Abstract
Erythropoietin (EPO) is a tissue-protective cytokine preventing vascular spasm, apoptosis, and inflammatory responses. Although best known for its role in hematopoietic lineages, EPO also affects other tissues, including those of the nervous system. Enthusiasm for recombinant human erythropoietin (rhEPO) as a potential neuroprotective therapeutic must be tempered, however, by the knowledge it also enlarges circulating red cell mass and increases platelet aggregability. Here we examined whether erythropoietic and tissue-protective activities of rhEPO might be dissociated by a variation of the molecule. We demonstrate that asialoerythropoietin (asialoEPO), generated by total enzymatic desialylation of rhEPO, possesses a very short plasma half-life and is fully neuroprotective. In marked contrast with rhEPO, this molecule at doses and frequencies at which rhEPO exhibited erythropoiesis, did not increase the hematocrit of mice or rats. AsialoEPO appeared promptly within the cerebrospinal fluid after i.v. administration; intravenously administered radioiodine-labeled asialoEPO bound to neurons within the hippocampus and cortex in a pattern corresponding to the distribution of the EPO receptor. Most importantly, asialoEPO exhibits a broad spectrum of neuroprotective activities, as demonstrated in models of cerebral ischemia, spinal cord compression, and sciatic nerve crush. These data suggest that nonerythropoietic variants of rhEPO can cross the blood–brain barrier and provide neuroprotection.
The widespread and highly efficacious use of recombinant human erythropoietin (rhEPO) for the treatment of EPO-deficient anemias reflects a triumph of biotechnology. As a typical member of the cytokine superfamily, EPO also performs other functions. Recently, EPO has been identified as an important endogenous mediator of the adaptive responses of tissues to metabolic stress, primarily by limiting the extent of injury. For example, EPO synthesized by hypoxic astrocytes may mediate the protective phenomenon of preconditioning in the nervous system in which exposure to a brief, nontoxic episode of ischemia dramatically increases the resistance of neurons to subsequent insults (1–3).
Given the large size of rhEPO, it was initially surprising to discover that rhEPO administered peripherally readily penetrates the blood–brain barrier and effectively reduces brain injury after insults (4). Additional evidence has also shown widespread efficacy of rhEPO in injury models of the spinal cord (5, 6), retina (7), and the heart (8). Mechanistically, EPO acts in a coordinated fashion at multiple levels, including limiting the production of tissue-injuring molecules [e.g., reactive oxygen species and glutamate (9–11)], reversal of vasospasm (12, 13), attenuation of apoptosis (5, 10, 14, 15), modulation of inflammation (4, 16), and recruitment of stem cells (17). A recent clinical trial supports the relevance of these animal models for human disease by demonstrating significant improvement in outcome of stroke patients who were administered rhEPO intravenously within 8 h of the onset of symptoms (18).
Animal models have demonstrated that single doses of rhEPO are remarkably effective for the treatment of acute injury (4–6, 19). Many clinical situations, however, will likely require multiple doses of rhEPO, which will lead to potentially harmful increases in the red cell mass. For example, animal models clearly show that EPO-dependent increases in hematocrit can cause and amplify brain injury (20). Pharmacological doses of rhEPO also stimulate the production of hyperreactive platelets (21) that can predispose to thrombosis (22), especially in the setting of injury. This potential complication of rhEPO therapy is relevant for humans as well (23, 24), because thrombotic events have been observed in the setting of “blood doping” by athletes (25). These considerations temper enthusiasm for rhEPO as a tissue-protective agent. Clearly, molecules retaining the beneficial tissue-protective actions of EPO, but not stimulating the bone marrow, are desirable.
One distinguishing feature between erythropoiesis and neuroprotection is that effective production of red cells requires the continuous presence of EPO, whereas a brief exposure is sufficient for neuroprotection in vitro (26). We reasoned that if neuroprotection is activated after a brief exposure to EPO, then a short-lived EPO could be translocated into tissue beds to initiate neuroprotection through EPO receptor (EPO-R) activation, but yet not survive long enough within the circulation to stimulate erythropoiesis. To produce a short-lived EPO, we completely removed the sialic acids that delay clearance in vivo (27, 28). In this article, we show that systemically administered asialoerythropoietin (asialoEPO) does not increase erythrocyte mass, yet is fully protective in animal models of stroke, spinal cord injury, and peripheral neuropathy.
Materials and Methods
Preparation and Characterization of AsialoEPO. The sialic acid of rhEPO (Dragon Pharmaceuticals, Vancouver) was removed by digestion with neuraminidase isolated from Streptococcus sp. 6646K (Seikagaku America, Rockville, MD, no. 120050). The reaction was performed at 37°C, pH 6.8, for 3 h using 0.05 units of enzyme per mg of rhEPO. The product (asialoEPO) was purified by anion-exchange chromatography and the homogeneity was confirmed by isoelectric focusing gel analysis. The final sialic acid content was determined according to the European Pharmacopoeia (51) method and acid hydrolysis followed by high-performance anion-exchange chromatography with pulsed amperometric detection (Dionex).
