibits mononuclear cell infiltration in the balloon-injured vessels and promotes reendothelialization. (A–D) The extent of mononuclear cell infiltration into the vessel wall was assessed at different time points (4 and 7 days) after balloon injury. Shown are photomicrographs of the rat carotid artery stained with an anti-CD11b (MAC1) immunostaining (×20) 4 days after balloon injury in the control (A) and TTM-treated (B) groups as well as 7 days after balloon injury in the control group (C) and TTM-treated animals (D). The recovery of the endothelial cells in the denuded area was assessed by staining with an anti-PECAM antibody (×20) 14 days after balloon injury in the control (E) and TTM-treated (F) groups and confirmed by Evan's blue staining (G and H).
Fig. 4.
TTM treatment decreases the level of IL-1α, S100A13, and pS expression in the balloon-injured vessel wall. (A–F) Photomicrographs of rat carotid artery 14 days after balloon injury as shown. (Magnification, ×20.) Tissue slices were subjected to immunohistochemical analysis by using an anti-IL-1α antibody (A, TTM-treated; D, control), anti-S100A13 antibody (B, TTM-treated; E, control), and the 1H6 antibody (C, TTM-treated; F, control). (G–I) Immunohistochemical analysis of pS expression in control (G) and PMA-treated (H) platelets with the anti-pS antibody. (I) Preadsorption of the anti-pS antibody with pS liposomes served as an additional control. (J) The selectivity of the anti-pS antibody was evaluated by immunoblot analysis on pS, cardiolipin (CL), phosphatidylglycerol (pG), phosphatidylinositol (pI), and phosphatidylcholine (pC) immobilized on a nitrocellulose membrane. (K) The reactivity of the anti-pS antibody was evaluated in a concentration-dependent manner by using immunodot blot analysis with a decreasing amount of pS.
The molten globule character of FGF1 (23) and IL-1α (19) suggest that these proteins may be endowed with the ability to insert and traverse acidic phospholipid-rich membranes. The transition of pS from the inner surface to the outer surface of the plasma membrane of platelets and endothelial cells is an important component of the intrinsic coagulation system (38), and the presence of pS on the outer surface of the plasma membrane may also be an important immediate-early component of the apoptotic program (39). We became interested in the appearance of pS on the outer surface of the plasma membrane, because pS-rich membrane structures may also represent a window for the stress-induced export of IL-1α and FGF1. Interestingly, we have obtained an IgG monoclonal antibody that is able to recognize pS by both ELISA (data not shown) and immunodot blot methods not only at reasonably low levels of the acidic phospholipid (Fig. 4K) but also with an apparent specificity for the pS polar head group (Fig. 4J). In addition, the anti-pS antibody is also able to recognize activated human platelets (Fig. 4 G and H), and this staining pattern is repressed significantly by preadsorption of the anti-pS antibody to pS micelles (Fig. 4I). Because the transition of pS from the inner to the outer leaflet of the plasma membrane may be involved in the release of IL-1α (17) and FGF1 (18) and this activity may be exaggerated by the export of IL-1α and FGF1 into the extracellular compartment, we anticipated that the presence of pS may be a useful reporter of IL-1α and FGF1 release, and TTM may be able to attenuate this response. Indeed, as shown in Fig. 4 C and F, animals pretreated with TTM for 2 weeks before balloon injury, exhibit little if any staining with the anti-pS antibody 2 weeks postinjury, whereas the control animals reveal a significant signal at this time point.
The staining pattern exhibited by the anti-pS antibody in the control group is noteworthy because we have observed considerable staining at the blood–tissue interface (Fig. 4F), a region at which platelet deposition is known to be exaggerated (36, 37, 40). Likewise, we were surprised by the absence of pS in the TTM-treated animals, because the data shown in Fig. 2B suggest that the denuded area at the blood–tissue interface is sealed with a monolayer of cells. Although reendothelialization after balloon angioplasty in the rat is known to occur at later time points, it was unanticipated that this cellular monolayer could have established itself with such rapidity. Because the MAC1 (Fig. 3 B and D) and pS (Fig. 4 C and F) staining profiles eliminated the possibility that this monolayer could be a combination of both mononuclear cells and platelets, we examined these tissues for the expression of PECAM, an endothelial cell marker. As shown in Fig. 3 E and F, although the vessels derived from the nontreated rats did not exhibit a PECAM-positive monolayer at the blood–tissue interface 2 weeks postinjury, the rats treated with TTM for 2 weeks before injury reported a PECAM-positive staining at the blood–tissue interface. Further, Evan's blue staining (Fig. 3 G and H) also exhibited considerable luminal repair in the TTM-treated rats at this time point, which is consistent with the suggestion that TTM administration was not only able to repress the restenotic program but also may have enabled reendothelialization of the denuded area with an unanticipated rapidity.
