Abstract
In sepsis, both necrotic and apoptotic cell death can occur. Apoptotic cells induce anergy that could impair the host response, whereas necrotic cells cause immune activation that might result in enhanced antimicrobial defenses. We determined whether adoptive transfer of apoptotic or necrotic cells impacted survival in a clinically relevant sepsis model. We also evaluated the effects of adoptive transfer of apoptotic or necrotic cells on the prototypical TH1 and TH2 cytokines IFN-γ and IL-4, respectively. C57BL6/J mice had adoptive transfer of apoptotic (irradiated) or necrotic (freeze thaw) splenocytes. Controls received saline. Apoptotic cells greatly increased mortality, whereas necrotic splenocytes markedly improved survival, P ≤ 0.05. The contrasting effects that apoptotic or necrotic cells exerted on survival were mirrored by opposite effects on splenocyte IFN-γ production with greatly decreased and increased production, respectively. Importantly, either administration of anti-IFN-γ antibodies or use of IFN-γ knockout mice prevented the survival benefit occurring with necrotic cells. This study demonstrates that the type of cell death impacts survival in a clinically relevant model and identifies a mechanism for the immune suppression that is a hallmark of sepsis. Necrotic cells (and likely apoptotic cells) exert their effects via modulation of IFN-γ
Sepsis is the leading cause of death in many intensive-care units and currently ranks as the 12th most common cause of death in America (1). Septic patients are severely immune suppressed as typified by their loss of delayed type hypersensitivity, inability to eradicate their primary infection, and a predisposition to develop secondary nosocomial infections (2–5, 6). A feature illustrative of the immune suppression in septic patients is their failure to respond to skin testing with antigens derived from microbes to which previous exposure occurred (positive controls) (2, 7). Animal studies indicate that the immune defect in sepsis may be critical to the pathogenesis and resultant mortality (8–10). Evidence to support this contention is also provided by a recent clinical trial using IFN-γ. Administration of this cytokine, which is a potent macrophage activator and an inducer of the TH1 response, improved survival in patients with sepsis (11).
A number of defects in the immune system have been reported in sepsis. These abnormalities include a shift from a proinflammatory TH1 to an antiinflammatory TH2 lymphocyte profile, a loss in cellular MHC II expression, and a profound apoptosis-induced depletion of CD4 T and B cells (5, 11–15). The sepsis-induced apoptosis of lymphocytes may be particularly important not only because of the extensive lymphocyte loss but also because of a potential immunosuppressive effect of apoptotic cells on the immune system. Recent work has demonstrated that uptake of apoptotic cells by phagocytic cells stimulates immune tolerance by the release of antiinflammatory cytokines and suppression of release of proinflammatory cytokines (16–20). In addition, uptake of apoptotic cells by macrophages and dendritic cells does not induce expression of costimulatory molecules (21, 22). Thus, T cells that come in contact with antigen-presenting cells that have ingested apoptotic cells may either become anergic or undergo apoptosis (23). Conversely, uptake of necrotic cells by phagocytic cells causes expression of costimulatory molecules and results in T cell activation and development of immunity (23).
Most of the studies that have examined the immunosuppressive effect of apoptotic cells have been conducted in isolated cells, and results from such studies may not reflect the in vivo condition. Furthermore, to our knowledge, no studies have examined the impact of apoptotic cells on sepsis, a disorder in which there is extensive lymphocyte and gastrointestinal epithelial cell apoptosis (10, 14, 24, 25). We hypothesized that the extensive degree of lymphocyte apoptosis that occurs in sepsis might be a major cause of immune suppression in the disorder. Thus, the purpose of this study was to determine whether adoptive transfer of apoptotic or necrotic cells would impact sepsis survival in a clinically relevant model. In addition, effects of necrotic or apoptotic cells on proinflammatory and antiinflammatory cytokine production, sepsis-induced lymphocyte apoptosis, and quantitative blood bacterial counts were conducted.
Materials and Methods
Sepsis Model: Cecal Ligation and Puncture (CLP). All mice used in the study were purchased from The Jackson Laboratory and were on a C57BL6/J background. In addition to immune-competent mice, IFN-γ knockout mice (IFN-γ-/-) (catalog no. 002287) and mice deficient in mature T and B cells, i.e., Rag 1-/- mice (catalog no. 002096), were studied. Male mice weighing 18–26 g were housed for at least 7 days before manipulations. The CLP murine model that reproduces many of the clinical features of sepsis in patients was used to induce intraabdominal peritonitis (26). Sham-operated mice were handled in the same manner, except that the cecum was not ligated or punctured. Animal studies were approved by the Animal Studies Committee at the Washington University School of Medicine.
Induction of Apoptosis and Necrosis. Mouse splenocytes were obtained by gently grinding moistened spleen sections between the ends of frosted microscope slides as described (9, 25). The resultant cell suspensions were filtered with a 70-μm filter and washed. Red blood cells were lysed by brief incubation with 0.14 M ammonium chloride solution, and cell debris was removed by Histopaque (Sigma, catalog no. 1083-1) centrifugation. Apoptosis was induced in isolated splenocytes by γ irradiation (10,000 rad). Splenocytes were confirmed to be apoptotic by flow cytometry and labeling with annexin V and 7-amino-actinomycin D. At 5 and 20 h after irradiation, ≈40% and 100% of splenocytes were apoptotic, respectively (data not shown). Necrosis was induced in splenocytes by two to three freeze thaw cycles. The splenocytes were contained in a 500-μl volume of PBS that after freezing and thawing was injected in toto retroorbitally into anesthetized mice. Therefore, the injected necrotic material consisted of the entire cell components. Microscopic evaluation of splenocytes exposed to freeze thaw cycles demonstrated cell fragments but no intact cells (data not shown).