For practical reasons, rhEPO and asialoEPO were considered to be of equivalent mass for dosing, as the sialic acid content accounted for ≤8% of the total weight of this ≈34,000-Da protein. Concentrations are expressed as molarity instead of units, as asialoEPO exhibits no erythropoietic activity in vivo. One international unit of erythropoietic activity is equal to ≈8 ng of rhEPO protein.
For binding assays, asialoEPO and rhEPO were radiolabeled according to a standard protocol by using chloramine T as described (29). The radioligand activity corresponded to ≈50 μCi/μg(1Ci = 37 GBq) with 50% bindability. Soluble Fc-EpoR fusion constructs (5 pM; R & D Systems), 4 pM radioligand, and graded concentrations (0–10,000 pM) of unlabeled EPO or asialoEPO were incubated for 30 min at 22°C in 100 μl of PBS before the addition of 100 μl of PBS with a 0.25-mg scintillation proximity assay (SPA) polyvinyl toluene protein A reagent (Amersham Pharmacia Biosciences, Amersham, U.K.). This reaction was incubated for an additional 2 h before luminescence recording by a scintillation counter.
Erythroleukemic UT-7 cells (30) were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany, no. ACC137). The assay of EPO derivatives was performed as described (31) over a 48-h period, and using WST-1 reduction (Roche Diagnostics, no. 1 644 807) to quantitate living cells. Signal-to-noise ratio of the assay was 8:15, and the half-maximal effective concentration of EPO variants was determined by a four-parameter-fit from concentration-response curves by using at least six drug concentrations. The P-19 survival assay was performed exactly as described (15). PC-12 cells were differentiated in the presence of nerve growth factor (NGF) and cell death was triggered by NGF withdrawal. EPO variants were used in this assay as described (32) and were present 24 h before and during the withdrawal period. For viability assessment the reduction of thiozole salts was used.
Animal Protocols. All procedures involving animals were approved by the animal research committees at each respective Institution and conducted in compliance with international laws and policies (33–36).
Pharmacokinetic Determination of Plasma and Cerebrospinal Fluid (CSF) Concentrations. CSF samples were obtained from male Sprague–Dawley rats by a needle placed into the cisterna magna using the methodology of Frankmann (37). Plasma half-life was determined from blood samples drawn serially from a catheter implanted in the carotid artery in awake Sprague–Dawley rats. After i.v., i.p., or s.c. administration of 44 μg/kg of body weight (bw) of asialoEPO/rhEPO, timed blood samples were collected with an Accusampler (Dilab, Lund, Sweden) over a 20-h period. AsialoEPO concentrations were determined by enzyme-linked immunoabsorbance assay kits (R & D Systems and Immuno-Biological Laboratories, Hamburg, Germany). Extensive evaluation confirmed that the antibodies used in these kits identified asialoEPO and rhEPO with equal sensitivity. The lower limit of quantification was 1.0 pM.
In Vivo Autoradiography. NMRI mice were injected i.v. with 100 μg/kg radioiodinated asialoEPO, and after 4 h the animals were perfused with PBS/heparin. The brains were removed, frozen on dry ice, and cryosectioned (20 μm). For cellular localization of radiolabel, slides were dipped in photographic emulsion and were exposed for 5 weeks before development.
Hemoglobin Responses to Repeated Doses of AsialoEPO in Vivo. The response of the bone marrow to asialoEPO versus rhEPO was assessed by repeated parenteral administration of equal doses of asialoEPO or rhEPO to BALB/c or C3H/hen female mice. Serum hemoglobin concentrations were determined by a hemoglobinometer using blood (<50 μl) withdrawn from the orbital sinus once weekly under isoflurane anesthesia. Animals were not iron supplemented.
Focal Ischemia Model. Ischemic stroke within the distribution of the middle cerebral artery (MCA) was produced as described (4). Briefly, under isoflurane anesthesia the ipsilateral carotid was ligated and the MCA was permanently occluded just distal to the bifurcation of the MCA into the rhinal artery. To produce a reversible ischemic event, the contralateral carotid was non-traumatically occluded for 90 min and then released. AsialoEPO was given intravenously with respect to the time of reperfusion at the dosages indicated in the text. The core temperature was maintained at 35–37°C until full recovery from anesthesia. Infarct volume was determined 24 h later by quantitative image analysis of tetrazolium salt-stained 1-mm slices of brain as described (4).