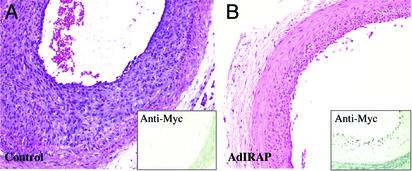
Because the administration of TTM before balloon injury significantly attenuated the vascular response to injury, which may involve the ability of TTM to repress the nonclassical export of the proinflammatory cytokine, IL-1α, we sought to demonstrate independently that the repression of IL-1 may impair restenosis in vivo. We used somatic gene transfer after balloon angioplasty to express the IRAP gene product, because IRAP contains a functional signal peptide sequence that would enable it to have unrestricted access to the extracellular compartment to inhibit with a high degree of specificity the proinflammatory function of IL-1 gene family prototypes (41). We prepared an IRAP-Myc fusion gene to evaluate the expression of IRAP after gene transfer, and as shown in Fig. 5, the administration of the IRAP-Myc gene in an adenoviral vector after balloon injury was able to significantly limit the degree of neointimal thickening. Immunohistochemical analysis with an anti-Myc antibody was readily able to detect IRAP-Myc protein expression 14 days postinjury (Fig. 5B Inset). An insertless adenoviral vector served as a control, and as shown in Fig. 5A there was no apparent effect of the adenovirus on neointimal thickening (Fig. 5), nor did the Myc antibody exhibit a Myc signal in these tissue sections (Fig. 5A Inset). These data suggest that the expression of IRAP during the response to injury is able to significantly impair the development of a neointima in the rat.
Fig. 5.
The effects of adenoviral IRAP gene transfer on the formation of neointima thickening in the rat. An adenoviral construct encoding a Myc-tagged IRAP sequence was administered at the day of the balloon injury as described in Methods. The administration of an insertless adenovirus served as control. Representative cross sections from the carotid artery of the adenoviral IRAP (B) and insertless adenoviral control (A) groups were stained with hematoxylin/eosin. (Magnification, ×10.) (Insets) Expression of the Myc epitope tag (Myc-IRAP) was evaluated by anti-Myc immunohistochemical analysis.
Discussion
The biological significance of nonclassical export of signal peptideless proteins has been difficult to access, because in vitro methods using established cell lines that are amenable to stable transfection were required to establish the pathways for release (18, 21). This issue was complicated further by the absence of data in vivo to confirm the in vitro mechanisms involved in the nonclassical release of signal peptideless polypeptides. Although we recently identified a Cu2+-dependent mechanism responsible for stress-induced release of the signal peptideless polypeptides IL-1α and FGF1 using established and stably transfected human and murine cell lines (19, 20), the biological significance of these observations was not readily apparent. Here we report the ability of freshly isolated human PBM cells to use intracellular Cu2+ for the stress-induced release of the endogenous precursor and mature forms of IL-1α, and similar data were also obtained for the stress-induced release of FGF1 (data not shown). Indeed, this observation represents a previously uncharacterized post-translational mechanism for the stress-induced release of signal peptideless polypeptides from normal human diploid mononuclear cells and confirms our data from established cell lines (17, 19). We also provide additional evidence concerning the role of extracellular IL-1 as a determinant in the development of restenosis in vivo. We demonstrate that inhibition of the IL-1 receptor by the somatic gene transfer of the IRAP gene immediately after vascular injury is able to attenuate the response to injury in the arterial vessel wall significantly. It has been extensively demonstrated that proinflammatory cytokines are induced very early after vascular injury and may play a crucial role in the attraction of mononuclear cells during the early stages of restenosis and experimental arteriosclerosis (42). Indeed, the observation that polymorphism of the IRAP gene is associated with reduced restenosis (43) is consistent with our data. It is also well established that the prototype members of the IL-1 gene family are potent inducers of the latent transcription factor NF-κB, which in turn may contribute to injury-induced lumen loss by the induction of a more prominent inflammatory response (44).