Mice that received the apoptotic or necrotic splenocytes (≈5 × 107) were compared with a third group of mice that received normal saline solution but no cells. All cell suspensions were injected retroorbitally.
Anti-IFN-γ Antibody. An anti-IFN-γ antibody (clone H22) (see ref. 27) was generously provided by Robert Schreiber, Washington University School of Medicine. (Note that this antibody is also available from PharMingen, catalog no. 557530.) The anti-IFN-γ antibody was administered via i.p. injection 24 h before adoptive transfer of cells and also immediately after CLP at a dose of 250 μg per mouse (27). The isotype control antibody was purchased from PharMingen (catalog no. 553969, clone no. A19-3).
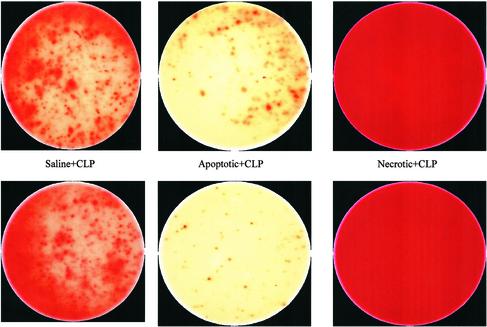
Cytokine Analysis. Enzyme-linked immunospot assay (ELISPOT) (Cellular Technology, Cleveland) was performed per the manufacturer's instructions to determine the effects of apoptotic, necrotic, and normal cells on stimulated T cell TH1 and TH2 cytokine production in splenocytes. Approximately 24 h after CLP or sham surgery, splenocytes were isolated by gently grinding the moistened organ between the ends of two frosted microscope slides followed by filtering through sterile mesh wire as described (9). Cells (1 × 106) were loaded on plates precoated with capture antibody (PharMingen) for IFN-γ or IL-4 and stimulated with anti-CD3 and anti-CD28 as described (25). Cell culture media was RPMI medium 1640 with 50 μM 2-mercaptoethanol and 2 mM glutamine, supplemented with 10% FCS. Cell incubation was maintained for 24 or 48 h for IFN-γ and IL-4, respectively. A secondary detecting antibody was added before avidin-horseradish peroxidase was applied. 3-Amino-9-ethylcarbazole was used as a substrate solution, and the reaction was stopped per the recommended protocol. Images of the individual wells were obtained, and the percent area of the well that was positively stained, indicated by red (see Fig. 2), was calculated by using an image analysis program (METAMORPH, Universal Imaging, West Chester, PA).
Fig. 2.
Color photomicrographs of ELISPOT for IFN-γ. Isolated splenocytes from mice that had adoptive transfer of apoptotic or necrotic cells followed by CLP were plated on individual wells for 24 h. Production of IFN-γ was determined by a colorimetric reaction involving avidin-horseradish peroxidase-mediated oxidation of a substrate to produce the red color. Increasing red color is indicative of increased production of IFN-γ. The six photomicrographs are from six mice in the same experiment. Note the dramatic increase in the production of IFN-γ in splenocytes from mice that had adoptive transfer of necrotic cells compared with the splenocytes from mice that had CLP but no adoptive transfer. Splenocytes from mice that had adoptive transfer of apoptotic cells had decreased production of IFN-γ compared with the mice that had CLP alone. See Table 1 for quantitative results. (Magnification: ×20.)
Detection of Apoptosis via Flow Cytometry. Briefly, blood was obtained from the different treatment groups ≈24 h after sham or CLP surgery and apoptosis was quantified by using a commercially available fluorescein-labeled annexin V/7-amino-actinomycin D kit (Apoptosis Detection Kit, R & D Systems) as described (9, 25). The lymphocyte phenotypes were identified by using fluorescently labeled mAbs directed against lymphocytes surface markers (PharMingen): B cells, CD4 T cells, CD8 T cells, and CD3 T cells. Flow cytometric analysis (10,000–50,000 events per sample) was performed on FACSCalibur (Becton Dickinson).
Microbiologic Analysis. Blood was obtained via aseptic conditions for quantitative bacteriologic counts as described (25). Blood (0.1 cc) was collected and diluted with sterile saline. This solution was plated in serial dilutions onto blood agar and MaConkey media and incubated at 37°C. Colonies were counted at 24 h.
Flow Cytometry to Detect Macrophage MHC II Expression. Flow cytometry was performed to examine the effect of adoptive cell transfer of apoptotic or necrotic cells on macrophage MHC II expression. Dissociated splenocytes were labeled with the macrophage marker anti-CD14 (PharMingen, catalog no. 553739) and a MHC II marker (PharMingen, catalog no. 557000). The labeled cells were examined with flow cytometry as described.
Statistical Analysis. Data reported are mean ± SEM. Data were analyzed by using one-way ANOVA with Tukey's multiple comparison test, using the statistical program PRISM (Graph-Pad). Survival studies were analyzed by Fisher's exact P. Significance was accepted at P ≤ 0.05.
