Abstract
Hyperlipidemia promotes the chronic inflammatory disease atherosclerosis through poorly understood mechanisms. Atherogenic lipoproteins activate platelets, but it is unknown whether platelets contribute to early inflammatory atherosclerotic lesions. To address the role of platelet aggregation in diet-induced vascular disease, we studied β3 integrin-deficient mice (lacking platelet integrin αIIbβ3 and the widely expressed nonplatelet integrin αvβ3) in two models of atherosclerosis, apolipoprotein E (apoE)-null and low-density lipoprotein receptor (LDLR)-null mice. Unexpectedly, a high-fat, Western-type (but not a low-fat) diet caused death in two-thirds of the β3-/-apoE-/- and half of the β3-/-LDLR-/- mice due to noninfectious pneumonitis. In animals from both models surviving high-fat feeding, pneumonitis was absent, but aortic atherosclerosis was 2- to 6-fold greater in β3-/- compared with β+/+ littermates. Expression of CD36, CD40L, and CD40 was increased in lungs of β3-/-LDLR-/- mice. Each was also increased in smooth muscle cells cultured from β3-deficient mice and suppressed by retroviral reconstitution of β3. These data show that the platelet defect caused by αIIbβ3 deficiency does not impair atherosclerotic lesion initiation. They also suggest that αvβ3 has a suppressive effect on inflammation, the loss of which induces atherogenic mediators that are amplified by diet-induced hyperlipidemia.
Atherosclerosis is the most common cause of death in developed countries. People with established vascular disease benefit from pharmacologic therapies directed at lipids, blood pressure, and clots, and interventions that restore blood flow to diseased arteries. These treatments are of no use to the large number of people whose first hint of coronary artery disease is sudden death, caused by thrombus formation at the site of a ruptured atherosclerotic plaque that was too small to cause symptoms (1). Plaques destined to cause clinical events probably result from months to years of chronic inflammation. Exactly how this process starts and evolves is unclear.
Inflammation is affected by hyperlipidemia, a robust risk factor for atherosclerosis (2). Atherogenic lipoproteins up-regulate adhesion molecules, induce the production of cytokines, elicit immune reactions against oxidized epitopes, and activate platelets. Inhibition of platelet function decreases clinical events in patients with complex atherosclerotic lesions (3). Platelets are also postulated to participate in the early stages of atherogenesis, but data addressing this notion are contradictory. In rabbits with diet-induced hyperlipidemia, platelets are recruited to lesion-prone sites before lesions develop (4). Mice with deficiency of von Willebrand factor, critical for platelet adhesion under high-flow conditions, have less atherosclerosis at discrete sites after eating a toxic diet (5). However, studies in von Willebrand-deficient pigs (6, 7) and humans (8, 9) provide conflicting data about the role of platelet adhesion in plaque formation. In humans, the antiplatelet and antiinflammatory drug aspirin is effective for the primary prevention of coronary disease in some groups but not others (10). Humans with Glanzmann thrombasthenia, a disorder of platelet aggregation caused by mutations in the αIIbβ3 integrin, do not seem to be protected from vascular disease (11).
Integrins are cell-surface heterodimers that mediate interactions between the extracellular environment and platelets, inflammatory cells, and the vasculature. The β3 integrin subfamily consists of two integrins, αIIbβ3 and αvβ3. αIIbβ3 (glycoprotein IIb/IIIa) is expressed on platelets and megakaryocytes and participates in thrombus formation. It is a target of inhibitors known to provide clinical benefit to patients with acute coronary syndromes undergoing percutaneous interventions (12). αvβ3 is a nonplatelet integrin expressed in blood vessels and some inflammatory cells (13). Human atherosclerotic lesions show extensive expression of αvβ3, predominantly in smooth muscle cells and endothelial cells, and vascular injury up-regulates αvβ3 on smooth muscle cells (14, 15). The β3 integrins could modulate atherosclerosis through platelet-dependent or vascular wall-dependent mechanisms, but the exact role of β3 in vascular disease is unclear.
To determine whether the β3 molecule is involved in diet-induced atherosclerosis, we studied β3-/- mice (16), a model for Glanzmann thrombasthenia, in two different models of atherosclerosis, apolipoprotein E (apoE)-/- and low-density lipoprotein receptor (LDLR)-/- mice. Given the platelet defect in β3-null mice and the widespread use of antiplatelet agents to prevent atherosclerosis, we expected to find less vascular disease in β3-/- mice. Surprisingly, β3-null animals had more atherosclerosis and a high frequency of fatal pneumonitis, suggesting that the β3 integrin has a tonic suppressive effect on diet-induced inflammatory events at sites as diverse as the vascular wall and the lung.
Materials and Methods
Animals. β3-deficient mice of mixed genetic background (C57BL/6 and 129Sv) were bred with apoE-null mice in the C57BL/6 background or LDLR-null mice in the C57BL/6 background to generate β3-/-apoE-/- and β3-/-LDLR-/- littermates that were used for experiments. Animals were genotyped at the β3 locus (16) by using a forward wild-type primer (5′-CTTAGACACCTGCTACGGGC), a reverse neo-specific primer (5′-CACGAGACTAGTGAGACGTG), and a reverse wild-type primer (5′-CCTGCCTGAGGCTGAGTG). Mice housed in a specific pathogen-free barrier facility were weaned at the age of 3 weeks to standard mouse chow providing 6% calories as fat. At the age of 6 weeks, sex-matched animals were started on a Western-type diet containing 0.15% cholesterol providing 42% calories as fat (TD 88137, Harlan, Madison, WI).
Cages were monitored daily. Dead mice were necropsied either immediately or after storage overnight at 4°C by the same experienced veterinarian (M.L.). In the apoE model, 18 spontaneous deaths were recorded for β3-null mice. Eight showed pulmonary inflammation. Ten could not be evaluated because of postmortem changes. Necropsies showed fatty liver in some mice, but heart, kidneys, intestinal tract, brain, and spleen were normal. To determine whether pulmonary inflammation was present in mice surviving the Western-type diet, lungs were perfused and formalin-fixed from male and female mice of both β3 genotypes in the apoE- and LDLR-null models at the time of isolation of aortas for atherosclerosis assays.