Because (i) extracellular IL-1 seems important for the response to injury in vivo and (ii)Cu2+ mediates the stress-induced release of IL-1α and FGF1 from human PBM cells in vitro, we suggest that efficient Cu2+ chelation may be an approach for management of arterial restenosis. Indeed, our data suggest that the Cu2+ chelator TTM is able to attenuate the development of neointimal thickening significantly and is able to promote the rapid reendothelialization of the denuded area. We further suggest that the reduced response to injury in the TTM-treated animals may be due to a repression of the infiltration of the vessel wall with either blood- and/or tissue-derived macrophages during the immediate-early stages in the development of the vascular response to injury. Indeed, the inhibition of mononuclear cell recruitment to mechanically injured arteries that are devoid of endothelium may also limit the delivery of essential cytokines and growth factors responsible for the initiation of migration of the vascular smooth muscle cell, a prerequisite for the development of a neointima (6, 45). Although a wide variety of growth factors and cytokines have been implicated as regulators of cell migration within the vasculature (46, 47), the potential regulatory function of the FGF family members has been described extensively (3, 48). Indeed, the introduction by somatic gene transfer of an engineered form of FGF1 to force secretion of this signal peptideless polypeptide through the endoplasmic reticulum-Golgi apparatus resulted in an exaggeration of vascular smooth muscle cell migration and proliferation as well as the formation of a prominent neointimal angiogenic response (2).
Because we observed a significant decrease in the immunohistochemical staining for FGF1, and IL-1α in the TTM-treated rats compared with controls and Cu2+-mediated heterotetramer formation between S100A13 and either IL-1α or FGF1 is responsible for their release in response to stress (19, 20), we suggest that the attenuation of the restenotic response may have been due to the ability of TTM to inhibit the release of IL-1α and FGF1 in vivo. This observation is consistent with the ability of TTM to inhibit the release of IL-1α and FGF1 from not only NIH 3T3 cells but also human U937 cells (19, 20) and PBM cells in vitro. Thus, these observations reinforce the premise that intracellular Cu2+ may play a key regulatory role in the nontraditional export of signal-less polypeptides in vivo and that effective drug-induced Cu2+ chelation may be an innovative approach for treating stress-dependent pathologies involving the function of IL-1α, FGF1, or both including cancer (27, 49), because TTM has been shown to be an effective antiangiogenic agent in vivo (27, 28). Likewise, recent studies have also suggested that Cu2+ chelation may represent an alternative approach to the management of β-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Because IL-1α expression is enhanced significantly in the brains of Alzheimer's disease patients (50) and is able to induce the expression of the β-amyloid precursor gene in human endothelial cells in vitro (51), the therapeutic effect of Cu2+ chelators in Alzheimer's pathology may be due to their ability to inhibit the release of IL-1α.
It is noteworthy that a major difference between the response to injury in the control, the IRAP gene transfer, and TTM-treated arteries was the apparent ability of vascular endothelial cells to establish reparation of the denuded area. Although we do not know the mechanism used by the TTM-treated animals to repair the denuded area, it is unlikely that IL-1α and FGF1 play a role in the promotion of this response. However, because IL-1α is able to repress endothelial cell migration (52), the absence of IL-1α may enable another angiogenic factor to function in a more astute manner. Because the vascular smooth muscle cell is a rich source of vascular endothelial growth factor (VEGF) (45) and VEGF is a prominent chemotactic signal for the endothelial cell, it will be interesting to determine whether members of the VEGF gene family are involved in the reparative process.