Results
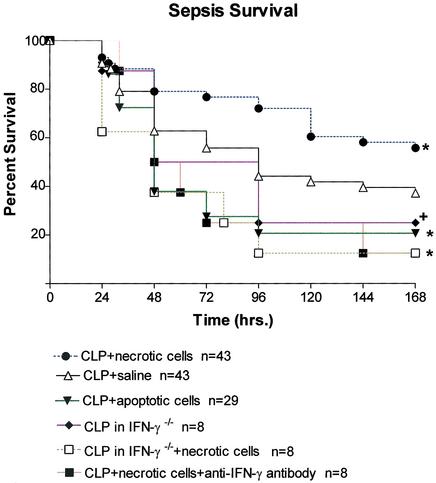
Adoptive Transfer of Necrotic Cells Improves Survival But Transfer of Apoptotic Cells Worsens Survival in Sepsis. Adoptive transfer of apoptotic or necrotic splenocytes immediately after CLP did not affect sepsis survival compared with CLP mice in the control group (data not shown). Adoptive transfer of splenocytes 5 days before CLP had major effects on sepsis mortality (Fig. 1). All data presented are from studies in which adoptive transfer was performed before sham or CLP surgery. Mice that had adoptive transfer of apoptotic splenocytes had the lowest survival (20.7%), which was statistically different from the 37.2% survival in the control group of mice that had CLP plus saline, P ≤ 0.05. Mice that had adoptive transfer of necrotic splenocytes had the best survival (55.8%), and this survival was also statistically significant when compared with mice that received saline (37.2%), P < 0.05.
Fig. 1.
Effect of apoptosis or necrosis on sepsis survival. Five days before CLP, mice had adoptive transfer of 5 × 107 splenocytes that had been irradiated (apoptotic) or subjected to freeze thawing (necrotic). Another group of mice were injected with saline. Survival was recorded for 7 days after CLP. Mice that had adoptive transfer of apoptotic cells had only a 20.7% survival rate, which was statistically different from survival in saline control mice (30.2%); *, P < 0.05. Mice that had adoptive transfer of necrotic splenocytes had the best survival rate (55.8%), which was different from that of saline control mice; +, P < 0.05. Mice that had adoptive transfer of necrotic cells but who were treated with anti-IFN-γ antibody had no protection, and their survival was only 12.5%. Survival in IFN-γ-/- that received necrotic cells was 12.5% as well.
IFN-γ Mediates the Protective Effect of Adoptive Transfer of Necrotic Cells. To determine whether the increase in IFN-γ was contributing to the improvement in survival in mice that had adoptive transfer of necrotic cells, two strategies were used: administration of an anti-IFN-γ antibody or IFN-γ knockout mice (IFN-γ-/-). Anti-IFN-γ antibody (250 μg per mouse) was administered 24 h before adoptive transfer of necrotic cells via i.p. injection. A second injection of the antibody was given immediately after CLP. Adoptive transfer of necrotic cells provided no protection in mice that were treated with anti-IFN-γ antibody; their survival was 12.5% at 7 days (Fig. 1). To determine whether the anti-IFN-γ antibody had any detrimental effects that were unrelated to its action to block IFN-γ, four mice had adoptive transfer of necrotic cells and treatment with the inactive isotype control for the anti-IFN-γ antibody by using the identical protocol. Five days later, mice had CLP. All four mice survived sepsis and were healthy when killed at 7 days.
The results of studies in IFN-γ-/- mice confirmed the findings with the anti-IFN-γ antibody (Fig. 1). Adoptive transfer of necrotic cells into IFN-γ-/- mice was without benefit and their survival was only 12.5% (Fig. 1).
Adoptive Transfer of Necrotic But Not Apoptotic Cells Increases Splenocyte IFN-γ There were no statistical differences in the percentage area of the ELISPOT plates positive for IFN-γ production for sham, sham plus necrotic cells, or sham plus apoptotic cells, i.e., 75.5 ± 3.9%, 74.9 ± 3.4%, and 69.0 ± 4.6% respectively (n = 15–17 mice per group) (Table 1). Sepsis, i.e., CLP alone, caused a decrease in the percent area of the plates positive for IFN-γ immunostaining to 40.4 ± 3.6% (Table 1). If apoptotic cells had been administered before CLP, the percent area of the plates positive for IFN-γ immunostaining was further reduced to 28.7 ± 3.9%, and this finding was statistically different compared with mice that had CLP alone (P < 0.05). In contrast, the percent area of the plates positive for IFN-γ immunostaining in mice that had adoptive transfer of necrotic cells before CLP was 63.8 ± 3.2%, and this finding was greater than the percent area for mice that had CLP alone (P < 0.05). The differences in the degree of colorimetric staining for the CLP, CLP plus apoptotic cells, and CLP plus necrotic cells were often apparent by gross visual inspection of the microphotographs of the various wells (Fig. 2).
Table 1. Effect of apoptotic or necrotic cells on cytokine production.
| Sham | Sham + necrotic cells | Sham + apoptotic cells | CLP | CLP + necrotic cells | CLP + apoptotic cells |
|---|---|---|---|---|---|
| IFN-γ | |||||
| 75.5 ± 3.9 (15) | 74.9 ± 3.4 (15) | 69.0 ± 4.6 (17) | 40.4 ± 3.6 (24) | 63.8 ± 3.2* (20) | 28.7 ± 3.9† (24) |
| IL-4 | |||||
| 32.5 ± 6.1 (8) | 32.8 ± 5.6 (7) | 35.5 ± 10.2 (6) | 40.7 ± 3.5 (12) | 40.5 ± 5.4 (13) | 45.9 ± 4.1 (13) |
Values are expressed as mean ± SEM. The concentrations of IFN-γ and IL-4 were determined via ELISPOT as discussed in Materials and Methods. The number of mice in each group is included in parentheses.
P < 0.01 CLP + necrotic greater than CLP.