Spinal Cord Compression. Spinal cord compression was performed under controlled core temperature (35–37°C) using an aneurysm clip (53-g closing force) applied for 1 min at thoracic level 3, avoiding compression of the paravertebral arteries as described (6). Animals subsequently received either three daily doses of asialoEPO, rhEPO (both at 10 μg/kg of bw i.v.) or saline, and biweekly doses thereafter. Motor function of lesioned animals was evaluated in a blinded fashion by using the methodology of Basso and colleagues (38, 39). Three days after injury, five animals from each group were killed and the spinal cords were removed and fixed in formalin for histological analysis. Paraffin embedded tissue was sectioned (6 μm) to determine the extent of injury at the following five distinct levels: the lesion epicenter, 1 cm above and 1 cm below the epicenter, and the cervical and lumbar enlargements. Specimens were stained by using anti-NeuN (Chemicon, Temecula, CA), an indicator of living neurons (40). Surviving neurons within gray matter (i.e., histological characteristics of normal structures and presenting clear immunoreactivity for NeuN) were counted in each section. The results of neuronal counting in five randomly selected sections were averaged.
Sciatic Nerve Compression. Sprague–Dawley rats (250–350 g) were anesthetized by using isoflurane and their core temperature was maintained at 35–37°C. Electrophysiological function of the sciatic nerve was assessed by recording compound muscle action potentials (CMAPs) at supramaximal stimulation (3 Hz, duration of 0.1 msec) delivered by means of stainless steel electrodes at midthigh. Two gold-plated balls (2-mm diameter) 1 cm apart were used for recording with the anode at the belly of the gastrocnemius muscle and the cathode at midtendon 6 mm from the calcaneus. CMAPs were acquired over 64 stimulations and were then averaged for five independent placements of the recording electrode.
After recording of baseline CMAP, the left sciatic nerve was exposed at midthigh and an aneurysm clip (53-g compression) was placed around the sciatic nerve for 2 min. Either 24 h or 15 min before (pretreatment) or immediately after the release of compression (posttreatment), a single dose of asialoEPO, rhEPO (both at 50 μg/kg of bw), or saline was administered intravenously and the surgical incision was closed. After drug administration, the CMAP was redetermined and, thereafter, was obtained successively over the next several weeks.
Neurological function was performed in a blinded fashion by averaging three determinations of rear foot toe splaying (the sciatic static index) according to the methodology of Bervar (41). In this assay, 0 is normal and negative scores indicate motor weakness.
Data Analysis. Statistical analysis was performed using ANOVA or by Student's t test where appropriate. Comparison of motor function over time was accomplished by a repeated measures analysis. Counts of surviving neurons in the spinal cord sections were compared by the Kruskal–Wallis one-way ANOVA by ranks. In vitro experiments were repeated in at least three independent preparations with essentially similar results.
Results
In Vitro Characterization of AsialoEPO. The asialoEPO obtained by enzymatic conversion of rhEPO with sialidase contained <0.02 sialic acids per molecule and migrated as one homogeneous band at pI ≈8.5. In contrast, the isoforms of rhEPO migrated at pI 3.3–4.5 (sialic acid content typically 10–14 per molecule; data not shown). Bioactivity of the final reaction product was verified by proliferation assays in TF-1 (data not shown) and UT-7 cells (Table 1) where the compound was found to be equipotent with rhEpo. The binding affinity to pure EpoR-Fc constructs was also in a similar range as that of rhEpo (Table 1). Bioactivity in neuronal-like cells was assessed by using P19 and PC-12 cells, in which asialoEPO protected from serum (P19) or NGF (PC-12) withdrawal to a similar extent (Table 1).
Table 1. Comparison of the affinity, potency, and predominating plasma half-life of asialoEPO and rhEPO.
| Percent protection
|
Plasma half-life, h†
|
||||||
|---|---|---|---|---|---|---|---|
| IC50 for sFc-EPO-R* binding, pM | EC50 for proliferation of UT-7, pM | P-19 | PC12 | i.v. | i.p. | s.c. | |
| rhEPO | 10 | 20 ± 10 | 51 | 31 ± 7 | 5.6 | 7.0 | 5.4 |
| asialoEPO | 14 | 20 ± 10 | 43 | 34 ± 4 | 0.023 | 0.5 | 2.5 |
Soluble Fc-EPO-R fusion construct.
Plasma half-lives are terminal phase except for i.v. asialoEPO, which is initial phase.
Pharmacokinetics. Intravenously injected asialoEPO exhibited a predominant plasma half-life of 1.4 min (Table 1) and was below the lower limit of quantification in the systemic circulation within 1–2 h, in contrast to rhEpo with a plasma half-life of ≈5.6 h. Administration of asialoEPO by i.p. or s.c. routes gave rise to effective plasma half-lives of 0.5 and 2.5 h, respectively. AsialoEPO appeared promptly in CSF after i.v. or i.p. injection and concentrations sufficient to bind appreciably to the EpoR (0.5–30 pM range) were reached. The maximum CSF concentrations followed the plasma peak with a delay of 30–60 min, depending on the plasma kinetics profile of different routes of administration (data not shown). Brain tissue penetration of asialoEPO was followed by using radiolabeled compound. Histological localization of the radiolabel after injection of 125I-asialoEPO showed a specific neuronal pattern, e.g., in the hippocampus (see Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org), similar to the one observed with 125I-rhEpo (data not shown) or by immunohistochemical localization of the EpoR (14, 42, 43).