Analytical Procedures. Cholesterol, triglycerides, and glucose were measured in serum from mice after a 4-h fast as described (17). Lipoproteins were analyzed by fast protein liquid chromatography with pooled serum samples (18).
Intimal Lesion Quantification. Atherosclerosis was measured by the en face technique (19). Pinned aortas were imaged with a digital camera and analyzed by using an image-processing program. The percentage of involvement of the intimal surface area is reported for the arch (encompassing the surface from the aortic valve to the left subclavian artery), the thoracic aorta (extending to the final intercostal artery), and the abdominal aorta (to the ileal bifurcation).
Preparation of Retrovirus and Infection of Smooth Muscle Cells. Human β3 integrin in the ΔU3 retroviral vector (ΔU3-hβ3; ref. 20) and a LacZ control vector (ΔU3nlsLZ) were transfected into separate aliquots of 293GPG packaging cells cultured in DMEM with 10% heat-inactivated FBS supplemented with tetracycline, puromycin, and G418 as described (20). Viruses were collected at regular intervals for up to 96 h after transfection.
Smooth muscle cells were cultured from the aortas of β3-/-apoE-/- and β3+/+apoE-/- mice by the explant technique. Vessels were cleaned of adventitia, washed in medium, and chopped into ≈1-mm cubes that were suspended in a small volume of medium and transferred to a T25 flask. Explants were dispersed onto the culture surface, and then the flask was tilted slowly, cap-side-up, with the tissue fragments attached to the vertical culture surface. Fresh medium was placed in the bottom of the flask, and then 2 h laterthe flask was returned to the usual culture position, allowing the fresh medium to cover the adherent aortic tissue. Smooth muscle cells growing from the explants were passaged, expanded, and verified to be smooth muscle cells by staining with a smooth muscle α-actin antibody. These cells were transduced with ΔU3-hβ3 or ΔU3nlsLZ for 24 h in the presence of medium containing polybrene (8 μg/ml, Sigma). Cells then were incubated for an additional 48 h in DMEM with 10% heat-inactivated FBS before harvesting.
Quantitative RT-PCR-Based Gene Expression. Total RNA prepared from lungs, cultured smooth muscle cells, or liver was used to prepare complementary DNA and subjected to quantitative PCR in a GeneAmp 5700 sequence detector (Applied Biosystems) as described (18). Aliquots of RNA not subjected to reverse transcription were included in each assay as negative controls. The primer pairs used were 5′-CAAGCTCCTTGGCATGGTAGA and 5′-TGGATTTGCAAGCACAATATGAA (CD36), 5′-GAACTTGGAGGTCCTACAGAAAGG and 5′-TGCCCATCACGACAGGAAT (CD40), 5′-AGCCAACCTTCCCCCAGAT and 5′-CACAGCAAAAAGCACAGATCCA (CD40L), 5′-CACCCCTTGAACCTCACTAAACA and 5′-AAAGGACATCGCAAAGATGACA [ATP binding cassette A1 (ABCA1)], and 5′-CGCTCCTGGAAGATGGTGAT and 5′-GGCAAATTCAACGGCACAGT (GAPDH). Results were normalized to GAPDH mRNA in each sample, which was unaffected by genotype.
Western Blotting. Proteins were extracted from lungs and cultured smooth muscle cells and subjected to SDS/PAGE in gradient gels under denaturing conditions. Protein signals on blots were detected by chemiluminescence. For CD36, the primary antibody was a rabbit polyclonal antibody (SC-9154, Santa Cruz Biotechnology) used at a dilution of 1:200, and the secondary antibody was donkey anti-rabbit (NA 934V, Amersham Biosciences) used at 1:5,000. For human β3, the primary antibody was mouse monoclonal antibody 7G2 used at 1:3,000. For the 70-kDa heat-shock cognate protein (HSC70), the primary antibody was a mouse monoclonal antibody from Santa Cruz Biotechnology (SC-7298) used at 1:1,000. For both monoclonal antibodies, the secondary antibody was sheep anti-mouse (NA 931, Amersham Biosciences) used at 1:5,000.
Results
Effects of High-Fat Feeding on Survival in β3-Null Mice. The first suggestion of a β3-associated dietary phenotype came from analysis of the numbers of pups surviving the high-fat diet of suckling. During a defined time period with the same number of breeders, 115 β3+/+apoE-/- mice were born, and 110 survived to weaning (96% survival). Over the same time period, 52 β3-/-apoE-/- littermate mice were born, an expectedly smaller number given the effects of β3 deficiency on placental defects and fetal loss (16). Thirty-two of these mice survived to weaning (62% survival, P < 0.001 vs. β3+/+apoE-/- by χ2 test).
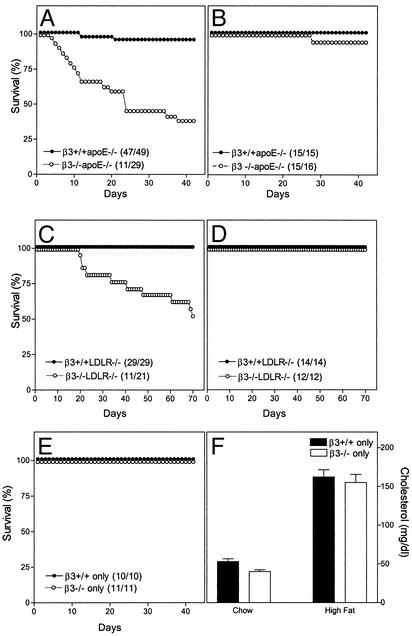
At the age of 6 weeks, mice were started on a Western-type diet containing 0.15% cholesterol and providing 42% calories as fat. Six weeks later, 96% of the β3+/+apoE-/- but only 38% of the β3-/-apoE-/- littermates were alive (Fig. 1A, P < 0.001 by Fisher's exact test). Mice started on chow at the age of 6 weeks showed no difference in survival over the ensuing 6 weeks (Fig. 1B). To confirm these data, the β3 mutation was moved into a different model, LDLR-null mice. β3+/+LDLR-/- and β3-/-LDLR-/- littermates were also started on the Western-type diet at the age of 6 weeks. Ten weeks later, 100% of the β3+/+LDLR-/- but only 52% of the β3-/-LDLR-/- mice were alive (Fig. 1C, P < 0.001 by Fisher's exact test). No deaths were observed when mice were fed a chow diet (Fig. 1D). For both models, death rates with high-fat feeding were the same in males and females. β3-null mice maintained without the apoE or LDLR mutation did not die when fed the Western diet (Fig. 1E) despite the fact that serum cholesterol levels tripled (Fig. 1F).