P < 0.05 CLP + apoptotic less than CLP.
In contrast to the decrease in IFN-γ production that occurred with CLP, IL-4 production was not different in sham versus CLP (Table 1). Furthermore, no differences in IL-4 production occurred in the three groups of mice that had CLP, i.e., CLP alone, CLP plus apoptotic cells, or CLP plus necrotic cells.
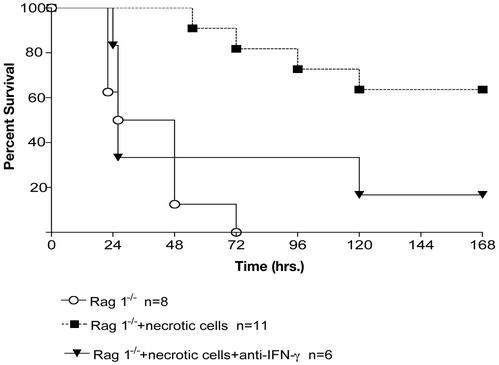
Adoptive Transfer of Necrotic Cells Improves Survival in Rag 1-/- Mice, and This Effect Is Blocked via Treatment with Anti-IFN-γ Antibody. To determine whether adoptive transfer of necrotic cells afforded protection in mice without an adaptive immune system, adoptive transfer was performed in Rag 1-/- mice that lack mature T and B cells. As reported (9), Rag 1-/- have an increased mortality in sepsis caused by CLP and all mice had died by 72 h (Fig. 3). In contrast, Rag 1-/- mice that had adoptive transfer of necrotic cells had a >60% survival at 7 days (Fig. 3). To determine whether INF-γ was contributing to the protective effect of necrotic cells in Rag 1-/- mice, anti-IFN-γ antibody was administered by using the protocol described. Similar to the findings in immune competent mice, anti-IFN-γ antibody administration in Rag 1-/- mice abrogated the protective effect of adoptive transfer of necrotic cells (Fig. 3).
Fig. 3.
Survival in Rag 1-/- mice. Five days before CLP, mice had adoptive transfer of 5 × 107 splenocytes that had undergone freeze-thaw cycles to induce necrosis or saline injection as a control. Another group of mice received anti-IFN-γ antibodies (see Materials and Methods) before adoptive transfer of necrotic cells. Mice that received necrotic cells had a statistically significant improvement in survival compared with mice receiving saline, P < 0.05. There was no improvement in survival in Rag 1-/- mice that were treated with anti-IFN-γ antibodies before receiving necrotic cells.
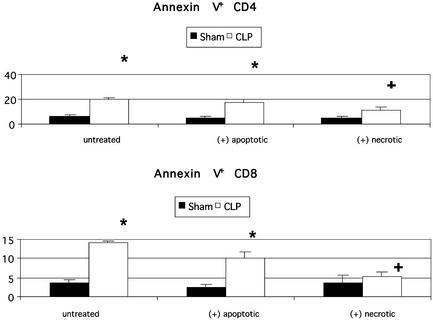
Effect of Adoptive Transfer of Cells on Sepsis-Induced Apoptosis. Using flow cytometry, cellular forward and side-scatter properties were used to identify the characteristic location of lymphocytes and gating was performed on this region. Backgating after lymphocyte labeling with anti-CD3 and anti-CD19 was used to confirm that this region was specific for lymphocytes. In shamoperated mice, the percentage of blood lymphocytes that were apoptotic (annexin V+/7-amino-actinomycin D-) varied from 4% to 5.5% and was not different in the three groups, i.e., sham alone, sham plus apoptotic cells, and sham plus necrotic cells, (n = 7–11 mice per group). Sepsis caused a marked increase in the percentage of circulating lymphocytes undergoing apoptosis (annexin V+/7-amino-actinomycin D-). The percentages of CD3-positive lymphocytes that were apoptotic were 15.7 ± 1.6 (n = 11 mice), 12.6 ± 0.4 (n = 7 mice), and 9.0 ± 2.1 (n = 7 mice) for CLP alone, CLP plus apoptotic cells, and CLP plus necrotic cells, respectively; these values for the three groups were not statistically significant. Analysis of apoptosis in CD4 and CD8 T lymphocytes did demonstrate an effect of adoptive transfer of necrotic but not apoptotic cells to decrease sepsis-induced lymphocyte apoptosis (Fig. 4).
Fig. 4.
Flow cytometric analysis of lymphocyte subsets and lymphocyte apoptosis. Mice had sham surgery or adoptive transfer of apoptotic or necrotic cells followed by CLP. Peripheral blood was obtained 24 h after sham or CLP surgery, and the percentage of lymphocytes composing CD4 T and CD8 T was determined. In addition, the percentage of cells undergoing apoptosis, i.e., annexin V positive, in the two lymphocyte subsets was determined. Sepsis caused a marked increase in lymphocyte apoptosis in CD4 and CD8 T cells in mice with CLP alone and CLP plus apoptotic cells; *, P < 0.01. The percent apoptosis in CD4 and CD8 T cells in mice that had adoptive transfer of necrotic cells followed by CLP was not statistically different from sham-operated mice but was different from apoptosis in mice that had CLP alone; +, P < 0.05.
