Abstract
Natural and synthetic agonists of the peroxisome proliferator-activated receptor γ (PPARγ) regulate adipocyte differentiation, glucose homeostasis, and inflammatory responses. Although effects on adipogenesis and glucose metabolism are genetically linked to PPARγ, the PPARγ dependence of antiinflammatory responses of these substances is less clear. Here, we have used a combination of mRNA expression profiling and conditional disruption of the PPARγ gene in mice to characterize programs of transcriptional activation and repression by PPARγ agonists in elicited peritoneal macrophages. Natural and synthetic PPARγ agonists, including the thiazolidinedione rosiglitazone (Ro), modestly induced the expression of a surprisingly small number of genes, several of which were also induced by a specific PPARδ agonist. The majority of these genes encode proteins involved in lipid homeostasis. In contrast, Ro inhibited induction of broad subsets of lipopolysaccharide and IFN-γ target genes in a gene-specific and PPARγ-dependent manner. At high concentrations, Ro inhibited induction of lipopolysaccharide target genes in PPARγ-deficient macrophages, at least in part by activating PPARδ. These studies establish overlapping transactivation and transrepression functions of PPARγ and PPARδ in macrophages and suggest that a major transcriptional role of PPARγ is negative regulation of specific subsets of genes that are activated by T helper 1 cytokines and pathogenic molecules that signal through pattern recognition receptors. These findings support a physiological role of PPARγ in regulating both native and acquired immune responses.
PPARγ is a member of the nuclear receptor superfamily of ligand-dependent transcription factors that regulates adipocyte differentiation and glucose homeostasis (1–3). Although the endogenous ligands that regulate peroxisome proliferator-activated receptor (PPAR) γ activity in vivo remain poorly characterized, several naturally occurring polyunsaturated fatty acids and their metabolites have been identified that activate PPARγ, including products that are generated through the actions of specific lipoxygenases (e.g., 13-hydroxyoctadecadienoic acid and 15-hydroxyeicosatetraenoic acid) and prostaglandin synthases (e.g., 15 deoxy-Δ12,14 prostaglandin J2) (4–7). In addition, numerous synthetic PPARγ agonists have been identified, including the thiazolidinedione class of drugs used clinically in the treatment of type 2 diabetes mellitus (1, 3).
Natural and synthetic PPARγ ligands have been also been shown to exert antiinflammatory effects in models of athero-sclerosis (8–10), inflammatory bowel disease (11, 12), and allergic encephalomyelitis (13–15). The investigation of potential antiinflammatory effects of PPARγ agonists in these settings was initially based on studies demonstrating that they could inhibit transcriptional activation of inflammatory response genes by activators such as lipopolysaccharide (LPS), IL-1β, and IFN-γ in macrophages and other cell types (16–18). PPARγ expression is dramatically up-regulated in macrophages and T cells during inflammatory responses, and can be induced by IL-4 and other immunoregulatory molecules (19–21). Overexpression of PPARγ potentiates the ability of diverse PPARγ agonists to inhibit the expression of inflammatory response genes, consistent with it mediating antiinflammatory effects (16, 22). However, 15-deoxyΔ12,14 prostaglandin J2 was found to inhibit NF-κB-dependent transcription by PPARγ-independent mechanisms (23, 24), and doses of thiazolidinediones that exert maximal inhibitory effects on LPS-inducible genes are significantly higher than would be expected based on their binding affinity for PPARγ in vitro (16). Furthermore, treatment of macrophages derived from PPARγ-null embryonic stem cells with high concentrations of synthetic PPARγ ligands was recently reported to inhibit the induction of the inducible nitric-oxide synthase (iNOS) and cyclooxygenase 2 (COX2) genes by IFN-γ to approximately the same extent as in macrophages derived from wild-type embryonic stem cells (25). These observations raise a number of questions regarding the roles of PPARγ in regulating macrophage gene expression during inflammatory responses.
To address these questions, we used a combination of microarray analysis and Cre-mediated disruption of the PPARγ gene in primary mouse macrophages to characterize PPARγ-dependent and -independent transcriptional programs regulated by PPARγ agonists in these cells. Surprisingly, relatively few genes were positively regulated by synthetic PPARγ ligands in thioglycollate-elicited peritoneal macrophages. Most of the genes in this group have roles in lipid metabolism and were also activated by a specific PPARδ agonist. The major action of synthetic PPARγ ligands was to inhibit induction of a subset of LPS and IFN-γ-dependent genes. This effect was largely PPARγ dependent at low concentrations of rosiglitazone (Ro), but became increasingly PPARγ independent at high concentrations. PPARγ-independent effects could be at least partially explained by activation of PPARδ, which can also potently inhibit LPS induction of COX2 and iNOS transcription. These observations support a primary role of PPARγ in mediating antiinflammatory effects of thiazolidinediones and reveal potential roles of PPARδ in contributing to this function.