Fig. 1.
Survival in β3-null mice after high-fat and chow feeding. Survival is presented for β3-/- (open symbols) and β3+/+ (filled symbols) littermate mice in the apoE-/- model (A and B), the LDLR-/- model (C and D), and alone (E and F). At the age of 6 weeks (day 0 on the horizontal axis), mice were started on a Western-type high-fat diet (A, C, and E) or continued on a low-fat chow diet (B and D). (F) Serum cholesterol levels inβ3+/+ only (filled bars) andβ3-/- only (open bars) mice at baseline on the chow diet and after eating the high-fat diet for 6 weeks.
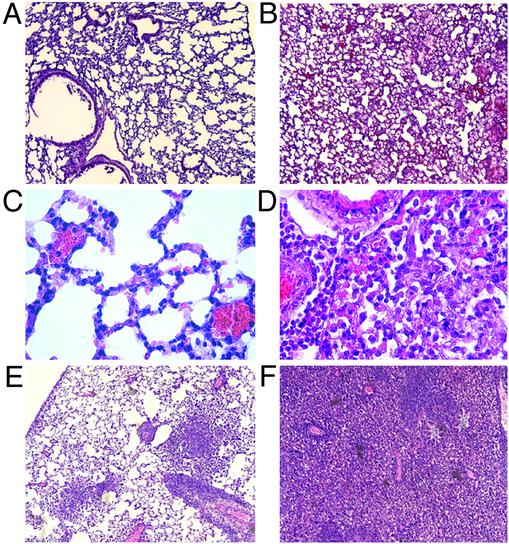
Pneumonitis was the likely cause of spontaneous deaths in high-fat-fed β3-null animals in both hyperlipidemic models. The lungs of β3-/-apoE-/- mice surviving high-fat feeding for 6 weeks were essentially normal (Fig. 2A). However, isolated small foci of mononuclear cells were present in lung after 6 weeks of high-fat feeding in both β3-/-apoE-/- (Fig. 2C) and β3+/+apoE-/- mice, confirming previous observations of low-grade pulmonary inflammation in the lungs of high-fat-fed apoE-null mice (21). Autopsies of eight different β3-/-apoE-/- mice found dead with high-fat feeding showed pneumonitis as the cause of death. Low (Fig. 2B) and higher (Fig. 2D) magnification views showed alveolar mononuclear cell infiltrates. Stains of lung tissue for pathogens (including Pneumocystis carinii) and cultures from sentinel animals were negative. In addition to macrophages and lymphocytes, alveolar spaces also contained fibrillar proteinaceous material that stained light pink with periodic acid-Schiff but was negative for fibrin (data not shown). Fig. 2 E and F show histology from two β3-/-LDLR-/- mice found dead after high-fat feeding. As with the apoE-null model, lungs of both β3-/-LDLR-/- and β3+/+LDLR-/- mice who survived high-fat feeding for 10 weeks were essentially normal (data not shown).
Fig. 2.
Lung histology after high-fat feeding. Hematoxylin/eosin staining of mouse lung. (A) β3-/-apoE-/- mouse killed after 6 weeks of eating a high-fat diet. Similar essentially normal lung findings were seen in a total of eight surviving apoE-/- mice (four β+/+ and four β3-/-) and four LDLR-/- mice (two β+/+ and two β-/-). (B) β3-/-apoE-/- mouse found dead after high-fat feeding. Similar findings were seen in a total of eight β3-/-apoE-/- mice found dead after high-fat feeding. (C) Higher-power view of a lung from a β3-/-apoE-/- survivor. (D) Higher-power view of lung from a β3-/-apoE-/- mouse found dead. (E and F) Two different β3-/-LDLR-/- mice found dead after high-fat feeding.
β3-null mice have a platelet defect, but iron stains of lung tissue were negative (data not shown), suggesting that pulmonary hemorrhage was not the cause of death. These mice are hyperlipidemic (see below), but Sudan black stains were only faintly positive and oil red O stains were negative (data not shown), suggesting that death was not caused by alveolar lipid.
Blood Counts, Serum Chemistries, and Diet-Induced Atherosclerosis. Western diet-fed β3-/-apoE-/- mice (hemoglobin 14.7 ± 0.7 g/dl, hematocrit 42.3 ± 2.3%, n = 7) were anemic compared with β3+/+apoE-/- mice (hemoglobin 16.7 ± 0.2 g/dl, hematocrit 48.8 ± 0.7%, n = 9, P < 0.01 for each vs. β3-/-apoE-/-). The hematocrit for β3+/+apoE-/- mice is the same as reported by others (22). White blood cell (7.3 ± 0.7 vs. 8.9 ± 0.6 thousands per mm3) and platelet (745 ± 199 vs. 805 ± 144 thousands per mm3) counts were unaffected by β3 genotype. Similar blood counts were found in the same animals on a chow diet and in LDLR-null mice.