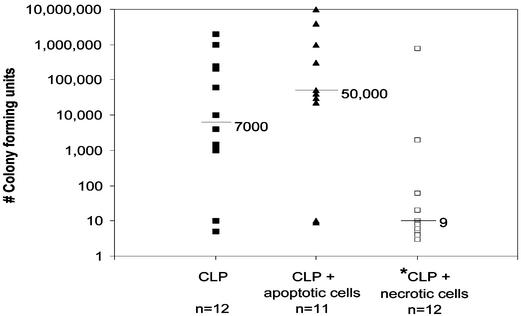
Adoptive Transfer of Necrotic Cells Decreased Blood Bacterial Counts. Quantitative blood bacterial counts were negative in blood from sham-operated mice. In septic mice, there was a wide variation in blood bacterial counts in the three groups, i.e., CLP alone, CLP plus apoptotic cells, and CLP plus necrotic cells (Fig. 5). The median values for the number of colony-forming units (CFUs) for CLP, CLP plus apoptotic, and CLP plus necrotic were 7,000, 50,000, and 9, respectively (Fig. 5). The median value for CLP plus necrotic cells was statistically lower than the two other groups (P < 0.05). Although the number of CFUs was greater in the CLP plus apoptotic group versus CLP alone, this value did not quite achieve statistical significance.
Fig. 5.
Microbiologic analysis of blood cultures. Mice had adoptive transfer of apoptotic or necrotic cells followed by CLP. Twenty four hours after CLP, blood cultures were obtained. The number of colony-forming units was determined and the data for the individual mice were plotted (each point represents one mouse). The median values for the number of colony-forming units were 7,000, 50,000, and 9 for CLP, CLP plus apoptotic cells, and CLP plus necrotic cells, respectively. The value for CLP plus necrotic cells was statistically different from the other groups, P < 0.05.
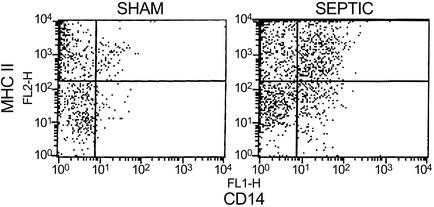
Flow Cytometric Analysis for Macrophage MHC II Expression. Flow cytometry showed an increase in the number of splenic macrophages in septic versus sham-operated mice (Fig. 6). The percentage of macrophages that were positive for MHC II expression via flow cytometry were not different in the three groups of septic mice, i.e., 76.1 ± 3.6% (n = 5), 77.3 ± 1.4% (n = 4), and 77.0 ± 1.9% (n = 6) for CLP, CLP plus apoptotic cells, and CLP plus necrotic cells, respectively.
Fig. 6.
Flow cytometry analysis of macrophages and MHC II expression. Dissociated splenocytes from a sham or CLP-operated mouse were labeled with antibodies to macrophages (anti-CD14) and MHC II. Cells underwent flow cytometry as described. Note the increase in both the number of macrophages in the septic versus sham-operated mouse and the increase in MHC II expression in the macrophages.
Discussion
A major finding in the current study is the dramatic effect of apoptotic versus necrotic cells on immune function and survival in sepsis. Uptake of apoptotic cells exacerbated the sepsis-induced decrease in IFN-γ (Table 1) and was associated with a worse survival outcome (Fig. 1). On the other hand, uptake of necrotic cells prevented the sepsis-induced decrease in IFN-γ (Table 1) and was associated with improved survival (Fig. 1). The immune mechanism responsible for the beneficial effect of adoptive transfer of necrotic cells on sepsis survival appears to be caused at least in part by increased production of IFN-γ. Several observations support this conclusion. First, administration of an IFN-γ-blocking antibody prevented the increase in sepsis survival that occurred with adoptive transfer of necrotic cells in both immune-competent mice (Fig. 1) and Rag 1-/- mice (Fig. 3). Second, adoptive transfer of necrotic cells into mice that were unable to produce IFN-γ, i.e., IFN-γ-/-, conferred no survival benefit (Fig. 1). Finally, mice that had adoptive transfer of apoptotic cells had the lowest splenocyte production of IFN-γ (Fig. 2) and also had the worse survival.
IFN-γ is produced primarily by T cells and natural killer cells (28). IFN-γ-/- mice have decreased resistance to bacterial, mycobacterial, and viral infections (28). These mice also have impaired TH1 immune response. It is interesting that the protective effect of adoptive transfer of necrotic cells also occurred in Rag 1-/- mice (Fig. 3) because these mice are lacking in mature T cells, cells that are major producers of IFN-γ. The fact that treatment with anti-IFN-γ antibody abrogated the protective effect of necrotic cells in Rag 1-/- mice suggests that other cells, most likely natural killer cells, were producing amounts of IFN-γ that were sufficient to provide protection in sepsis.
Animal studies that have examined the role of IFN-γ in sepsis have not provided a uniform picture of its beneficial or adverse effects (28–31). Miles et al. (29) administered IFN-γ to mice undergoing CLP and reported increased mortality. Alternatively, Zantl et al. (30) documented an essential role for IFN-γ in a mouse peritonitis model. Those investigators used a peritonitis model in IFN-γ receptor-deficient mice and reported that 7 of 11 IFN-γ receptor-positive mice survived, whereas all 15 mice with an inactivated receptor for IFN-γ died. One potential explanation for the differences in the two studies could be the fact that, in the former study, exogenous IFN-γ was administered and in the latter study, endogenous IFN-γ was inhibited. It is also possible that IFN-γ may have beneficial or adverse effects depending on its concentration and whether the stage of sepsis is characterized by a hyperinflammatory or hypoinflammatory state. A recent investigation in a small group of septic patients showed that administration of IFN-γ restored monocyte tumor necrosis factor α production and improved survival (11).