Our data also suggest the absence of pS in injured tissue after Cu2+-chelation therapy. This may be an important component of the nonclassical pathways of protein export and in our system may involve the well described function of members of the S100 gene family as binding proteins for acidic phospholipids including pS (53). In addition, recent evidence has demonstrated that a hallmark of stress-induced cell injury may be the translocation of pS to the outer leaflet of the plasma membrane (54). Further, the absence of pS expression in the artery of TTM-treated rats may also be a function of the antioxidant potential of the Cu2+ chelator, which may also repress the effect of reactive oxygen species at the place of injury. Reactive oxygen species are thought to induce pS translocation either through the oxidation of thiol-containing transport molecules or by the peroxidation of lipids (54). Because our anti-pS monoclonal antibody specifically recognizes the loss of pS asymmetry in the plasma membrane in activated platelets and possibly in the injured vasculature as well, this reagent may provide a tool for the visualization and perhaps targeting of cells undergoing stress-induced responses in vivo.
Acknowledgments
We acknowledge the administrative assistance of N. Albrecht in the construction of this manuscript. This work was supported in part by National Institutes of Health Grants HL 35627, RR15555, and HL 32348 (to T.M.).
Abbreviations: PBM, peripheral blood mononuclear; FGF, fibroblast growth factor; pS, phosphatidylserine; TTM, tetrathiomolybdate; IRAP, IL-1 receptor antagonist protein; PMA, phorbol 12-myristate 13-acetate; PECAM, platelet-endothelial cell adhesion molecule.
References
- 1.Libby, P., Schwartz, D., Brogi, E., Tanaka, H. & Clinton, S. K. (1992) Circulation 86, Suppl. 6, III47–III52. [PubMed] [Google Scholar]
- 2.Nabel, E. G., Yang, Z. Y., Plautz, G., Forough, R., Zhan, X., Haudenschild, C. C., Maciag, T. & Nabel, G. J. (1993) Nature 362, 844–846. [DOI] [PubMed] [Google Scholar]
- 3.Lindner, V., Majack, R. A. & Reidy, M. A. (1990) J. Clin. Invest. 85, 2004–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby, P. & Tanaka, H. (1997) Prog. Cardiovasc. Dis. 40, 97–106. [DOI] [PubMed] [Google Scholar]
- 5.Lindner, V. (1998) Pathobiology 66, 311–320. [DOI] [PubMed] [Google Scholar]
- 6.Danenberg, H. D., Welt, F. G., Walker, M., III, Seifert, P., Toegel, G. S. & Edelman, E. R. (2002) Circulation 105, 2917–2922. [DOI] [PubMed] [Google Scholar]
- 7.Bayes-Genis, A., Campbell, J. H., Carlson, P. J., Holmes, D. R., Jr., & Schwartz, R. S. (2002) Atherosclerosis (Berlin) 163, 89–98. [DOI] [PubMed] [Google Scholar]
- 8.Wang, X., Romanic, A. M., Yue, T. L., Feuerstein, G. Z. & Ohlstein, E. H. (2000) Biochem. Biophys. Res. Commun. 271, 138–143. [DOI] [PubMed] [Google Scholar]
- 9.Olson, N. E., Chao, S., Lindner, V. & Reidy, M. A. (1992) Am. J. Pathol. 140, 1017–1023. [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar, S., McDonnell, P. C., Lehr, R., Tierney, L., Tzimas, M. N., Griswold, D. E., Capper, E. A., Tal-Singer, R., Wells, G. I., Doyle, M. L. & Young, P. R. (2000) J. Biol. Chem. 275, 10308–10314. [DOI] [PubMed] [Google Scholar]