Materials and Methods
Cell Culture. Thioglycollate-elicited macrophages were isolated by peritoneal lavage 3 days after peritoneal injection of 2.5 ml of 3% thioglycollate (Difco). Cells were plated in RPMI and 10% FBS and washed after 5 h, the medium was removed, and cells were fed with fresh medium containing 0.5% FBS. The generation of PPARγ–/– macrophages by crossing PPARγf/f mice with Mx-Cre transgenic mice was carried out as described by Akiyama et al. (26). LPS (Sigma) was used at a concentration of 100 ng/ml and IFN-γ (Genzyme) at a concentration of 100 units/ml.
Analysis of PPARγ Deletion Efficiency. For PPARγ RNA analysis, total RNA was converted into cDNA by random priming (SuperScript First-Strand cDNA Synthesis kit, Invitrogen) and then amplified for 35 cycles with primers flanking the floxed exon 2. The following primers were used: sense, TGCCTATGAGCACTTCACAAGA; antisense, CTTCTGAAACCGACAGTACTGA.
Expression Array Profiling. Cells were lysed with TRIzol (Invitrogen), and total RNA was purified by using RNeasy columns (Qiagen, Valencia, CA). cRNA was generated from 10 μg of total RNA by using the SuperScript kit (Invitrogen) and the High Yield RNA transcription labeling kit (Enzo Diagnostics). Fragmented cRNA was hybridized to Affymetrix (Santa Clara, CA) arrays according to the manufacturer's instructions. Data were analyzed with the MICROARRAY suite (Affymetrix), GENESPRING (Silicon Genetics), and in-house software developed by Sasik et al. (27).
Northern Blot Analysis. RNA analysis by Northern blotting followed the procedure in ref. 28. Five to 10 μg of total RNA was separated by gel electrophoresis and transferred to nylon (SuPerCharge, Schleicher & Schuell). Before hybridization, membranes were UV cross-linked (Stratagene) and stained with Methylene Blue (Molecular Research Center, Cincinnati). Probes were generated by RT-PCR, followed by random priming labeling (Invitrogen) and hybridization with QuikHyb (Stratagene).
Promoter Studies. Transient transfections were performed as described by using Lipofectamine (Invitrogen) to transfect RAW 264.7 (16). Cells were transfected with 1 μg of the 3xAOX-TK-luciferase reporter plasmid containing three copies of the PPAR response element present in the Acyl CoA oxidase promoter. One microgram of β-galactosidase expression vector was also cotransfected as a control for transfection efficiency. PPAR ligands were used at the indicated concentrations in 0.5% FBS, and cells were harvested 36 h later for analysis of luciferase activity.
Results
Ro Induces a Small Set of Genes in Peritoneal Macrophages. We initially examined the transcriptional responses of thioglycollate-elicited macrophages to the synthetic PPARγ ligand Ro. To optimize conditions for microarray experiments, the induction of CD36, a known PPARγ target gene, was evaluated under a variety of time course and culture conditions. A maximum induction of CD36 mRNA of 2- to 3-fold was observed in thioglycollate-elicited macrophages after 24 h of Ro treatment, consistent with previous reports (29). Variations in serum content or the use of charcoal-stripped serum did not alter the fold of induction in response to Ro (data not shown). PPARγ activity can be inhibited by MAP kinase-dependent phosphorylation of S112 (30, 31), whereas PKA activity has been observed to increase PPARα-dependent transcription (32). We therefore examined the effects of MAP kinase inhibitors and activators of PKA phosphorylation on Ro-dependent induction of CD36. None of these agents significantly increased the 2- to 3-fold response of CD36 to Ro (data not shown). We also investigated the transcriptional responses of CD36 to different PPARγ agonists, including nonsteroidal antiinf lammatory drugs, etodolac, 15d-PGJ2, and 13-hydroxyoctadecadienoic acid. These agents elicited maximal 2- to 3-fold increases in CD36 expression. Microarray and subtractive hybridization experiments were therefore performed by using 0.5% serum, because these conditions also permitted robust responses of peritoneal macrophages to LPS stimulation.
Initial studies of broad transcriptional responses of thioglycollate-elicited peritoneal macrophages to Ro used Affymetrix Mu11 and U74 microarrays. A representative experiment using U74A microarrays to compare levels of gene expression in Ro-treated (10 μM) and control macrophages is illustrated in Fig. 1 A and C. Ro treatment resulted in the induction of only eight mRNAs by more than a factor of two. A somewhat larger set of induced genes, including CD36, was obtained when the cut-off for induction was lowered to 1.8-fold. Of this expanded set, only CD36, adipose differentiation-related protein (ADRP), carnitine palmitoyl transferase 1a (Cpt1a), enoyl coenzyme A hydratase 1 (Ech1), peroxisomal biogenesis factor 11a (Pex11a), α-mannosidase II, and ATP-biding cassette, subfamily G1 (ABCG1) were confirmed as Ro-induced target genes by Northern blotting experiments (color-coded data points in Fig. 2A). Similar results were obtained in a parallel set of microarray experiments performed by using bone marrow-derived macrophages treated with IL-4 to induce PPARγ expression (data not shown).