A second significant finding in the study is the impact of apoptotic cells to worsen survival in sepsis. Survival in mice receiving apoptotic cells was approximately half that of mice that received no cells (Fig. 1). This effect of adoptive transfer of apoptotic cells to decrease sepsis survival is particularly striking considering that mice that had adoptive transfer of necrotic cells had a significant improvement in survival compared with saline-treated mice. Apoptosis is a major cause of death in lymphocytes and gastrointestinal epithelial cells in patients with sepsis and trauma (14, 32–34). Immunohistochemical studies of spleens from patients dying of sepsis demonstrated focal regions in which 25–50% of cells were positive for markers of apoptosis (14, 34). A recent study of circulating white blood cells from patients with sepsis showed that 15–20% of circulating T and B cells were undergoing apoptosis (32). This finding contrasted with an ≈3–4% degree of apoptosis in lymphocytes from control patients. Considered together, the present findings suggest that the extensive apoptosis that occurs in sepsis may be responsible for or contribute to the profound immune suppression that characterizes the disorder (5, 6, 33, 34).
Although the present investigation demonstrated that adoptive transfer of cells 5 days before CLP but not immediately after CLP impacted survival, it is important to note several points. First, patients with sepsis frequently have a prolonged septic phase that often lasts days to weeks (14). Second, increased lymphocyte apoptosis occurs throughout the duration of the septic course and is greatest during septic shock (14, 32). Thus, increased apoptosis occurring in the early phases of sepsis may lead to more profound immune suppression as the disease progresses. Also, the experimental design in the present study did not include examination of effects at earlier time points, i.e., adoptive transfer of cells 1–2 days before sepsis, and it is possible that adoptive transfer at earlier time points would have had effects similar to the current study. The fact that adoptive transfer of cells had major effects in Rag 1-/- mice, mice that have no adaptive immune system, suggests that the more rapidly acting innate immune system is also involved.
Another important finding in the present study relates to the effect of sepsis and apoptotic or necrotic cells on the TH1 versus TH2 cytokine profile. Activated CD4 T cells can be directed to secrete either of two distinct and antagonistic sets of cytokines (35). In one case, CD4 T cells will secrete cytokines with proinflammatory properties including tumor necrosis factor α, IFN-γ, and IL-2. Alternatively, CD4 T cells will secrete cytokines with antiinflammatory (TH2) properties, e.g., IL-4, IL-10, and transforming growth factor β. The factors that determine whether CD4 T cells have a TH1 or TH2 response are not entirely known but are thought to be influenced by the type of pathogen, amount of bacterial innoculum, and site of infection (35). Sepsis caused an impaired TH1 response as evidenced by the decrease in IFN-γ in CLP versus sham-operated mice (Table 1). In contrast, sepsis did not impair the TH2 response as evidenced by the nonstatistically significant trend toward an increase in IL-4 production (Table 1). The effect of sepsis to cause a blunted TH1 but increased TH2 response has been reported by numerous groups (5, 12, 33) and this change in immune function has been postulated to contribute to the increased mortality in the disorder. Adoptive transfer of apoptotic or necrotic cells appears to modulate this TH1 versus TH2 response.
A great deal of research has focused on the impact of apoptotic cells on the inflammatory/immune response. Recent studies have shown that apoptotic cells not only fail to induce inflammation but actively suppress an inflammatory/immune response (16–22, 36). In a series of elegant studies, Fadok, Henson, and associates (17–19, 37, 38) have shown that the type of cell death is instrumental in regulating the balance between immune activation versus immune tolerance in antigen-presenting cells. They documented that phagocytosis of apoptotic neutrophils inhibited the production of proinflammatory TH1 cytokines and increased production of antiinflammatory TH2 cytokines transforming growth factor β1 (TGF-β1) and prostaglandin E2 (17). Their studies indicate that the phosphatidylserine receptor could be the molecular switch that determines whether inflammation or immunity occurs (38). Barker et al. (21) compared the differential effect of necrotic versus apoptotic cell uptake on antigen presentation by macrophages. Macrophages that had ingested necrotic neutrophils had markedly increased expression of CD40 (a T cell costimulatory molecule) compared with macrophages that had ingested apoptotic neutrophils. Conversely, macrophages that had engulfed apoptotic neutrophils had increased production of TGF-β1. In the present study, stimulation of splenocyte suspensions with endotoxin did not reveal any difference in the degree of TGF-β1 production in the various groups of mice (unpublished observations).
Other differences in the three groups of CLP mice included differences in the degree of circulating lymphocyte apoptosis (Fig. 4). Adoptive transfer of necrotic cells before CLP caused a larger decrease in the degree of sepsis induced T cell apoptosis than did apoptotic cells (Fig. 4). The potential mechanisms for these observed effects are unknown. Given the rapid clearance of apoptotic cells (39), the relatively small difference in the degree of circulating T cell apoptosis in CLP mice receiving apoptotic or necrotic cells may represent a large effect.
INF-γ is a potent activator of macrophages and increases macrophage MHC II expression. Flow cytometry demonstrated an increase in MHC II expression in macrophages from septic versus sham-operated mice (Fig. 6) but no differences in macrophage MHC II expression in spleens from the CLP, CLP plus apoptotic cells, or CLP plus necrotic cells. It is possible that differences in macrophage MHC II expression may exist at other time points, or differences may have been present in circulating monocytes but not in tissue macrophages. Nevertheless, the present study did not demonstrate a significant effect of adoptive transfer of apoptotic or necrotic cells on MHC II expression in macrophages.