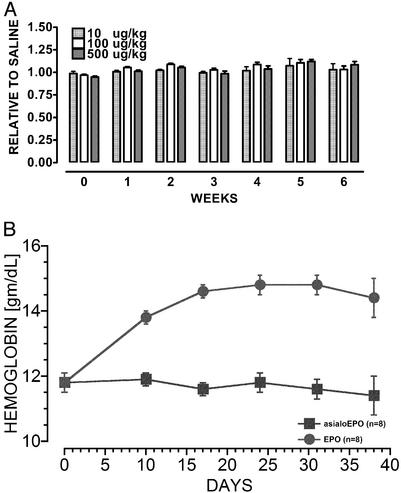
Effect of AsialoEPO on the Serum Hemoglobin Concentration. A wide range of asialoEPO dosage regimens was explored, none of which affected hemoglobin concentrations. For example, i.v. injection of asialoEPO from 10 to 500 μg/kg of bw every 3 days was equivalent to saline (Fig. 1A). In contrast, rhEPO exhibited a pronounced dose-dependent effect (data not shown). Because asialoEPO persists longer in the plasma when given i.p., this regimen was also tested for changes in hematocrit. Administration of asialoEPO (50 μg/kg of bw i.p.) twice weekly did not effect hemoglobin concentration (Fig. 1B). In contrast, equivalent doses of rhEPO raised the hemoglobin concentration as expected.
Fig. 1.
(A) Biweekly i.v. injections of asialoEPO over a wide dosage range do not change hemoglobin concentration in mice (n = 8 for each group; 50 μg/kg of bw and 200 μg/kg of bw omitted for clarity). (B) Biweekly i.p. administration of asialoEPO at a neuroprotective dose (100 μg/kg of bw) does not change the hemoglobin concentration. In contrast, an equal dose of rhEPO raises the hemoglobin concentration.
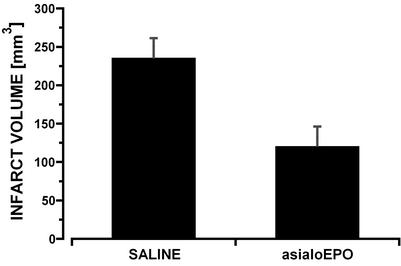
Focal Ischemia. In pilot experiments, asialoEPO administered as a single dose i.p. was equipotent with rhEPO in reducing damage in a MCAO model of cerebral ischemia in the range of 5–50 μg/kg of bw (data not shown). In further experiments, asialoEPO was then tested after i.v. administration, i.e., the protocol resulting in the shortest possible plasma half-life. AsialoEPO given as an i.v. bolus (44 μg/kg of bw) at the restoration of blood flow (i.e., 90 min after occlusion) reduced infarct volume by ≈50% (P < 0.01) at 24 h (Fig. 2), similar to rhEPO (data not shown). Thus, the presence of EPO-like activity in plasma for only a few minutes can elicit neuroprotection.
Fig. 2.
AsialoEPO administered i.v. versus saline reduces infarct size in a rat reperfusion model of focal stroke (n = 6 for each group; P < 0.01).