Fig. 1.
Ro modestly induces a small set of genes in thioglycollate-elicited macrophages. (A) Scatter plot of mRNA expression levels as assessed by hybridization of cRNA from macrophages treated for 24 h with Ro (10 μM) or control solvent. Genes confirmed to be induced by secondary analysis are indicated by colored data points. (B) RT-PCR analysis of PPARγ mRNA from peritoneal macrophages of PPARγf/f and Mx-Cre+/PPARγf/f mice treated with polyinosinic-polycytidylic acid (pIpC) illustrates quantitative excision of exon 2. (C) Dendogram of genes represented on the Affymetrix U74A microarray found to be reproducibly induced by Ro in wild-type peritoneal macrophages. Red indicates up-regulation and green indicates down-regulation with respect to levels of expression in untreated PPARγ+/+ macrophages. The colors of gene names in C correspond to the colors of data points in A.
Fig. 2.
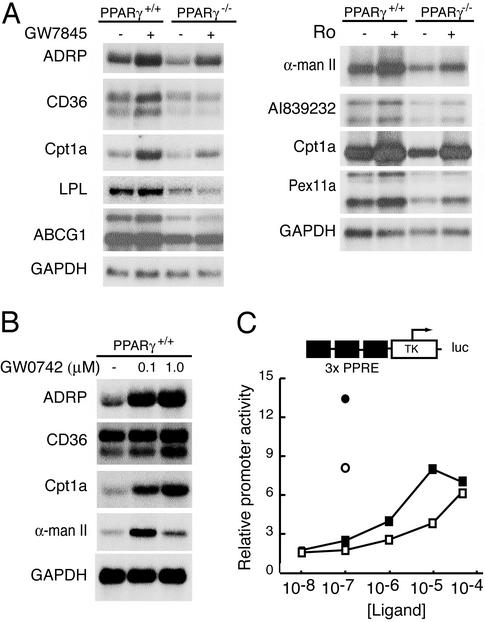
PPARγ and PPARδ positively regulate an overlapping set of genes involved in lipid metabolism. (A) Secondary analysis of positively regulated genes by Northern blotting. PPARγ+/+ or PPARγ–/– macrophages were treated for 24 h with control solvent or 1 μM concentrations of either Ro or the PPARγ-specific agonist GW7845. RNA was harvested and 10 μg was analyzed by Northern blotting with specific probes for the indicated mRNAs. (B) PPARδ activates an overlapping set of genes. PPARγ+/+ macrophages were treated for 24 h with control solvent, or 0.1 μMor1 μM of the PPARδ-specific agonist GW0742. RNA was analyzed by Northern blotting as described above. (C) Ro can induce gene expression through PPARδ. RAW 264.7 cells that lack PPARγ were transfected with a PPAR-responsive reporter gene. Cells were cotransfected with CMV expression vectors and treated with either GW0742 or Ro as follows: •, GW0742 + CMV-PPARδ; ○, GW0742 + CMV vector; ▪, Ro + CMV-PPARδ; □, Ro + CMV vector. Luciferase activity was assayed 36 h after drug treatment.
These experiments raised the question of whether endogenous ligands might prevent the identification of positively regulated PPARγ target genes. Experiments were therefore performed by using PPARγ-deficient macrophages. Mice bearing a floxed allele of PPARγ (PPARγf/f) were mated with mice carrying a Cre transgene under the control of the pIpC-inducible Mx promoter to generate Mx-Cre+/PPARγf/f mice (26). To control for potential effects of pIpC injection, both PPARγf/f and Mx-Cre+/PPARγf/f mice were injected i.p. with pIpC every 2 days for a total of three injections. This treatment causes floxing out of the PPARγ allele and loss of exon 2 in macrophages derived from Mx-Cre+/PPARγf/f mice but not in macrophages derived from PPARγf/f mice lacking the Mx-Cre transgene. Recombination is nearly quantitative, as documented by Southern blotting experiments (26), resulting in a frame shift in the mature transcript and loss of detectable PPARγ protein by Western blotting (26). Efficiency of recombination was confirmed in each macrophage preparation by RT-PCR analysis of PPARγ mRNA, as illustrated in Fig. 1B. We shall refer to macrophages in which recombination has not occurred as PPARγ+/+ and Mx-Cre+ macrophages in which exon 2 has been deleted as PPARγ–/–.
After plating, PPARγ–/– and PPARγ+/+ macrophages were treated with control solvent or Ro at a concentration of 10 μM for 24 h. To investigate the PPARγ dependence of inhibitory effects of Ro on inflammatory responses, additional groups of cells also received LPS alone or LPS and Ro. Hybridization data from the U74A microarray indicated that several of the genes demonstrated to be positively regulated by Ro in PPARγ+/+ macrophages were underexpressed in PPARγ–/– macrophages, consistent with the presence of endogenous ligands for PPARγ or constitutive transcriptional activity. This pattern of expression was confirmed for several of these genes by Northern blotting experiments (Fig. 2 A).