- 11.Sims, J. E., Nicklin, M. J., Bazan, J. F., Barton, J. L., Busfield, S. J., Ford, J. E., Kastelein, R. A., Kumar, S., Lin, H., Mulero, J. J., et al. (2001) Trends Immunol. 22, 536–537. [DOI] [PubMed] [Google Scholar]
- 12.Burgess, W. H. & Maciag, T. (1989) Annu. Rev. Biochem. 58, 575–606. [DOI] [PubMed] [Google Scholar]
- 13.Friesel, R. & Maciag, T. (1999) Thromb. Haemostasis 82, 748–754. [PubMed] [Google Scholar]
- 14.Thomas, K. A., Rios-Candelore, M., Gimenez-Gallego, G., DiSalvo, J., Bennett, C., Rodkey, J. & Fitzpatrick, S. (1985) Proc. Natl. Acad. Sci. USA 82, 6409–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang, J. D., Cousens, L. S., Barr, P. J. & Sprang, S. R. (1991) Proc. Natl. Acad. Sci. USA 88, 3446–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lomedico, P. T., Gubler, U., Hellmann, C. P., Dukovich, M., Giri, J. G., Pan, Y. C., Collier, K., Semionow, R., Chua, A. O. & Mizel, S. B. (1984) Nature 312, 458–462. [DOI] [PubMed] [Google Scholar]
- 17.Tarantini, F., Micucci, I., Bellum, S., Landriscina, M., Garfinkel, S., Prudovsky, I. & Maciag, T. (2001) J. Biol. Chem. 276, 5147–5151. [DOI] [PubMed] [Google Scholar]
- 18.Tarantini, F., Gamble, S., Jackson, A. & Maciag, T. (1995) J. Biol. Chem. 270, 29039–29042. [DOI] [PubMed] [Google Scholar]
- 19.Mandinova, A., Soldi, R., Graziani, I., Bagala, C., Bellum, S., Landriscina, M., Tarantini, F., Prudovsky, I. & Maciag, T. (2003) J. Cell Sci., in press. [DOI] [PubMed]
- 20.Landriscina, M., Bagala, C., Mandinova, A., Soldi, R., Micucci, I., Bellum, S., Prudovsky, I. & Maciag, T. (2001) J. Biol. Chem. 276, 25549–25557. [DOI] [PubMed] [Google Scholar]
- 21.LaVallee, T. M., Tarantini, F., Gamble, S., Mouta Carreira, C., Jackson, A. & Maciag, T. (1998) J. Biol. Chem. 273, 22217–22223. [DOI] [PubMed] [Google Scholar]
- 22.Landriscina, M., Soldi, R., Bagala, C., Micucci, I., Bellum, S., Tarantini, F., Prudovsky, I. & Maciag, T. (2001) J. Biol. Chem. 276, 22544–22552. [DOI] [PubMed] [Google Scholar]
- 23.Mach, H. & Middaugh, C. R. (1995) Biochemistry 34, 9913–9920. [DOI] [PubMed] [Google Scholar]
- 24.Hemker, H. C., van Rijn, J. L., Rosing, J., van Dieijen, G., Bevers, E. M. & Zwaal, R. F. (1983) Blood Cells 9, 303–317. [PubMed] [Google Scholar]
- 25.Raju, K. S., Alessandri, G., Ziche, M. & Gullino, P. M. (1982) J. Natl. Cancer Inst. 69, 1183–1188. [PubMed] [Google Scholar]
- 26.Volker, W., Dorszewski, A., Unruh, V., Robenek, H., Breithardt, G. & Buddecke, E. (1997) Atherosclerosis (Berlin) 130, 29–36. [DOI] [PubMed] [Google Scholar]
- 27.Brewer, G. J., Dick, R. D., Grover, D. K., LeClaire, V., Tseng, M., Wicha, M., Pienta, K., Redman, B. G., Jahan, T., Sondak, V. K., et al. (2000) Clin. Cancer Res. 6, 1–10. [PubMed] [Google Scholar]
- 28.Pan, Q., Kleer, C. G., van Golen, K. L., Irani, J., Bottema, K. M., Bias, C., De Carvalho, M., Mesri, E. A., Robins, D. M., Dick, R. D., et al. (2002) Cancer Res. 62, 4854–4859. [PubMed] [Google Scholar]
- 29.Pogliaghi, G., Tacchini, L., Anzon, E., Radice, L. & Bernelli-Zazzera, A. (1995) FEBS Lett. 372, 181–184. [DOI] [PubMed] [Google Scholar]
- 30.Schosinsky, K. H., Lehmann, H. P. & Beeler, M. F. (1974) Clin. Chem. 20, 1556–1563. [PubMed] [Google Scholar]
- 31.Clowes, A. W., Reidy, M. A. & Clowes, M. M. (1983) Lab. Invest. 49, 208–215. [PubMed] [Google Scholar]