If the present findings demonstrating a marked immune suppressive effect by apoptotic cells are substantiated by other in vivo studies of sepsis, it may have a major impact on several areas of therapy of critical illness. For example, transfusion of non-leukoreduced blood (which has large numbers of apoptotic leukocytes) has been shown to be associated with increased infectious complications (40). A recent large Canadian study showed that critically ill patients who were managed with a lower threshold for blood transfusion (and thereby received more blood transfusions) had a worse survival compared with critically ill patients who were managed with a higher threshold for blood transfusion (41). Many of these critically ill patients were septic. An advisory committee to the Food and Drug Administration has recently recommended universal leukoreduction of all red blood cell products. Findings from the current study showing that administration of apoptotic cells (the overwhelming number of which were leukocytes) worsened sepsis survival provide some theoretical support for the use of leukoreduction of red blood cell products to avoid immune suppression. Work by Freire-de-Lima et al. (42) documenting that a single injection of apoptotic cells increased parasitaemia in mice infected with Trypanosoma cruzi highlights the profound impact of apoptotic cell on the ability of the immune system to control infection.
In conclusion, the present findings showing that adoptive transfer of apoptotic cells worsens survival, whereas adoptive transfer of necrotic cells improves survival in a clinically relevant model of sepsis, emphasize the potential significance of the type of cell death on the host's immune defenses. Apoptosis that occurs in patients with sepsis and patients with injuries that predispose to sepsis, e.g., trauma and burn injury, may be an important mechanism of immune suppression and mortality.
Acknowledgments
This work was supported by National Institutes of Health Grants GM44118 and GM55194 and the Alan A. and Edith L. Wolff Foundation.
Abbreviations: CLP, cecal ligation and puncture; ELISPOT, enzyme-linked immunospot assay.
References
- 1.Angus, D. C., Linde-Zwirble, W. T., Lidicker, J., Clermont, G., Carcillo, J. & Pinsky, M. R. (2001) Crit. Care Med. 29, 1303-1310. [DOI] [PubMed] [Google Scholar]
- 2.Meakins, J. L., Pietsch, J. B., Bubenick, O., Kelly, R., Rode, H., Gordon, J. & MacLean, L. D. (1977) Ann. Surg. 186, 241-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyons, A., Kelly, J. L., Rodrick, M. L., Mannick, J. A. & Lederer, J. A. (1997) Ann. Surg. 226, 450-458; discussion 458-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegrini, J. D., De, A. K., Kodys, K., Puyana, J. C., Furse, R. K. & Miller-Graziano, C. (2000) J. Surg. Res. 88, 200-206. [DOI] [PubMed] [Google Scholar]
- 5.Lederer, J. A., Rodrick, M. L. & Mannick, J. A. (1999) Shock 11, 153-159. [DOI] [PubMed] [Google Scholar]
- 6.Puyana, J. C., Pellegrini, J. D., De, A. K., Kodys, K., Silva, W. E. & Miller, C. L. (1998) J. Trauma 44, 1037-1045; discussion 1045-1046. [DOI] [PubMed] [Google Scholar]
- 7.Pietsch, J. B., Meakins, J. L. & MacLean, L. D. (1977) Surgery 82, 349-355. [PubMed] [Google Scholar]
- 8.Ertel, W., Meldrum, D. R., Morrison, M. H., Ayala, A. & Chaudry, I. H. (1990) Surgery 108, 154-160. [PubMed] [Google Scholar]
- 9.Hotchkiss, R. S., Swanson, P. E., Knudson, C. M., Chang, K. C., Cobb, J. P., Osborne, D. F., Zollner, K. M., Buchman, T. G., Korsmeyer, S. J. & Karl, I. E. (1999) J. Immunol. 162, 4148-4156. [PubMed] [Google Scholar]
- 10.Chung, C. S., Xu, Y. X., Wang, W., Chaudry, I. H. & Ayala, A. (1998) Arch. Surg. 133, 1213-1220. [DOI] [PubMed] [Google Scholar]
- 11.Docke, W. D., Randow, F., Syrbe, U., Krausch, D., Asadullah, K., Reinke, P., Volk, H. D. & Kox, W. (1997) Nat. Med. 3, 678-681. [DOI] [PubMed] [Google Scholar]
- 12.Wichmann, M. W., Ayala, A. & Chaudry, I. H. (1998) Crit. Care Med. 26, 1372-1378. [DOI] [PubMed] [Google Scholar]
- 13.Williams, M. A., Withington, S., Newland, A. C. & Kelsey, S. M. (1998) J. Infect. Dis. 178, 1421-1433. [DOI] [PubMed] [Google Scholar]
- 14.Hotchkiss, R. S., Tinsley, K. W., Swanson, P. E., Schmieg, R. E., Jr., Hui, J. J., Chang, K. C., Osborne, D. F., Freeman, B. D., Cobb, J. P., Buchman, T. G. & Karl, I. E. (2001) J. Immunol. 166, 6952-6963. [DOI] [PubMed] [Google Scholar]