Surprisingly, although Ro-dependent induction of CD36 was almost completely lost in PPARγ–/– macrophages, several Ro target genes retained partial or full induction, including ADRP and Cpt1a (Figs. 1C and 2A). This result is unlikely to be due to incomplete inactivation of the floxed PPARγ alleles, because nonrearranged alleles could not be detected in these cells by Southern blotting or PCR analysis and PPARγ protein could not be detected by Western blotting (ref. 26; Fig. 1B). Two lines of evidence suggest that PPARγ-independent induction of these genes is due to activation of PPARδ. First, microarray experiments comparing macrophages treated with Ro or the PPARδ-specific agonist GW0742 exhibited a largely concordant pattern of induced genes (data not shown). This was confirmed for several genes by Northern blotting experiments (Fig. 2B). Second, 10–50 μM concentrations of Ro activated a PPAR-responsive promoter in RAW264.7 cells, which express PPARδ but not PPARγ (16), in a manner that was modestly enhanced by overexpression of PPARδ (Fig. 2C). In contrast, transfection of a PPARγ expression plasmid in these cells resulted in half maximal induction of the PPAR-responsive promoter at a concentration of 50 nM Ro (ref. 16; data not shown).
Ro Inhibits a Subset of LPS-Inducible Genes by a PPARγ-Dependent Mechanism. To characterize the program of PPARγ-dependent inhibition of inflammatory gene expression, we initially examined the time-dependent effects of Ro treatment before stimulation with LPS. Whereas LPS induction of both the iNOS and COX2 genes was inhibited by coincident treatment with Ro, more significant levels of inhibition were observed by pretreatment for 4–18 h (data not shown). Of the ≈8,000 genes represented on the U74A microarray, treatment of macrophages for 6 h with LPS resulted in reproducible induction of 107 genes by 3-fold or greater (see Table 1, which is published as supporting information on the PNAS web site, www.pnas.org). This profile is similar to that recently reported for the response of human macrophages to LPS (33). By using conditions that resulted in maximum inhibition of iNOS induction, Ro was found to reduce the LPS response of 14 of these genes by >50% (13%). By using a lower stringency of 40% repression, 27 transcripts (25%) were repressed in wild-type cells. The majority of these genes, exhibiting raw expression values >200, are illustrated in Fig. 3A. In contrast, >25% of the LPS-induced genes exhibited <10% reduction in response to Ro, indicating that the effects of Ro are promoter-specific. We confirmed this pattern of regulation for repressed and non-repressed genes by Northern blotting experiments, examples of which are illustrated in Fig. 3B.
Fig. 3.
Inhibition of LPS-dependent gene expression by Ro. (A) Dendogram of genes in which LPS induction was inhibited >40% by Ro. The color scheme for increased or decreased expression is the same as described in Fig. 1. (B) Confirmation of negative regulation of LPS target genes by Northern blotting.
At the concentration of Ro used for microarray experiments (10 μM), inhibitory effects on LPS-induced genes exhibited partial dependence on PPARγ. For example, Ro treatment resulted in a 67% reduction in LPS-dependent expression of iNOS in PPARγ+/+ macrophages and a 48% reduction in PPARγ–/– macrophages (Fig. 3 and Table 1). In addition, the magnitude of the response of several genes to LPS, exemplified by iNOS and Tyki, was significantly greater in PPARγ–/– macrophages than in control macrophages (Figs. 3B and 4A, Table 1). Because of the ability of Ro to activate PPARδ at a concentration of 10 μM, dose–response experiments were performed in PPARγ+/+ and PPARγ–/– macrophages. At a concentration of 1 μM, inhibitory effects of Ro on LPS induction of iNOS and IL-12 p40 were almost entirely PPARγ dependent (Fig. 4A). In contrast, at a concentration of 50 μM, inhibitory effects of Ro were PPARγ-independent, a result consistent with previous reports (25). Inhibitory effects of a 10 μM concentration of Ro exhibited partial PPARγ dependence, as was observed in microarray experiments. To test the possibility that inhibitory effects of high concentrations of Ro were mediated by PPARδ, the ability of the PPARδ-specific ligand GW0742 to inhibit LPS-dependent transcription was evaluated. GW0742 strongly inhibited LPS induction of iNOS over concentration ranges that are consistent with its intrinsic binding affinity for PPARδ (Fig. 4B). Neither Ro nor GW0742 inhibited induction of IL-1β or tumor necrosis factor (TNF) α, indicating similar profiles of promoter-specific inhibitory activity.
Fig. 4.