- 32.Richardson, M., Gerrity, R. G., Alavi, M. Z. & Moore, S. (1982) Arteriosclerosis (Dallas) 2, 369–379. [DOI] [PubMed] [Google Scholar]
- 33.Hardy, S., Kitamura, M., Harris-Stansil, T., Dai, Y. & Phipps, M. L. (1997) J. Virol. 71, 1842–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stamenova, M., Kehayov, I., Kyurkchiev, S. & Despodova, T. (1990) Folia Biol. (Prague) 36, 81–90. [PubMed] [Google Scholar]
- 35.Williams, M. S., Coller, B. S., Vaananen, H. J., Scudder, L. E., Sharma, S. K. & Marmur, J. D. (1998) Circulation 98, 742–748. [DOI] [PubMed] [Google Scholar]
- 36.Holvoet, P. & Collen, D. (1997) Curr. Opin. Lipidol. 8, 320–328. [DOI] [PubMed] [Google Scholar]
- 37.Peerschke, E. I. & Ghebrehiwet, B. (2001) Immunol. Rev. 180, 56–64. [DOI] [PubMed] [Google Scholar]
- 38.Thiagarajan, P. & Benedict, C. R. (1997) Circulation 96, 2339–2347. [DOI] [PubMed] [Google Scholar]
- 39.Fabisiak, J. P., Kagan, V. E., Tyurina, Y. Y., Tyurin, V. A. & Lazo, J. S. (1998) Am. J. Physiol. 274, L793–L802. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz, R. S., Holmes, D. R., Jr., & Topol, E. J. (1992) J. Am. Coll. Cardiol. 20, 1284–1293. [DOI] [PubMed] [Google Scholar]
- 41.Arend, W. P. (2001) Semin. Arthritis Rheum. 30, 1–6. [DOI] [PubMed] [Google Scholar]
- 42.Libby, P. (2002) Nature 420, 868–874. [DOI] [PubMed] [Google Scholar]
- 43.Francis, S. E., Camp, N. J., Burton, A. J., Dewberry, R. M., Gunn, J., Stephens-Lloyd, A., Cumberland, D. C., Gershlick, A. & Crossman, D. C. (2001) Heart 86, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindner, V. & Collins, T. (1996) Am. J. Pathol. 148, 427–438. [PMC free article] [PubMed] [Google Scholar]
- 45.Hojo, Y., Ikeda, U., Maeda, Y., Takahashi, M., Takizawa, T., Okada, M., Funayama, H. & Shimada, K. (2000) Atherosclerosis (Berlin) 150, 63–70. [DOI] [PubMed] [Google Scholar]
- 46.Myllarniemi, M., Frosen, J., Calderon Ramirez, L. G., Buchdunger, E., Lemstrom, K. & Hayry, P. (1999) Cardiovasc. Drugs Ther. 13, 159–168. [DOI] [PubMed] [Google Scholar]
- 47.Smith, J. D., Bryant, S. R., Couper, L. L., Vary, C. P., Gotwals, P. J., Koteliansky, V. E. & Lindner, V. (1999) Circ. Res. 84, 1212–1222. [DOI] [PubMed] [Google Scholar]
- 48.Hanna, A. K., Fox, J. C., Neschis, D. G., Safford, S. D., Swain, J. L. & Golden, M. A. (1997) J. Vasc. Surg. 25, 320–325. [DOI] [PubMed] [Google Scholar]
- 49.Khan, M. K., Miller, M. W., Taylor, J., Gill, N. K., Dick, R. D., Van Golen, K., Brewer, G. J. & Merajver, S. D. (2002) Neoplasia 4, 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bush, A. I. (2002) Neurobiol. Aging 23, 1031–1038. [DOI] [PubMed] [Google Scholar]
- 51.Goldgaber, D., Harris, H. W., Hla, T., Maciag, T., Donnelly, R. J., Jacobsen, J. S., Vitek, M. P. & Gajdusek, D. C. (1989) Proc. Natl. Acad. Sci. USA 86, 7606–7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meier, C. A., Chicheportiche, R., Juge-Aubry, C. E., Dreyer, M. G. & Dayer, J. M. (2002) Cytokine 18, 320–328. [DOI] [PubMed] [Google Scholar]
- 53.Donato, R. (1999) Biochim. Biophys. Acta 1450, 191–231. [DOI] [PubMed] [Google Scholar]
- 54.Ran, S. & Thorpe, P. E. (2002) Int. J. Radiat. Oncol. Biol. Phys. 54, 1479–1484. [DOI] [PubMed] [Google Scholar]