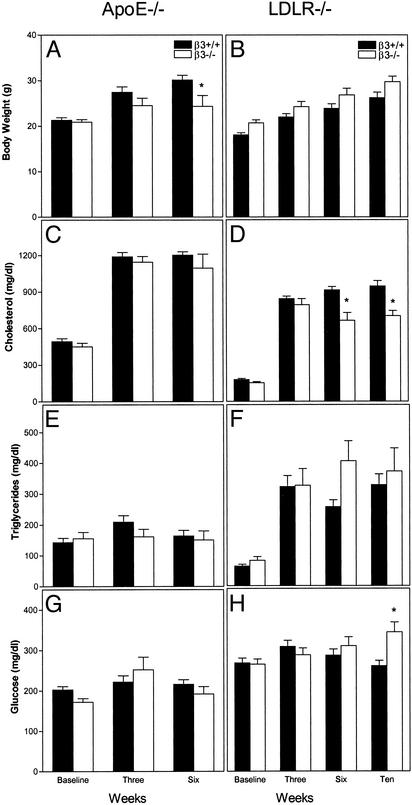
Body weights and fasting levels of cholesterol, triglycerides, and glucose are shown in Fig. 3 for β3+/+ (filled bars) and β3-/- (open bars) mice in apoE-null (Left) and LDLR-null (Right) models. Data for equal numbers of males and females are presented together, because there were no sex-specific differences for any variable. ApoE-null mice were studied at baseline and after 3 and 6 weeks of the Western-type diet. LDLR-null animals were studied for 10 weeks on the same diet, because atherosclerosis is less aggressive in this model. In both models, body weight for animals of each β3 genotype increased with high-fat feeding (Fig. 3 A and B), although β3-/-apoE-/- mice weighed less than their β3+/+apoE-/- littermates at 6 weeks (P = 0.0293; Fig. 3A). Cholesterol levels increased with high-fat feeding (Fig. 3 C and D). There was no effect of β3 genotype on cholesterol in apoE-null mice, but levels were ≈25% lower at both 6 (P = 0.0002) and 10 (P = 0.0017) weeks in β3-/-LDLR-/- mice (Fig. 3D).
Fig. 3.
Body weight and serum chemistries in mice. Data are presented forβ3+/+ (filled bars) and β3-/- littermate (open bars) mice in the apoE-null (A, C, E, and G) and LDLR-null (B, D, F, and H) models. Animals were studied at the age of 6 weeks on a chow diet (baseline) and then again 3 and 6 weeks after high-fat feeding for the apoE-null model and 3, 6, and 10 weeks after high-fat feeding for the LDLR-null model. Results are presented as mean ± SEM for decreasing numbers of mice over time due to spontaneous deaths.*, P value in comparison to β3+/+ mice at the same time point by unpaired two-tailed t test: A, P = 0.0293; D, P = 0.0002 at 6 weeks and 0.0017 at 10 weeks; H, P = 0.0026.
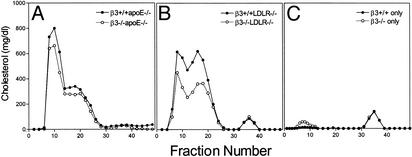
Size-exclusion chromatography of lipoproteins demonstrated the presence of atherogenic particles in the apoE-null model after 6 weeks (Fig. 4A) and in the LDLR-null model after 10 weeks (Fig. 4B). Lipoprotein profiles for Western diet-fed apoE+/+LDLR+/+ mice in the presence and absence of β3 are shown in Fig. 4C.
Fig. 4.
Size-exclusion chromatography of lipoproteins. Pooled serum samples from β3-/- (open symbols) and β3+/+ (filled symbols) fasted mice in the apoE-null (A) and LDLR-null (B) models were separated by chromatography, and individual fractions were assayed for cholesterol content. Mice were fed a Western-type diet for 6 (A) or 10 (B) weeks. Profiles are representative of three independent comparisons of different cohorts of mice. (C) Profiles for apoE+/+LDLR+/+ β3-/- (open symbols) and apoE+/+LDLR+/+β3+/+ (filled symbols) mice on the same Western-type diet.
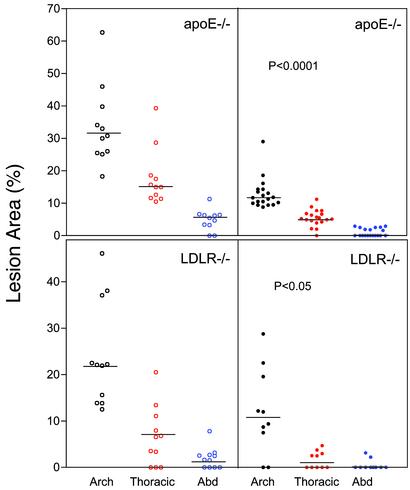
The tendency for lower cholesterol levels and anemia (22) in β3-deficient mice would be predicted to decrease atherosclerosis. However, β3-null mice (Fig. 5 Left) had more atherosclerosis than β3+/+ littermates (Fig. 5 Right) in both the apoE-null (Upper) and LDLR-null models (Lower). Lesions were 2.6-fold greater at the arch, 3.3-fold greater at the thoracic aorta, and 5.6-fold greater at the abdominal aorta (all P < 0.0001) in β3-/-apoE-/- as compared with β3+/+apoE-/- mice. Lesions were 2.0-fold greater at the arch (P = 0.0152) and 4.1-fold greater at the thoracic aorta (P = 0.0306) in β3-/-LDLR-/- as compared with β3+/+LDLR-/- mice.
Fig. 5.
Aortic intimal lesions in mice. β3-/- (Left) and β3+/+ (Right) littermate mice in the apoE-null (Upper) and LDLR-null (Lower) models were studied after eating a Western-type diet for 6 (apoE model) or 10 (LDLR model) weeks. Horizontal lines in the data sets indicate medians. For apoE-null mice, P < 0.0001 represents comparisons at the aortic arch (open black symbols forβ3-/- and filled black symbols for β3+/+), thoracic aorta (open red symbols for β3-/- and filled red symbols for β3+/+), and abdominal aorta (abd) (open blue symbols for β3-/- and filled blue symbols forβ3+/+). For LDLR-null mice, P < 0.05 represents comparisons at the aortic arch (open black symbols for β3-/- and filled black symbols for β3+/+), and thoracic aorta (open red symbols for β3-/- and filled red symbols for β3+/+). Comparisons were performed by using either parametric (unpaired t)or nonparametric (Mann–Whitney) tests depending on data distribution.