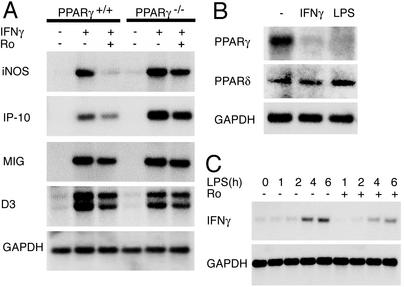
PPARγ and PPARδ mediate inhibitory effects of Ro. (A) Inhibition of LPS-dependent expression of iNOS and IL-12 p40 by Ro is PPARγ dependent at 1 μM and PPARγ independent at 50 μM. PPARγ+/+ and PPARγ–/– macrophages were treated with LPS (100 ng/ml) for 6 h in the presence of the indicated concentrations of Ro. Ten micrograms of total RNA was analyzed for expression of the indicated genes by Northern blotting. (B) PPARδ potently inhibits LPS-dependent induction of iNOS and COX2. PPARγ+/+ macrophages were treated with LPS for6hinthe presence of the indicated concentrations of the PPARδ agonist GW0742. Ten micrograms of total RNA was analyzed for expression of the indicated genes by Northern blotting.
Ro Represses IFN-γ-Dependent Transcription at Multiple Levels. Many of the transcripts susceptible to PPARγ-dependent inhibition have been shown to be inducible by IFN-γ. These include cytokines, chemokines, and cytokine receptors (e.g., Scyb9, Scyb10, and IL-15Rα), members of the IFN inducible with tetratricopeptide repeats family (e.g., Ifit1, Ifit2, and Ifit; IFN-inducible transcripts Ifi203, Ifi204, Ifi204, and Mx), other classes of inflammatory mediators (e.g., iNOS, HB-EFG, and VCAM-1), and the AP-1 transcription factor JunB. These observations raised the question of whether Ro would also inhibit responses of these genes to IFN-γ. Evaluation of a subset of these genes by Northern blotting experiments indicated that Ro could inhibit IFN-γ-induced mRNA of iNOS, IFN-inducible protein of 10 kDa (IP-10), and monokine induced by IFN-γ (MIG) (Fig. 5A). IP-10 and MIG are chemokines that are important for the recruitment of T cells into tissues during the inflammatory response; linking innate and adaptive immunity (34). The observation that Ro inhibited the expression of the p40 subunit of IL-12 also raised the possibility that PPARγ might inhibit the production of IFN-γ itself. Consistent with this, Ro reduced the response of IFN-γ to LPS in wild-type macrophages (Fig. 5C). Intriguingly, treatment of thioglycollate macrophages with LPS or IFN-γ resulted in down-regulation of PPARγ expression but not PPARδ expression (Fig. 5B).
Fig. 5.
Mutual antagonism of IFN-γ and PPARγ signaling pathways. (A) Ro inhibits IFN-γ induction of iNOS in a PPARγ-dependent manner. PPARγ+/+ and PPARγ–/– macrophages were treated with IFN-γ (100 units/ml) for 6 h in the presence of Ro (10 μM). (B) PPARγ expression but not PPARδ expression is inhibited in peritoneal macrophages by IFN-γ and LPS. PPARγ+/+ macrophages were treated for 6 h with either IFN-γ or LPS before analysis of mRNA. (C) Ro inhibits induction of IFN-γ expression in macrophages by LPS. Cells were treated for the indicated times with LPS in the presence or absence of Ro (10 μM). In all experiments depicted here, 10 μg of total RNA was analyzed for expression of the indicated genes by Northern blotting.
Discussion
PPARγ-Dependent and -Independent Actions of PPARγ Agonists. In the present studies, we characterized transcriptional responses to the PPARγ agonist Ro in wild-type and PPARγ–/– macrophages. Surprisingly, very few genes were positively regulated by Ro in these cells, despite relatively high levels of PPARγ protein expression. In contrast to the transcriptional program induced by thiazolidinediones in preadipocytes, in which scores of genes are induced, in some cases, by >50-fold, there were no genes that were reproducibly induced by >3-fold in thioglycollate-elicited macrophages. It is possible that highly induced genes were not represented on the microarrays used in these studies. However, subtractive hybridization cloning identified the majority of the genes that were modestly induced on these arrays but did not identify additional highly induced genes (data not shown).
Genes that were reproducibly induced by Ro in macrophages included CD36, the lipid droplet-associated protein ADRP, the ATP-binding cassette half transporter ABCG1, the peroxisomal enzymes enoyl coenzyme A hydratase 1 (Ech1) and peroxisomal biogenesis factor 11a (Pex11a), α mannosidase II, and carnitine palmitoyl transferase (Cpt1a). The products of these genes play roles in lipid transport and metabolism, and their induction by Ro is consistent with the general functions of the PPAR subfamily as fatty acid-regulated transcription factors. Several of these genes exhibited residual induction by Ro in PPARγ–/– macrophages, even at concentrations as low as 1 μM. This result appears to be due to activation of PPARδ because a very similar profile of gene activation was observed for the highly specific PPARδ agonist GW0742. The extent to which PPARγ and PPARδ regulate overlapping as opposed to distinct sets of target genes will require additional studies in wild-type and receptor-deficient macrophages.