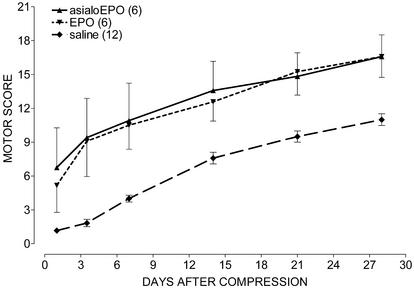
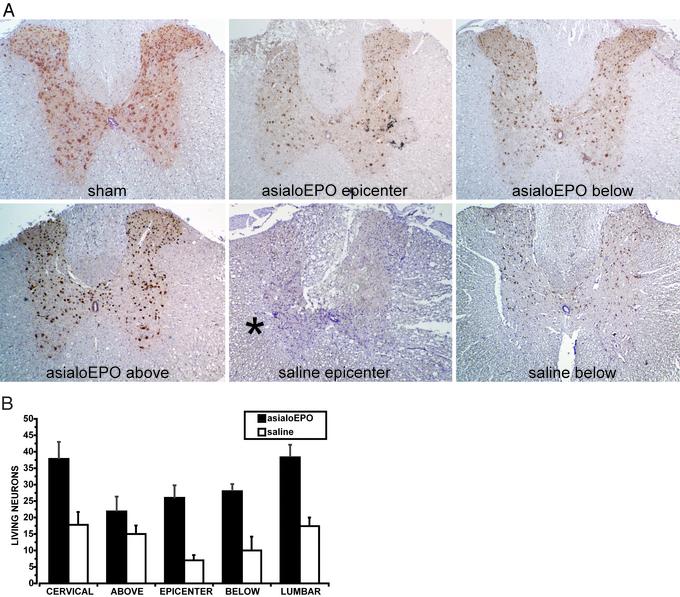
Spinal Cord Compression. Both asialoEPO and rhEPO administered (10 μg/kg of bw i.v.) for the first 3 days after spinal cord compression, and biweekly thereafter, were associated with an immediate and equivalent improvement of motor function in contrast to saline (Fig. 3; P < 0.001). The histological finding after 3 days differed strikingly between the groups. Notably, asialoEPO administration was associated with a restriction of injury to the epicenter alone, with nearly normal architecture both above and below (Fig. 4A). In contrast, the saline-treated group exhibited extensive cytoarchitectural disruption and edema (Fig. 4A) throughout the cord. The histological appearance of the white matter remained normal in asialoEPO-treated animals, consistent with their superior motor scores. Finally, the number of surviving (NeuN-positive) neurons was significantly higher in the asialoEPO-treated rats (P < 0.05) compared with the saline group (Fig. 4B).
Fig. 3.
AsialoEPO is as effective as rhEPO in restoring motor function after spinal cord compression. Agents were administered i.v. (10 μg/kg of bw) once daily for 3 days after compression and biweekly thereafter.
Fig. 4.
(A) Spinal cord histology 3 days after a 2-min compressive injury. NeuN immunohistochemistry identifies living neurons after asialoEPO (10 μg/kg i.v.) or saline administration immediately after injury. Sections were obtained at the lesion epicenter, 1 cm above, and 1 cm below. Note the extensive disruption of the central gray in the saline-treated cord (Lower Center). White matter (*) is also disrupted and edematous compared with animals receving asialoEPO. Shown are representative sections obtained from five animals in each group. (B) Summary of the mean living neurons number in the five levels investigated after asialoEPO, or saline treatment. Animals treated with asialoEPO presented with a significantly high number of living neurons compared with the saline group (P < 0.05 at all levels).
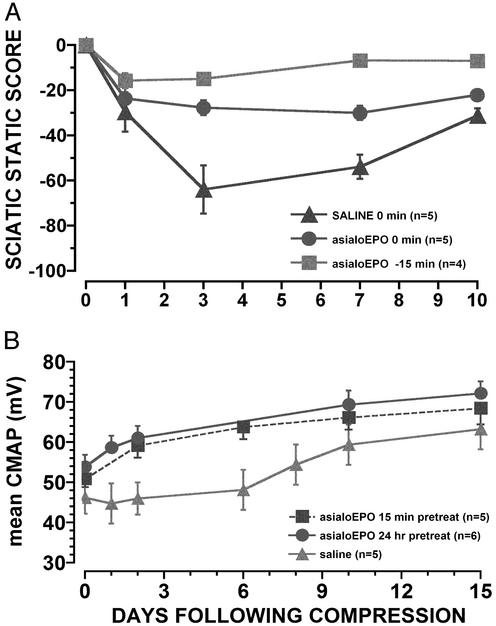
Sciatic Nerve Crush Model. Saline administration after sciatic nerve compression was associated with decreased motor function as assessed by the sciatic static index and CMAP amplitude, reaching a nadir 2–4 days after injury (Fig. 5) and normalizing by 3–4 weeks. AsialoEPO administered 15 min before or immediately after the release of compression reduced the degree of injury compared with saline control. (P < 0.01; Fig. 5A). Additional study was undertaken to define better the onset of neuroprotection. In one experiment, a single dose (50 μg/kg of bw) of asialoEPO or saline was administered 24 h or 15 min before nerve compression. The immediate postcompression CMAPs (day 0, Fig. 5B) recorded exhibited less functional loss (P < 0.05) for asialoEPO pretreatment (Fig. 5B). Similar observations were obtained with motor score testing, with the asialoEPO animals exhibiting significant improvement at the earliest time points evaluated (data not shown).
Fig. 5.
(A) Motor function after compressive injury of the sciatic nerve in the rat. AsialoEPO (50 μg/kg of bw i.v.) is more efficacious when administered 15 min before compression (P < 0.01; repeated measures analysis). (B) Average maximum CMAPs illustrate that 24-h or 15-min pretreatment with asialoEPO (50 μg/kg of bw i.v.) attenuates injury to an equivalent extent.