Atherosclerosis extent was also examined at ≈11 months of age in a limited number of retired apoE-/- breeders fed chow throughout life. Lesions were 2.4-fold greater at the arch (70.0 ± 2.7% vs. 30.0 ± 2.9%, n = 3, P = 0.0005) and 3-fold greater at the thoracic aorta (51.3 ± 3.2% vs. 17.0 ± 7.6%, n = 3, P = 0.0138) in β3-/-apoE-/- as compared with β3+/+apoE-/- mice.
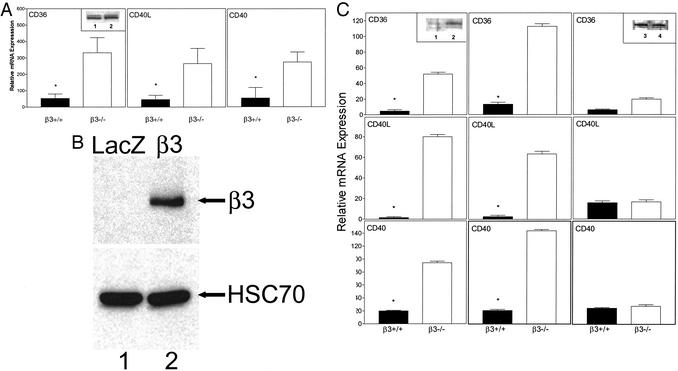
CD36, CD40L, and CD40 Expression in Lung and Cultured Aortic Smooth Muscle Cells. β3 interacts with several proinflammatory molecules. CD40L binds to αIIbβ3 on platelets in a β3-dependent fashion (23). CD36 is associated with αIIbβ3 on platelets and cooperates with αvβ3 in phagocytosis of apoptotic cells (24, 25). In normal lungs from high-fat-fed LDLR mice (Fig. 6A), CD36, CD40L, and CD40 (the receptor for CD40L) expression was increased 5- to 6-fold in β3-/- (open bars) as compared with β3+/+ (filled bars) mice (P < 0.05 for each). CD36 protein was increased in β3-/- (Inset, lane 2) as compared with β3+/+ (Inset, lane 1) lung. The difference did not correlate with mRNA levels, but CD36 expression is known to be regulated translationally (26).
Fig. 6.
(A) Expression of CD36, CD40L, and CD40 in mouse lung. RNA was prepared from the lungs of LDLR-null mice after eating a Western-type diet for 10 weeks. β3+/+ (filled bars) and β3-/- (open bars) samples were assayed for message levels of CD36, CD40L, and CD40 by quantitative RT-PCR with lungs from between four and six mice per genotype.*, P < 0.05 as compared with β3-/- mice. Data (relative to GAPDH levels in the same samples) are expressed as mean ± SEM. (Inset) CD36 Western blot of lung tissue from β3+/+LDLR-/- (lane 1) and β3-/-LDLR-/- (lane 2) mice. The two bands, ≈88 and ≈91 kDa, correspond to isoforms of CD36 due to differential glycosylation. (B) Reconstitution of β3 in β3-/- smooth muscle cells. Primary cultures of aortic smooth muscles cells from β3-/-apoE-/- mice were infected with the control retrovirus ΔU3nlsLZ (LacZ, lane 1) or a retrovirus containing the human β3 integrin ΔU3-hβ3(β3, lane 2). After cell harvest, protein extracts were prepared and Western blotted by using antibodies to human β3 and the control protein HSC70. (C) Expression of CD36, CD40L, and CD40 in smooth muscle cells. β3+/+ (filled bars) and β3-/- (open bars) cells from apoE-null mice were studied at baseline (Left), after transduction with a control retrovirus (Center), or after transduction with a human β3 retrovirus (Right). (Insets) Western blots of CD36 at baseline (β3+/+ in lane 1 and β3-/- in lane 2) and after reconstitution of β3(β3+/+ in lane 3 and β3-/- in lane 4). Vertical axes indicate relative mRNA expression in arbitrary units normalized to GAPDH in the same sample. For each data set, results are representative of six independent experiments with cells derived from two different apoE-null mice for each β3 genotype. (Left and Center)*, P < 0.001 vs. β3-/- cells. After β3 reconstitution, CD36, CD40L, and CD40 expression was suppressed in β3-/- cells compared with baseline (P < 0.001 for each).
To determine whether reconstitution of β3 affects expression of these inflammatory markers, we established primary cultures of smooth muscle cells from the aortas of apoE-null mice. β3 protein was reconstituted in β3-null cells by using a retrovirus expressing the full-length human β3 integrin. Its expression was detected by Western blotting in β3-/-apoE-/- smooth muscle cells infected with the β3 retrovirus (Fig. 6B, lane 2) but not in the same cells infected with a control retrovirus (Fig. 6B, lane 1).
Message levels for CD36, CD40L, and CD40 (Fig. 6C Left) were substantially higher in β3-/-apoE-/- (open bars) as compared with β3+/+apoE-/- (filled bars) smooth muscle cells (P < 0.001 for each). Protein levels of CD36 were also higher in β3-/-apoE-/- (Inset, lane 2) as compare with β3+/+apoE-/- (Inset, lane 1) cells. Cells of each genotype showed the same relative levels of expression after infection with the LacZ retrovirus (Fig. 6C Center). However, infection with the β3 retrovirus suppressed mRNA expression of CD36, CD40L, and CD40 in β3-/- cells (Fig. 6C Right) (P < 0.001 compared with baseline values for each) and resulted in similar levels of CD36 protein in β3-/- (Inset, lane 4) and β3+/+ (Inset, lane 3) cells.
ABCA1 Expression in β3-Deficient Mice. Given the potential role of reverse cholesterol transport in atherosclerosis, we measured ABCA1 expression in liver, a tissue with message levels of ABCA1 similar to macrophages in mice (27). ABCA1 (normalized to GAPDH) message levels were no different in β3-/-apoE-/- (115 ± 19 arbitrary units, n = 3) compared with β3+/+apoE-/- (82 ± 21 arbitrary units, n = 3, P = 0.3087) mice.