Although PPARγ has been reported to positively regulate a liver X receptor (LXR) α/ABCA1 pathway in human monocyte/macrophage cell lines and murine embryonic stem cell-derived macrophages (35, 36), LXRα or ABCA1 genes were only weakly induced in response to Ro in these studies. The basis for the surprisingly restricted program of transcriptional activation by PPARγ is unclear. Other nuclear receptors that are thought to use similar sets of coactivators, such as LXRs, are capable of robust induction of a broad set of target genes in these same cells (data not shown). It is possible that under different environmental conditions, e.g., foam cell formation, additional factors become expressed or active that enable a broader program of PPARγ-dependent transcriptional activation in macrophages.
In contrast to the very restricted program of transcriptional activation, Ro inhibited induction of a significantly larger set of LPS target genes. The inhibitory effects of Ro were almost completely PPARγ dependent when used at a concentration of 1 μM, but became less PPARγ dependent at higher concentrations. Several lines of evidence suggest that this PPARγ-independent effect is due to activation of PPARδ. PPARδ is expressed in macrophages and a specific PPARδ agonist inhibited LPS induction of iNOS and COX2 at concentrations that are consistent with its intrinsic binding affinity for PPARδ. PPARδ and PPARγ agonists also exhibited a similar profile of promoter specificity for inhibition of LPS target genes.
These findings demonstrate potent transrepressive activity of PPARδ and provide an explanation for why the dose–response curves for inhibition of proinflammatory responses by PPARγ agonists do not agree with their binding affinities for PPARγ. At low concentrations of Ro, repression is primarily mediated by PPARγ. As Ro concentrations are increased, PPARδ becomes activated and further repression is achieved. The basis for promoter-specific inhibition of LPS target genes is not clear, but the availability of large sets of genes that are regulated in a similar manner should facilitate the application of bioinformatics approaches to the search for potential common regulatory mechanisms. Ro does not inhibit the nuclear entry or DNA-binding activity of p65 in these cells (data not shown), a result consistent with the observation that many LPS target genes that require NF-κB for activation are not inhibited by Ro.
Roles of PPARγ in Native and Acquired Immunity. LPS is representative of a diverse group of pathogen-associated molecules that regulate gene expression by binding to pattern recognition receptors, such as TLR4, that play essential roles in native immunity. The subset of LPS-responsive genes that are inhibited by PPARγ includes many genes that promote native responses and the evolution of acquired immunity (e.g., IL-12, IP-10, and monokine induced by IFN-γ). These observations suggest that PPARγ plays a role in modulating the program of macrophage activation after exposure to pathogens. PPARγ is expressed at low levels in resident peritoneal macrophages but is highly expressed in macrophages recovered from peritoneal exudates 3 days after an inflammatory stimulus (16). The mechanisms responsible for this induction are not known, but the timing corresponds to the resolution phase of many acute inflammatory responses. Components of bacterial pathogens may persist after the execution of cytopathic programs (e.g., NO production by iNOS), and PPARγ may be important in inhibiting persistent macrophage activation by these substances.
Many of the LPS-inducible genes inhibited by Ro have previously been documented to also be targets of IFN-γ. Secondary analysis of several of these genes demonstrated that Ro could inhibit their responses to IFN-γ in a PPARγ-dependent manner. In addition, Ro was found to significantly inhibit the LPS response of the p40 subunit of IL-12 (which is an important positive regulator of IFN-γ production by T helper 1 cells), a result consistent with previous findings (37, 38). Consistent with this, Ro inhibited the induction of IFN-γ in response to LPS. Similar effects have been described in T cells (39, 40). Thus, Ro inhibits both the production of IFN-γ and the cellular response to it. In concert with previous observations that PPARγ is strongly induced by the T helper 2 cytokine IL-4 (20), the present studies support a physiologic role of PPARγ in regulating specific activation programs of macrophages.
In concert, these studies support a role of PPARγ as a mediator of antiinflammatory effects of PPARγ agonists. Genetic evidence for antiinflammatory effects of PPARγ in disease models remains limited but includes the recent observation that mice heterozygous for a null PPARγ allele develop much more severe adjuvant-induced arthritis than wild-type mice (41). Negative regulation of gene expression may also be the basis for some of the insulin-sensitizing effects of Ro observed in diabetic patients. Ro treatment has recently been shown to reduce circulating concentrations of markers of low grade inflammation such as C-reactive protein (42). Given the broad expression of PPARδ and potent inhibitory effects of PPARδ-specific ligands on LPS target genes observed in these studies, it will also be of interest to determine the extent to which PPARδ regulates inflammatory and immunity processes in vivo.