Discussion
Fully desialated rhEPO was prepared, and as reported (27, 28), disappeared very rapidly from the systemic circulation after i.v. injection. As anticipated, the affinity for EPO-R was similar to that of rhEPO when examined by using a variety of in vitro systems. Despite its short plasma half-life, asialoEPO appeared promptly within the CSF at concentrations within the in vitro neuroprotective range. In vivo autoradiography, performed by using sections obtained 4 h after administration of radioiodinated asialoEPO, confirmed that i.v.-administered drug actually reaches hippocampal and cortical neurons, typical cells expressing EPO-R in the normal brain. The anatomical pathway from the circulation across the blood–brain barrier to the labeled neurons visualized has not been established, but could involve the specialized glial cells that ensheath the capillaries (4).
Because of a short plasma survival of asialoEPO only a small proportion of the erythrocyte precursors are committed to enter the erythrocyte pool. Administration of asialoEPO, therefore, never increased hemoglobin concentration. Although it is inevitable that some erythrocyte precursors are recruited into the circulating pool, modulation of endogenous EPO concentrations (i.e., transient suppression) will tend to maintain red cell mass at the systemic set point. Although not directly assessed, similar kinetics for thrombocyte maturation (44–46) imply that asialoEPO would not appreciably increase the fraction of reactive platelets within the circulation.
Based on the observed transfer of asialoEPO into the brain, it is no surprise that asialoEPO is effective in the treatment of a variety of neurological injuries. The very brief duration of its action supports the concept of a triggering of neuroprotection, as has been suggested by in vitro models (26). The similar efficacy of asialoEPO and rhEPO observed in the stroke model confirm that only a very brief exposure is required to initiate a gene expression program (e.g., antiapoptosis) in vivo as well.
Longer followup periods of the stroke model will be necessary to determine whether late differences (i.e., other than effects on early apoptosis) exist and can be effected by additional exposure to asialoEPO. However, three consecutive daily doses of asialoEPO are as efficacious as rhEPO in the spinal cord compression model, because spinal cord injury occurs in distinct temporal phases that span several weeks (47, 48). The first is acute in onset (hours) and represents the immediate reaction to direct tissue injury, including cellular necrosis, hemorrhage, ischemia, and free radical formation. Histological evaluation shows that asialoEPO markedly attenuates this phase. Secondary injury occurs later (8–14 days) during which time axonal dysfunction becomes permanent because of the programmed cell death of oligodendrocytes. The observations reported here favor a model wherein primary and secondary injury are causally related. Thus, modulation of the primary injury phase profoundly affects the secondary phase, without need for additional therapy. Further experiments will be necessary to determine whether a later administration of asialoEPO (such as in a multiple dosing regimen) further improves motor recovery, such as might happen if asialoEPO is also effective at preventing the delayed oligodendrocyte apoptosis.
The sciatic nerve model also supports the relevance of a triggering mechanism by EPO. However, the immediate protective effect of treatment in the sciatic neuropathy model shown by the 15-min pretreatment protocol cannot be explained by an effect on gene expression, but may reflect maintenance of vascular autoregulation, as has been reported for experimental subarachnoid hemorrhage (40, 49). Further, an immediate difference in function for asialoEPO-treated animals despite disruption of axoplasm suggests that secondary causes, such as edema formation, are also likely reduced. Further study is needed to evaluate these possibilities. It has been reported that the sciatic nerve expresses very high levels of EPO-R (50). Since EPO-R expression is up-regulated after injury (50), postinjury treatment paradigms may yield additional interesting results.
Identification of a tissue-protective erythropoietin derivative with a brief plasma half-life potentially offers important advantages over rhEPO and would allow for multiple or chronic dosing strategies in neurodegenerative and other diseases. Another approach is to develop molecules that differ in mediating classical erythropoietic functions as compared with tissue-protective actions. Efforts to produce and validate candidate drugs that would constitute initial members of such a class of tissue-protective therapeutics may yield interesting results.
Supplementary Material
Acknowledgments
We thank Deborah Diaz and Daniel Gomez for providing invaluable technical assistance in the performance of these studies.
Abbreviations: EPO, erythropoietin; rhEPO, recombinant human EPO; EPO-R, EPO receptor; CSF, cerebrospinal fluid; bw, body weight; CMAP, compound muscle action potential.