Discussion
Hyperlipidemia promotes atherosclerosis, as does inflammation, and inflammatory markers might rival atherogenic lipoproteins as predictors of vascular events (28). The processes linking hyperlipidemia and inflammation are obscure. In this article we show that the absence of the integrin β3 enhances the susceptibility of two distinct organs, aorta and lung, to inflammation caused by diet-mediated hyperlipidemia. Proatherogenic and proinflammatory mediators known to be induced by hyperlipidemia are elevated in β3-deficient mice and suppressed by reconstitution of β3. Our results indicate that β3-mediated signaling may limit inflammation induced by hypercholesterolemia.
β3 integrin-deficient mice in two different models of genetic hyperlipidemia, apoE deficiency and LDLR deficiency, have a high frequency of death induced by high-fat feeding (Fig. 1). A likely contributor to the phenotype is noninfectious pneumonitis, characterized by macrophage and lymphocyte infiltration but not by alveolar lipids or hemorrhage (Fig. 2). This observation is unexpected but not unprecedented. Dietary cholesterol accelerates pulmonary inflammation in a mouse model of asthma (29), and lipid levels may modulate several limbs of the inflammatory response (30, 31, 32). Previous data suggest a role for integrins in the suppression of pulmonary inflammation. Mice with deficiency of the β6 integrin have pulmonary and skin inflammation on a chow diet (33). Inactivation of thrombospondin-1, a major ligand for the αvβ3 integrin (absent in the β3-null mice of the current study), causes noninfectious pneumonia in chow-fed mice (34). Granulocyte/macrophage colony-stimulating factor (GM-CSF) is a powerful inducer of αvβ3 expression, and low-fat-fed GM-CSF-null mice have pulmonary inflammation and features of alveolar proteinosis (35, 36).
In both the apoE-null and LDLR-null models, β3-deficient mice surviving Western-type feeding had normal lungs but more aortic atherosclerosis than their β3+/+ littermates. In LDLR-/- mice, increased atherosclerosis occurred despite decreased cholesterol levels (Figs. 3D and 4B). The reason for this decrease is unknown. However, thrombospondin-1 binds to the LDLR-related protein (LRP) as well as αvβ3 (37), raising the possibility that compensatory induction of LRP in the absence of αvβ3 would lower cholesterol levels in LDLR-null mice but not apoE-null mice (lacking the key apolipoprotein required for LRP binding).
Increased vascular disease in the absence of members of the β3 integrin family is not inconsistent with previous studies. Femoral arteries in β3-null mice are not protected from leukocyte recruitment and intimal hyperplasia after guide-wire injury (38). Humans with Glanzmann thrombasthenia due to deficiency of αIIbβ3 alone or both αIIbβ3 and αvβ3 develop carotid atherosclerosis (11). Despite the unquestioned efficacy of short-term i.v. inhibition of αIIbβ3, long-term oral inhibition of αIIbβ3 curiously increases mortality in humans (39). These observations prompted the speculation that defects in β3 signaling could result in compensatory increases in inflammatory mediators (38, 39).
Our results validate this prediction by showing that in the absence of β3, the inflammatory mediators CD36, CD40L, and CD40 (the CD40L receptor) are increased in lung and in cells derived from the vascular wall (Fig. 6). We specifically assayed these proteins, because they are known to interact with β3. CD36 (40) is expressed on platelets, smooth muscle cells, and monocyte/macrophages and at numerous other sites (with the exception of large vessel endothelium). Among its many functions, CD36 clears apoptotic cells (in conjunction with αvβ3; ref. 25) and binds oxidized lipoproteins. CD40L (also known as CD154) and its receptor, CD40 (41), are expressed on lymphocytes, platelets, and the cells of the vasculature including smooth muscle cells. CD40L is a ligand for αIIbβ3, critical for Ig class switching, and stimulates inflammatory cytokine production in nonimmune cells.
CD36 and CD40/CD40L are proinflammatory in models of lung disease. Blocking CD36 decreases pulmonary inflammation in mice treated with bleomycin (42). Disrupting the CD40/CD40L interaction decreases pulmonary inflammation in mice after exposure to ionizing radiation (43).
CD36 and CD40/CD40L are proatherogenic. CD36-deficient mice in the apoE-null model are protected from diet-induced atherosclerosis (44). In both the apoE-null and LDLR-null models, disrupting the CD40/CD40L interaction decreases the initiation and progression of atherosclerotic lesions (45, 46, 47, 48). We documented increased CD36 and CD40/CD40L in smooth muscle cells from β3-null mice. Smooth muscle cells contain the greatest mass of αvβ3 in the normal vasculature. Arterial smooth muscle cell inflammation promotes intimal proliferation and foam cell formation in the setting of dietary hypercholesterolemia (32).
Both CD36 and CD40/CD40L are induced by hypercholesterolemia (49, 50). Our findings suggest that β3 serves to limit inflammation, and that its absence up-regulates expression of inflammatory proteins that are induced further by dietary lipids. CD40 signaling may be particularly important in this process. CD40, a member of the tumor necrosis factor receptor family, initiates a signaling cascade that includes p38 mitogen-activated protein kinase (51). Active p38 mitogen-activated protein kinase may have many detrimental vascular effects and is known to induce pathways that promote cellular lipid uptake (52).
β3 was shown recently to have an unexpected suppressive effect on angiogenesis (53). Blood vessel sprouting is accelerated in β3-null mice perhaps because of increased expression of the vascular endothelial growth factor receptor Flk-1. Flk-1 is proatherogenic in apoE-null mice (54), providing an additional potential mechanism underlying the enhanced atherosclerosis in β3-null animals.
Our observations suggest a previously unrecognized link between dietary hypercholesterolemia and integrin-modulated inflammation and raise the possibility that β3 agonists selected to promote the suppressive effects of β3 signaling on inflammation could be used to treat vascular disease.
Acknowledgments
We thank Robert P. Mecham for teaching us to culture smooth muscle cells from mouse aorta and F. Patrick Ross and Steven L. Teitelbaum for critical reagents and invaluable advice. This study was supported by National Institutes of Health Grants HL58427, AG20091, and AG14658, Clinical Nutrition Research Unit Grant DK56341, and Diabetes Research and Training Center Grant DK20579. L.Z. was supported by the Four Schools Physician-Scientist program. C.B.-M. was supported by an American Diabetes Association/Johnson & Johnson mentor-based post-doctoral fellowship. C.F.S. has served as a consultant for Pharmacia. Pharmacia did not support this work.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: apoE, apolipoprotein E; LDLR, low-density lipoprotein receptor; ABCA1, ATP binding cassette A1.