Supplementary Material
Acknowledgments
We thank the Salk Institute Microarray Core Facility and the University of California at San Diego BIOGEM Core Facility for assistance with microarray experiments; Jean Lozach for assistance with microarray data analysis; Dr. Timothy Willson and GlaxoSmithKline for GW7845 and GW0742; Audrey Briscoe and Hyon Lee for excellent technical assistance; and A. Zulueta for assistance with preparation of the manuscript. This work was supported by grants from the American Heart Association Western Affiliate (predoctoral fellowship to J.S.W. and beginning grant-in-aid to M.R.), the National Institutes of Health (ES10337 to C.K.G.), and the Stanford Reynolds Center (to C.K.G.).
Abbreviations: COX2, cyclooxygenase 2; iNOS, inducible NO synthase; LPS, lipopolysaccharide; PPAR, peroxisome proliferator-activated receptor; Ro, rosiglitazone.
See commentary on page 6295.
References
- 1.Willson, T. M., Lambert, M. H. & Kliewer, S. A. (2001) Annu. Rev. Biochem. 70, 341–367. [DOI] [PubMed] [Google Scholar]
- 2.Chawla, A., Repa, J., Evans, R. & Mangelsdorf, D. (2001) Science 294, 1866–1870. [DOI] [PubMed] [Google Scholar]
- 3.Spiegelman, B. M. (1998) Diabetes 47, 507–514. [DOI] [PubMed] [Google Scholar]
- 4.Xu, H. E., Lambert, M. H., Montana, V. G., Parks, D. J., Blanchard, S. G., Brown, P. J., Sternbach, D. D., Lehmann, J. M., Wisely, G. B., Willson, T. M., et al. (1999) Mol. Cell 3, 397–403. [DOI] [PubMed] [Google Scholar]
- 5.Forman, B. M., Tontonoz, P., Chen, J., Brun, R. P., Spiegelman, B. M. & Evans, R. M. (1995) Cell 83, 803–812. [DOI] [PubMed] [Google Scholar]
- 6.Kliewer, S. A., Lenhard, J. M., Willson, T. M., Patel, I., Morris, D. C. & Lehmann, J. M. (1995) Cell 83, 813–819. [DOI] [PubMed] [Google Scholar]
- 7.Nagy, L., Tontonoz, P., Alvarez, J. G. A., Chen, H. & Evans, R. M. (1998) Cell 93, 229–240. [DOI] [PubMed] [Google Scholar]
- 8.Claudel, T., Leibowitz, M. D., Fievet, C., Tailleux, A., Wagner, B., Repa, J. J., Torpier, G., Lobaccaro, J. M., Paterniti, J. R., Mangelsdorf, D. J., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, A., Brown, K., Silvestre, M., Willson, T., Palinski, W. & Glass, C. (2000) J. CIin. Invest. 106, 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Z., Ishibashi, S., Perrey, S., Osuga, J., Gotoda, T., Kitamine, T., Tamura, Y., Okazaki, H., Yahagi, N., Iizuka, Y., et al. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 372–377. [DOI] [PubMed] [Google Scholar]
- 11.Desreumaux, P., Dubuquoy, L., Nutten, S., Peuchmaur, M., Englaro, W., Schoonjans, K., Derijard, B., Desvergne, B., Wahli, W., Chambon, P., et al. (2001) J. Exp. Med. 193, 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su, C. G., Wen, X., Bailey, S. T., Jiang, W., Rangwala, S. M., Keilbaugh, S. A., Flanigan, A., Murthy, S., Lazar, M. A. & Wu, G. D. (1999) J. Clin. Invest. 104, 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niino, M., Iwabuchi, K., Kikuchi, S., Ato, M., Morohashi, T., Ogata, A., Tashiro, K. & Onoe, K. (2001) J. Neuroimmunol. 116, 40–48. [DOI] [PubMed] [Google Scholar]
- 14.Diab, A., Deng, C., Smith, J. D., Hussain, R. Z., Phanavanh, B., Lovett-Racke, A. E., Drew, P. D. & Racke, M. K. (2002) J. Immunol. 168, 2508–2515. [DOI] [PubMed] [Google Scholar]
- 15.Feinstein, D. L., Galea, E., Gavrilyuk, V., Brosnan, C. F., Whitacre, C. C., Dumitrescu-Ozimek, L., Landreth, G. E., Pershadsingh, H. A., Weinberg, G. & Heneka, M. T. (2002) Ann. Neurol. 51, 694–702. [DOI] [PubMed] [Google Scholar]
- 16.Ricote, M., Li, A. C., Willson, T. M., Kelly, C. J. & Glass, C. K. (1998) Nature 391, 79–82. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, C., Ting, A. T. & Seed, B. (1998) Nature 391, 82–86. [DOI] [PubMed] [Google Scholar]