References
- 1.Ruscher, K., Freyer, D., Karsch, M., Isaev, N., Megow, D., Sawitzki, B., Priller, J., Dirnagl, U. & Meisel, A. (2002) J. Neurosci. 22, 10291-10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimm, C., Wenzel, A., Groszer, M., Mayser, H., Seeliger, M., Samardzija, M., Bauer, C., Gassmann, M. & Reme, C. E. (2002) Nat. Med. 8, 718-724. [DOI] [PubMed] [Google Scholar]
- 3.Dawson, T. M. (2002) Lancet 359, 96-97. [DOI] [PubMed] [Google Scholar]
- 4.Brines, M. L., Ghezzi, P., Keenan, S., Agnello, D., de Lanerolle, N. C., Cerami, C., Itri, L. M. & Cerami, A. (2000) Proc. Natl. Acad. Sci. USA 97, 10526-10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celik, M., Gokmen, N., Erbayraktar, S., Akhisaroglu, M., Konakc, S., Ulukus, C., Genc, S., Genc, K., Sagiroglu, E., Cerami, A. & Brines, M. (2002) Proc. Natl. Acad. Sci. USA 99, 2258-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorio, A., Gokmen, N., Erbayraktar, S., Yilmaz, O., Madaschi, L., Cichetti, C., Di Giulio, A. M., Vardar, E., Cerami, A. & Brines, M. (2002) Proc. Natl. Acad. Sci. USA 99, 9450-9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Junk, A., Mammis, A., Savitz, S. I., Singh, M., Roth, S., Malhotra, S., Rosenbaum, P., Cerami, A., Brines, M. & Rosenbaum, D. (2002) Proc. Natl. Acad. Sci. USA 99, 10659-10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvillo, L., Latini, R., Kajstura, J., Leri, A., Anversa, P., Ghezzi, P., Cerami, A. & Brines, M. (2003) Proc. Natl. Acad. Sci. USA 100, 4802-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawakami, M., Sekiguchi, M., Sato, K., Kozaki, S. & Takahashi, M. (2001) J. Biol. Chem. 276, 39469-39475. [DOI] [PubMed] [Google Scholar]
- 10.Digicaylioglu, M. & Lipton, S. A. (2001) Nature 412, 641-647. [DOI] [PubMed] [Google Scholar]
- 11.Calapai, G., Marciano, M. C., Corica, F., Allegra, A., Parisi, A., Frisina, N., Caputi, A. P. & Buemi, M. (2000) Eur. J. Pharmacol. 401, 349-356. [DOI] [PubMed] [Google Scholar]
- 12.Squadrito, F., Altavilla, D., Squadrito, G., Campo, G. M., Arlotta, M., Quartarone, C., Saitta, A. & Caputi, A. P. (1999) Br. J. Pharmacol. 127, 482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasso, G. (2001) J. Neurosurg. Sci. 45, 7-14. [PubMed] [Google Scholar]
- 14.Juul, S. E., Anderson, D. K., Li, Y. & Christensen, R. D. (1998) Pediatr. Res. 43, 40-49. [DOI] [PubMed] [Google Scholar]
- 15.Siren, A. L., Fratelli, M., Brines, M., Goemans, C., Casagrande, S., Lewczuk, P., Keenan, S., Gleiter, C., Pasquali, C., Capobianco, A., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 4044-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agnello, D., Bigini, P., Villa, P., Mennini, T., Cerami, A., Brines, M. & Ghezzi, P. (2002) Brain Res. 952, 128-134. [DOI] [PubMed] [Google Scholar]
- 17.Shingo, T., Sorokan, S. T., Shimazaki, T. & Weiss, S. (2001) J. Neurosci. 21, 9733-9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehrenreich, H., Hasselblatt, M., Dembowski, C., Cepek, L., Lewczuk, P., Stiefel, M., Rustenbeck, H. H., Breiter, N., Jacob, S., Knerlich, F., et al. (2002) Mol. Med. 8, 495-505. [PMC free article] [PubMed] [Google Scholar]
- 19.Bernaudin, M., Marti, H. H., Roussel, S., Divoux, D., Nouvelot, A., MacKenzie, E. T. & Petit, E. (1999) J. Cereb. Blood Flow Metab. 19, 643-651. [DOI] [PubMed] [Google Scholar]
- 20.Wiessner, C., Allegrini, P. R., Ekatodramis, D., Jewell, U. R., Stallmach, T. & Gassmann, M. (2001) J. Cereb. Blood Flow Metab. 21, 857-864. [DOI] [PubMed] [Google Scholar]
- 21.Wolf, R. F., Peng, J., Friese, P., Gilmore, L. S., Burstein, S. A. & Dale, G. L. (1997) Thromb. Haemostasis 78, 1505-1509. [PubMed] [Google Scholar]
- 22.Wolf, R. F., Gilmore, L. S., Friese, P., Downs, T., Burstein, S. A. & Dale, G. L. (1997) Thromb. Haemostasis 77, 1020-1024. [PubMed] [Google Scholar]