References
- 1.Schmermund, A., Schwartz, R. S., Adamzik, M., Sangiorgi, G., Pfeifer, E. A., Rumberger, J. A., Burke, A. P., Farb, A. & Virmani, R. (2001) Atherosclerosis (Shannon, Irel.) 155, 499-508. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg, D. (2002) Nat. Med. 8, 1211-1217. [DOI] [PubMed] [Google Scholar]
- 3.Antiplatelet Trialists' Collaboration (1994) Br. Med. J. 308, 81-106. [PMC free article] [PubMed] [Google Scholar]
- 4.Theilmeier, G., Michiels, C., Spaepen, E., Vreys, I., Collen, D., Vermylen, J. & Hoylaerts, M. F. (2002) Blood 99, 4486-4493. [DOI] [PubMed] [Google Scholar]
- 5.Methia, N., Andre, P., Denis, C. V., Economopoulos, M. & Wagner, D. D. (2001) Blood 98, 1424-1428. [DOI] [PubMed] [Google Scholar]
- 6.Griggs, T. R., Bauman, R. W., Reddick, R. L., Read, M. S., Koch, G. G. & Lamb, M. A. (1986) Arteriosclerosis (Dallas) 6, 155-165. [DOI] [PubMed] [Google Scholar]
- 7.Fuster, V., Fass, D. N., Kaye, M. P., Josa, M., Zinsmeister, A. R. & Bowie, E. J. (1982) Circ. Res. 51, 587-593. [DOI] [PubMed] [Google Scholar]
- 8.Sramek, A., Reiber, J. H., Gerrits, W. B. & Rosendaal, F. R. (2001) Circulation 104, 762-767. [DOI] [PubMed] [Google Scholar]
- 9.Bilora, F., Boccioletti, V., Zanon, E., Petrobelli, F. & Girolami, A. (2001) Clin. Appl. Thromb. Hemost. 7, 311-313. [DOI] [PubMed] [Google Scholar]
- 10.Lauer, M. S. (2002) N. Engl. J. Med. 19, 1468-1474. [DOI] [PubMed] [Google Scholar]
- 11.Shpilberg, O., Rabi, I., Schiller, K., Walden, R., Harats, D., Tyrrell, K. S., Coller, B. & Seligsohn, U. (2002) Circulation 105, 1044-1048. [DOI] [PubMed] [Google Scholar]
- 12.Topol, E. J., Mark, D. B., Lincoll, A. M., Cohen, E., Burton, J., Kleiman, N., Talley, D., Sapp, S., Booth, J., Cabot, C. F., et al. (1999) Lancet 354, 2019-2024. [DOI] [PubMed] [Google Scholar]
- 13.Byzova, T. V., Rabbani, R., D'Souza, S. E. & Plow, E. F. (1998) Thromb. Haemostasis 80, 726-734. [PubMed] [Google Scholar]
- 14.Hoshiga, M., Alpers, E. E., Smith, L. L., Giachelli, C. M. & Schwartz, S. M. (1995) Circ. Res. 77, 1129-1135. [DOI] [PubMed] [Google Scholar]
- 15.Stouffer, G. A., Hu, Z., Sajid, M., Li, H., Jin, G., Nakada, M. T., Hanson, S. R. & Runge, M. S. (1998) Circulation 97, 907-915. [DOI] [PubMed] [Google Scholar]
- 16.Hodivala-Dilke, K. M., McHugh, K. P., Tsakiris, D. A., Rayburn, H., Crowley, D., Ullman-Cullere, M., Ross, F. P., Coller, B. S., Teitelbaum, S. & Hynes, R. O. (1999) J. Clin. Invest. 103, 229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Towler, D. A., Bidder, M., Latiffe, T., Coleman, T. & Semenkovich, C. F. (1998) J. Biol. Chem. 273, 30427-30434. [DOI] [PubMed] [Google Scholar]
- 18.Tordjman, K., Bernal-Mizrachi, C., Zemany, L., Weng, S., Feng, C., Zhang, F., Leone, T. C., Coleman, T., Kelly, D. P. & Semenkovich, C. F. (2001) J. Clin. Invest. 107, 1025-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semenkovich, C. F., Coleman, T. & Daugherty, A. (1998) J. Lipid Res. 39, 1141-1151. [PubMed] [Google Scholar]
- 20.Feng, X., Novack, D. V., Faccio, R., Ory, D. S., Aya, K., Boyer, M. I., McHugh, K. P., Ross, F. P. & Teitelbaum, S. L. (2001) J. Clin. Invest. 107, 1137-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang, S. H., Reddick, R. L., Burkey, B. & Maeda, N. (1994) J. Clin. Invest. 96, 937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul, A., Calleja, L., Vilella, E., Martinez, R., Osada, J. & Joven, J. (1999) Atherosclerosis (Shannon, Irel.) 147, 61-68. [DOI] [PubMed] [Google Scholar]
- 23.Andre, P., Prasad, K. S., Denis, C. V., He, M., Papalia, J. M., Hynes, R. O., Phillips, D. R. & Wagner, D. D. (2002) Nat. Med. 8, 247-252. [DOI] [PubMed] [Google Scholar]
- 24.Dorahy, D. J., Berndt, M. C., Shafren, D. R. & Burns, G. F. (1996) Biochem. Biophys. Res. Commun. 218, 575-581. [DOI] [PubMed] [Google Scholar]
- 25.Savill, J., Hogg, N. & Halsett, C. (1991) Chest 99, 6S-7S. [DOI] [PubMed] [Google Scholar]
- 26.Griffin, E., Re, A., Hamel, N., Fu, C., Bush, H., McCaffrey, T. & Asch, A. S. (2001) Nat. Med. 7, 840-846. [DOI] [PubMed] [Google Scholar]
- 27.Su, Y. R., Linton, M. F. & Fazio, S. (2002) J. Lipid Res. 43, 2180-2187. [DOI] [PubMed] [Google Scholar]
- 28.Ridker, P. M., Rifai, N., Rose, L., Buring, J. E. & Cook, N. R. (2002) N. Engl. J. Med. 20, 1557-1565. [DOI] [PubMed] [Google Scholar]