- 15.Chung, C. S., Xu, Y. X., Chaudry, I. H. & Ayala, A. (1998) J. Surg. Res. 77, 63-70. [DOI] [PubMed] [Google Scholar]
- 16.Voll, R. E., Herrmann, M., Roth, E. A., Stach, C., Kalden, J. R. & Girkontaite, I. (1997) Nature 390, 350-351. [DOI] [PubMed] [Google Scholar]
- 17.Fadok, V. A., Bratton, D. L., Konowal, A., Freed, P. W., Westcott, J. Y. & Henson, P. M. (1998) J. Clin. Invest. 101, 890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fadok, V. A., Bratton, D. L., Rose, D. M., Pearson, A., Ezekewitz, R. A. & Henson, P. M. (2000) Nature 405, 85-90. [DOI] [PubMed] [Google Scholar]
- 19.McDonald, P. P., Fadok, V. A., Bratton, D. & Henson, P. M. (1999) J. Immunol. 163, 6164-6172. [PubMed] [Google Scholar]
- 20.Chen, W., Frank, M. E., Jin, W. & Wahl, S. M. (2001) Immunity 14, 715-725. [DOI] [PubMed] [Google Scholar]
- 21.Barker, R. N., Erwig, L., Pearce, W. P., Devine, A. & Rees, A. J. (1999) Pathobiology 67, 302-305. [DOI] [PubMed] [Google Scholar]
- 22.Sauter, B., Albert, M. L., Francisco, L., Larsson, M., Somersan, S. & Bhardwaj, N. (2000) J. Exp. Med. 191, 423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green, D. R. & Beere, H. M. (2000) Nature 405, 28-29. [DOI] [PubMed] [Google Scholar]
- 24.Coopersmith, C., Stromberg, P. E., Dunne, W. M., Davis, C. G., Amiot, D. M., II, Buchman, T. G., Karl, I. E. & Hotchkiss, R. S. (2002) J. Am. Med. Assoc. 287, 1716-1721. [DOI] [PubMed] [Google Scholar]
- 25.Hotchkiss, R. S., Chang, K. C., Swanson, P. E., Tinsley, K. W., Hui, J. J., Klender, P., Xanthoudakis, S., Roy, S., Black, C., Grimm, E., et al. (2000) Nat. Immunol. 1, 496-501. [DOI] [PubMed] [Google Scholar]
- 26.Chaudry, I. H., Wichterman, K. A. & Baue, A. E. (1979) Surgery 85, 205-211. [PubMed] [Google Scholar]
- 27.Schreiber, R. D., Hicks, L. J., Celada, N. A., Buchmeier, N. A. & Gray, P. W. (1985) J. Immunol. 134, 1609-1618. [PubMed] [Google Scholar]
- 28.Pearl, J. E., Saunders, B., Ehlers, S., Orme, I. M. & Cooper, A. M. (2001) Cell. Immunol. 211, 43-50. [DOI] [PubMed] [Google Scholar]
- 29.Miles, R. H., Paxton, T. P., Dries, D. J. & Gamelli, R. L. (1994) J. Trauma 36, 607-611. [DOI] [PubMed] [Google Scholar]
- 30.Zantl, N., Uebe, A., Neumann, B., Wagner, H., Siewert, J. R., Holzmann, B., Heidecke, C. D. & Pfeffer, K. (1998) Infect. Immun. 66, 2300-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ono, S., Ueno, C., Aosasa, S., Tsujimoto, H., Seki, S. & Mochizuki, H. (2001) Am. J. Surg. 182, 491-497. [DOI] [PubMed] [Google Scholar]
- 32.Le Tulzo, Y., Pangault, C., Gacouin, A., Guilloux, V., Tribut, O., Amlot, L., Tattevin, P., Thomas, R., Fauchet, R. & Drenou, B. (2002) Shock 18, 487-494. [DOI] [PubMed] [Google Scholar]
- 33.Oberholzer, C., Oberholzer, A., Clare-Salzler, M. & Moldawer, L. L. (2001) FASEB J. 15, 879-892. [DOI] [PubMed] [Google Scholar]
- 34.Hotchkiss, R. S., Schmieg, R. E., Jr., Swanson, P. E., Freeman, B. D., Tinsley, K. W., Cobb, J. P., Karl, I. E. & Buchman, T. G. (2000) Crit. Care Med. 28, 3207-3217. [DOI] [PubMed] [Google Scholar]
- 35.Abbas, A. K., Murphy, K. M. & Sher, A. (1996) Nature 383, 787-793. [DOI] [PubMed] [Google Scholar]
- 36.Matzinger, P. (2001) Scand. J. Immunol. 54, 4-9. [DOI] [PubMed] [Google Scholar]
- 37.Fadok, V. A., Warner, M. L., Bratton, D. L. & Henson, P. M. (1998) J. Immunol. 161, 6250-6257. [PubMed] [Google Scholar]
- 38.Henson, P. M., Bratton, D. L. & Fadok, V. A. (2001) Nat. Rev. Mol. Cell Biol. 2, 627-633. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy, N. J. & Evan, G. I. (1998) Curr. Top. Dev. Biol. 36, 259-278. [DOI] [PubMed] [Google Scholar]
- 40.van de Watering, L. M., Hermans, J., Houbiers, J. G., van den Broek, P. J., Bouter, H., Boer, F., Harvey, M. S., Huysmans, H. A. & Brand, A. (1998) Circulation 97, 562-568. [DOI] [PubMed] [Google Scholar]
- 41.Hebert, P. C., Wells, G., Blajchman, M. A., Marshall, J., Martin, C., Pagliarello, G., Tweeddale, M., Schweitzer, I. & Yetisir, E. (1999) N. Engl. J. Med. 340, 409-417. [DOI] [PubMed] [Google Scholar]
- 42.Freire-de-Lima, C. G., Nascimento, D. O., Soares, M. B., Bozza, P. T., Castro-Faria-Neto, H. C., de Mello, F. G., DosReis, G. A. & Lopes, M. F. (2000) Nature 403, 199-203. [DOI] [PubMed] [Google Scholar]