- 23.Stohlawetz, P. J., Dzirlo, L., Hergovich, N., Lackner, E., Mensik, C., Eichler, H. G., Kabrna, E., Geissler, K. & Jilma, B. (2000) Blood 95, 2983-2989. [PubMed] [Google Scholar]
- 24.Beguin, Y. (1999) Haematologica 84, 541-547. [PubMed] [Google Scholar]
- 25.Fisher, J. W. (2003) Exp. Biol. Med. (Maywood) 228, 1-14. [DOI] [PubMed] [Google Scholar]
- 26.Morishita, E., Masuda, S., Nagao, M., Yasuda, Y. & Sasaki, R. (1997) Neuroscience 76, 105-116. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda, M. N., Sasaki, H., Lopez, L. & Fukuda, M. (1989) Blood 73, 84-89. [PubMed] [Google Scholar]
- 28.Imai, N., Higuchi, M., Kawamura, A., Tomonoh, K., Oh-Eda, M., Fujiwara, M., Shimonaka, Y. & Ochi, N. (1990) Eur. J. Biochem. 194, 457-462. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen, L. O., Stryhn, A., Holter, T. L., Etzerodt, M., Gerwien, J., Nissen, M. H., Thogersen, H. C. & Buus, S. (1995) Eur. J. Immunol. 25, 1609-1616. [DOI] [PubMed] [Google Scholar]
- 30.Komatsu, N., Nakauchi, H., Miwa, A., Ishihara, T., Eguchi, M., Moroi, M., Okada, M., Sato, Y., Wada, H., Yawata, Y., et al. (1991) Cancer Res. 51, 341-348. [PubMed] [Google Scholar]
- 31.Leveque, E., Nagel, M. D. & Haye, B. (1996) Hematol. Oncol. 14, 137-146. [DOI] [PubMed] [Google Scholar]
- 32.Koshimura, K., Murakami, Y., Sohmiya, M., Tanaka, J. & Kato, Y. (1999) J. Neurochem. 72, 2565-2572. [DOI] [PubMed] [Google Scholar]
- 33.European Economic Council Directive 86/609 (12December1987) in Official Journal of Law, p. 358.
- 34.Law by Decree No. 116 (18February1992) Gazzetta Ufficiale della Repubblica Italiana, Suppl. 40.
- 35.Law by Circular No. 8 (14July1994) Gazzetta Ufficiale della Repubblica Italiana, Suppl. 40.
- 36.Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl. Acad. Press, Washington, DC).
- 37.Frankmann, S. P. (1986) Physiol. Behav. 37, 489-493. [DOI] [PubMed] [Google Scholar]
- 38.Basso, D. M., Beattie, M. S. & Bresnahan, J. C. (1996) Exp. Neurol. 139, 244-256. [DOI] [PubMed] [Google Scholar]
- 39.Basso, D. M., Beattie, M. S. & Bresnahan, J. C. (1995) J. Neurotrauma 12, 1-21. [DOI] [PubMed] [Google Scholar]
- 40.Grasso, G., Buemi, M., Alafaci, C., Sfacteria, A., Passalacqua, M., Sturiale, A., Calapai, G., De Vico, G., Piedimonte, G., Salpietro, F. M. & Tomasello, F. (2002) Proc. Natl. Acad. Sci. USA 99, 5627-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bervar, M. (2000) J. Neurosci. Methods 102, 109-116. [DOI] [PubMed] [Google Scholar]
- 42.Nagai, A., Nakagawa, E., Choi, H. B., Hatori, K., Kobayashi, S. & Kim, S. U. (2001) J. Neuropathol. Exp. Neurol. 60, 386-392. [DOI] [PubMed] [Google Scholar]
- 43.Siren, A. L., Knerlich, F., Poser, W., Gleiter, C. H., Bruck, W. & Ehrenreich, H. (2001) Acta Neuropathol. 101, 271-276. [DOI] [PubMed] [Google Scholar]
- 44.Tange, T. & Miyazaki, H. (1996) Pathol. Int. 46, 968-976. [DOI] [PubMed] [Google Scholar]
- 45.Eaton, D. L. & de Sauvage, F. J. (1995) Curr. Opin. Hematol. 2, 167-171. [DOI] [PubMed] [Google Scholar]
- 46.Li, J. & Kuter, D. J. (2001) Int. J. Hematol. 74, 365-374. [DOI] [PubMed] [Google Scholar]
- 47.Liu, X. Z., Xu, X. M., Hu, R., Du, C., Zhang, S. X., McDonald, J. W., Dong, H. X., Wu, Y. J., Fan, G. S., Jacquin, M. F., et al. (1997) J. Neurosci. 17, 5395-5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bethea, J. R., Castro, M., Keane, R. W., Lee, T. T., Dietrich, W. D. & Yezierski, R. P. (1998) J. Neurosci. 18, 3251-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Springborg, J. B., Ma, X., Rochat, P., Knudsen, G. M., Amtorp, O., Paulson, O. B., Juhler, M. & Olsen, N. V. (2002) Br. J. Pharmacol. 135, 823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campana, W. M. & Myers, R. R. (2001) FASEB J. 15, 1804-1806. [DOI] [PubMed] [Google Scholar]
- 51.European Pharmocopoeia (2000) in 2001 Supplement (Council of Europe, Strasbourg, France), pp. 277-281.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.