- 29.Yeh, Y.-F. & Huang, S.-L. (2001) Int. Arch. Allergy Immunol. 125, 329-334. [DOI] [PubMed] [Google Scholar]
- 30.Hughes, D. A., Townsend, P. J. & Haslam, P. L. (1992) Clin. Exp. Immunol. 87, 279-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herringer, D. D., Gerritsen, M. E. & Granger, D. N. (1997) Circ. Res. 81, 274-281. [DOI] [PubMed] [Google Scholar]
- 32.Ludewig, B., Freigang, S., Jaggi, M., Kurrer, M. O., Pei, Y.-C., Vlk, L., Odermatt, B., Zinkernagel, R. M. & Hengartner, H. (2000) Proc. Natl. Acad. Sci. USA 97, 12752-12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munger, J. S., Huang, X., Kawakatsu, H., Griffiths, M., Dalton, S. L., Wu, J., Pittet, J.-F., Kaminski, N., Garat, C., Matthay, M. A., et al. (1999) Cell 96, 319-328. [DOI] [PubMed] [Google Scholar]
- 34.Lawler, J., Sunday, M., Thibert, V., Duquette, M., George, E. L., Rayburn, H. & Hynes, R. O. (1998) J. Clin. Invest. 101, 982-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Nichilo, M. O. & Burns, G. F. (1993) Proc. Natl. Acad. Sci. USA 90, 2517-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dranoff, G., Crawford, A. D., Sadelain, M., Ream, B., Rashid, A., Bronson, R. T., Dickersin, G. R., Bachurski, C. J., Mark, E. L. & Whitsett, J. A. (1994) Science 264, 713-716. [DOI] [PubMed] [Google Scholar]
- 37.Mikhailendo, I., Krylov, D., Argraves, K. M., Roberts, D. D., Liau, G. & Strickland, D. K. (1997) J. Biol. Chem. 272, 6784-6791. [DOI] [PubMed] [Google Scholar]
- 38.Smyth, S. S., Reis, E. D., Zhang, W., Fallon, J. T., Gordon, R. E. & Coller, B. S. (2001) Circulation 103, 2501-2507. [DOI] [PubMed] [Google Scholar]
- 39.Chew, D. P., Bhatt, D. L., Sapp, S. & Topol, E. J. (2001) Circulation 103, 201-206. [DOI] [PubMed] [Google Scholar]
- 40.Febbraio, M., Hajjar, D. P. & Silverstein, R. L. (2001) J. Clin. Invest. 108, 785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phipps, R. P. (2000) Proc. Natl. Acad. Sci. USA 97, 6930-6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yehualaeshet, T., O'Connor, R., Begleiter, A., Murphy-Ullrich, J. E., Silverstein, R. & Khalil, N. (2000) Am. J. Respir. Cell Mol. Biol. 23, 204-212. [DOI] [PubMed] [Google Scholar]
- 43.Adawi, A., Zhang, Y., Baggs, R., Rubin, P., Williams, J., Finkelstein, J. & Phipps, R. P. (1998) Clin. Immunol. Immunopathol. 89, 222-230. [DOI] [PubMed] [Google Scholar]
- 44.Febbraio, M., Podrez, E. A., Smith, J. D., Hajjar, D. D., Hazen, S. L., Hoff, H. F., Sharma, K. & Silverstein, R. L. (2000) J. Clin. Invest. 105, 1049-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mach, F., Schonbeck, U., Sukhova, G. K., Atkinson E. & Libby, P. (1998) Nature 394, 200-203. [DOI] [PubMed] [Google Scholar]
- 46.Lutgens, E., Gorelik, L., Daemen, M. J., de Muinck, E. D., Grewal, I. S., Koteliansky, V. E. & Flavell, R. A. (1999) Nat. Med. 5, 1313-1316. [DOI] [PubMed] [Google Scholar]
- 47.Schonbeck, U., Sukhova, G. K., Shimizu, K., Mach, F. & Libby, P. (2000) Proc. Natl. Acad. Sci. USA 97, 7458-7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lutgens, E., Cleutjens, K. B., Heeneman, S., Koteliansky, V. E., Burkly, L. C. & Daemen, M. J. (2000) Proc. Natl. Acad. Sci. USA 97, 7464-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenwalt, D. E., Scheck, S. H. & Rhinehart-Jones, T. (1995) J. Clin. Invest. 96, 1382-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cipollone, F., Mezzetti, A., Porreca, E., Di Febbo, C., Nutini, M., Fazia, M., Falco, A., Cuccurullo, F. & Davi, G. (2002) Circulation 106, 399-401. [DOI] [PubMed] [Google Scholar]
- 51.Pullen, S. S., Dang, T. T. A., Crute, J. J. & Kehry, M. R. (1999) J. Biol. Chem. 274, 14246-14254. [DOI] [PubMed] [Google Scholar]
- 52.Puigserver, P., Rhee, J., Lin, J., Wu, Z., Yoon, J. C., Zhang, C. Y., Krauss, S., Mootha, V. K., Lowell, B. B. & Spiegelman, B. M. (2001) Mol. Cell 8, 971-982. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds, L. E., Wyder, L., Lively, J. C., Taverna, D., Robinson, S. D., Huang, X., Sheppard, D., Hynes, R. O. & Hodivala-Dilke, K. M. (2002) Nat. Med. 8, 27-34. [DOI] [PubMed] [Google Scholar]
- 54.Luttun, A., Tjwa, M., Moons, L., Wu, Y., Angelillo-Scherrer, A., Liao, F., Nagy, J. A., Hooper, A., Priller, J., De Klerck, B., et al. (2002) Nat. Med. 8, 831-840. [DOI] [PubMed] [Google Scholar]