- 18.Marx, N., Schönbeck, U., Lazar, M. A., Libby, P. & Plutzky, J. (1998) Circ. Res. 83, 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricote, M., Huang, J., Fajas, L., Li, A., Welch, J., Najib, J., Witztum, J. L., Auwerx, J., Palinski, W. & Glass, C. K. (1998) Proc. Natl. Acad. Sci. USA 95, 7614–7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, J. T., Welch, J. S., Ricote, M., Binder, C. J., Willson, T. M., Kelly, C., Witztum, J. L., Funk, C. D., Conrad, D. & Glass, C. K. (1999) Nature 400, 378–382. [DOI] [PubMed] [Google Scholar]
- 21.Daynes, R. A. & Jones, D. C. (2002) Nat. Rev. Immunol. 2, 748–759. [DOI] [PubMed] [Google Scholar]
- 22.Li, M., Pascual, G. & Glass, C. (2000) Mol. Cell. Biol. 20, 4699–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straus, D. S., Pascual, G., Li, M., Welch, J., Ricote, M., Hsiang, C. H., Sengchanthalansgsy, L. L., Ghosh, G. & Glass, C. K. (2000) Proc. Natl. Acad. Sci. USA 97, 4844–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi, A., Kapahi, P., Natoli, G., Takahashi, T., Chen, Y., Karin, M. & Santoro, M. G. (2000) Nature 403, 103–108. [DOI] [PubMed] [Google Scholar]
- 25.Chawla, A., Barak, Y., Nagy, L., Liao, D., Tontonoz, P. & Evans, R. M. (2001) Nat. Med. 7, 48–52. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama, T. E., Sakai, S., Lambert, G., Nicol, C. J., Matsusue, K., Pimprale, S., Lee, Y. H., Ricote, M., Glass, C. K., Brewer, H. B., Jr., & Gonzalez, F. J. (2002) Mol. Cell. Biol. 22, 2607–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasik, R., Calvo, E. & Corbeil, J. (2002) Bioinformatics 18, 1633–1640. [DOI] [PubMed] [Google Scholar]
- 28.Ausubel, F. M. (2001) Current Protocols in Molecular Biology (Wiley, New York).
- 29.Tontonoz, P., Nagy, L., Alvarez, J. G. A., Thomazy, V. A. & Evans, R. M. (1998) Cell 93, 241–252. [DOI] [PubMed] [Google Scholar]
- 30.Hu, E., Kim, J. B., Sarraf, P. & Spiegelman, B. M. (1996) Science 274, 2100–2103. [DOI] [PubMed] [Google Scholar]
- 31.Adams, M., Reginato, M. J., Shao, D. S., Lazar, M. A. & Chatterjee, V. K. (1997) J. Biol. Chem. 272, 5128–5132. [DOI] [PubMed] [Google Scholar]
- 32.Lazennec, G., Canaple, L., Saugy, D. & Wahli, W. (2000) Mol. Endocrinol. 14, 1962–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nau, G. J., Richmond, J. F., Schlesinger, A., Jennings, E. G., Lander, E. S. & Young, R. A. (2002) Proc. Natl. Acad. Sci. USA 99, 1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luster, A. D. (2002) Curr. Opin. Immunol. 14, 129–135. [DOI] [PubMed] [Google Scholar]
- 35.Chinetti, G., Lestavel, S., Bocher, V., Remaley, A. T., Neve, B., Torra, I. P., Teissier, E., Minnich, A., Jaye, M., Duverger, N., et al. (2001) Nat. Med. 7, 53–58. [DOI] [PubMed] [Google Scholar]
- 36.Chawla, A., Boisvert, W. A., Lee, C. H., Laffitte, B. A., Barak, Y., Joseph, S. B., Liao, D., Nagy, L., Edwards, P. A., Curtiss, L. K., et al. (2001) Mol. Cell 7, 161–171. [DOI] [PubMed] [Google Scholar]
- 37.Gosset, P., Charbonnier, A. S., Delerive, P., Fontaine, J., Staels, B., Pestel, J., Tonnel, A. B. & Trottein, F. (2001) Eur. J. Immunol. 31, 2857–2865. [DOI] [PubMed] [Google Scholar]
- 38.Alleva, D. G., Johnson, E. B., Lio, F. M., Boehme, S. A., Conlon, P. J. & Crowe, P. D. (2002) J. Leukocyte Biol. 71, 677–685. [PubMed] [Google Scholar]
- 39.Cunard, R., Ricote, M., DiCampli, D., Archer, D. C., Kahn, D. A., Glass, C. K. & Kelly, C. J. (2002) J. Immunol. 168, 2795–2802. [DOI] [PubMed] [Google Scholar]
- 40.Marx, N., Kehrle, B., Kohlhammer, K., Grub, M., Koenig, W., Hombach, V., Libby, P. & Plutzky, J. (2002) Circ. Res. 90, 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Setoguchi, K., Misaki, Y., Terauchi, Y., Yamauchi, T., Kawahata, K., Kadowaki, T. & Yamamoto, K. (2001) J. Clin. Invest. 108, 1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haffner, S. M., Greenberg, A. S., Weston, W. M., Chen, H., Williams, K. & Freed, M. I. (2002) Circulation 106, 679–684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.