Abstract
Hypoxic–ischemic brain injury in premature infants results in cerebral white matter lesions with prominent oligodendroglial injury and loss, a disorder termed periventricular leukomalacia (PVL). We have previously shown that glutamate receptors mediate hypoxic–ischemic injury to oligodendroglial precursor cells (OPCs) in a model of PVL in the developing rodent brain. We used primary OPC cultures to examine the mechanism of cellular toxicity induced by oxygen–glucose deprivation (OGD) to simulate brain ischemia. OPCs were more sensitive to OGD-induced toxicity than mature oligodendrocytes, and OPC toxicity was attenuated by nonselective [2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX), 6-cyano-7-nitroquinoxaline-2,3-dione], α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-preferring (GYKI 52466), kainate-preferring (γ-d-glutamylaminomethanesulfonic acid), or Ca2+-permeable AMPA/kainate receptor antagonists (joro spider toxin, JSTx) administered either during or after OGD. Furthermore, NBQX or JSTx blocked OGD-induced Ca2+ influx. Relevant to recurrent hypoxic–ischemic insults in developing white matter, we examined the effects of sublethal OGD preconditioning. A prior exposure of OPCs to sublethal OGD resulted in enhanced vulnerability to subsequent excitotoxic or OGD-induced injury associated with an increased Ca2+ influx. AMPA/kainate receptor blockade with NBQX or JSTx either during or after sublethal OGD prevented its priming effect. Furthermore, OGD preconditioning resulted in a down-regulation of the AMPA receptor subunit GluR2 on cell surface that increased Ca2+ permeability of the receptors. Overall, these data suggest that aberrantly enhanced activation of Ca2+-permeable AMPA/kainate receptors may be a major mechanism in acute and repeated hypoxic–ischemic injury to OPCs in disorders of developing cerebral white matter, such as PVL.
Hypoxic-ischemic insults to the premature infant, particularly during the period from 24 to 32 weeks postconception, result in prominent cerebral white matter injury and permanent areas of hypomyelination, a disorder termed periventricular leukomalacia (PVL). PVL is a predominant age-specific form of brain injury in the premature infant and is the leading cause of subsequent neurological disability, such as spastic motor deficits (cerebral palsy) and cognitive deficits (1). The incidence of low birth weight infants is increasing because of improved survival rates of premature babies, yet currently no specific treatment exists for this disorder. The neuropathological hallmarks of PVL are focal and diffuse white matter lesions with prominent oligodendrocyte (OL) injury and loss (1).
During the developmental period of greatest risk for PVL, human cerebral white matter is populated primarily by OL precursor cells (OPCs) (2). OPCs have been well characterized both in vitro and in vivo (3–6). These cells express non-N-methyl-d-aspartate (NMDA) glutamate receptors, including α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors, but not functional NMDA receptors (7). Glutamate agonists or oxygen–glucose deprivation (OGD) induce excitotoxic injury to differentiated OLs (8–10), immature OLs (11), and OL cell lines (12–14) in vitro, and AMPA/kainate receptor blockade is protective against hypoxic–ischemic white matter injury in the developing rat brain (15).
The occurrence of hypoxic–ischemic insults in human premature infants is usually unpredictable, often making pretreatment less feasible. The efficacy of specific AMPA/kainate antagonists as a posttreatment strategy for PVL, however, remains unclear. Previous studies have focused only on a monophasic insult to OLs, yet repeated episodes of hypoxic–ischemic injury commonly occur clinically in human premature infants (1). OLs that have survived one ischemic episode may be exposed to a subsequent insult. In neurons, hypoxic–ischemic preconditioning is known to afford protection against subsequent, more severe insults (16, 17). However, the consequences and implications of sublethal insults to OLs are unknown, as is the involvement of AMPA/kainate receptors.
Materials and Methods
Cell Culture, Pharmacological Treatment, Oxygen-Glucose Deprivation, and Cell Viability/Differentiation/Proliferation Assays. Primary OPCs were isolated from mixed glial cultures of newborn Sprague–Dawley rat forebrains by using a selective detachment procedure (18, 19), further purified by differential adhesion (20), and maintained in a chemically defined medium (19, 21). Unless otherwise indicated, pharmacological agents were applied 10 min before exposure of the cells to kainate or OGD. Cell death was quantitatively assessed by using a trypan blue exclusion method (22) or by measuring the extent of lactate dehydrogenase release (13, 14). Cell lineage progression was monitored immunocytochemically with stage-specific OL markers: A2B5, O4, O1, and myelin basic protein (MBP) (20). Cell proliferation was measured by determining the incorporation of BrdUrd into replicating DNA of cells. See Supporting Materials and Methods, which is published as supporting information on the PNAS web site, www.pnas.org.
45Ca2+ Uptake. Cultures were incubated with 45CaCl2 (8 μCi/ml; 1 Ci = 37 GBq) at room temperature for 10 min, then washed with Hanks' balanced salts solution (HBSS) and lysed with 1% Triton X-100 in HBSS. Radioactivity in the whole lysate was counted by liquid scintillation.
Biochemical Measurements of Surface Expressed Receptors. Biotinylation of cell-surface protein in combination with immunoblotting of total versus surface receptors were performed as described in ref. 23 and in Supporting Materials and Methods.
Data Analysis. All data represent the mean ± SEM of at least three separate experiments, and all assays were performed in triplicate. Statistical differences were assessed by one-way analysis of variance with Tukey post hoc analysis for multiple comparisons. Student's t test was used when only two independent groups were compared.
Results
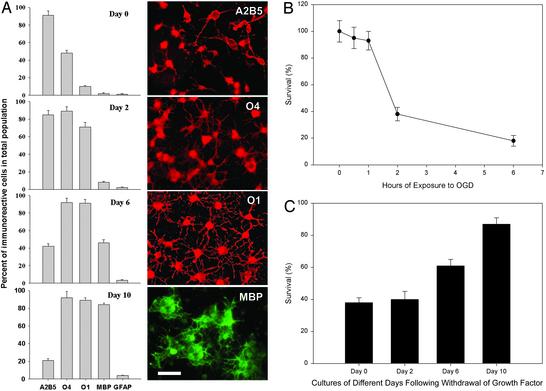
Oligodendroglial Sensitivity to OGD-induced Toxicity in Vitro Is Developmentally Regulated. Immunocytochemical characterization and representative cell images of stage-specific OL cultures are shown in Fig. 1A. We first examined OGD-induced toxicity to these cultures. The time course of vulnerability of OPCs (undifferentiated cultures) showed that 2-h OGD exposure killed ≈60% of the cells by 24 h (Fig. 1B), and thus this duration of OGD was used for further studies. Stage-specific cultures were obtained by allowing the OPC cultures to differentiate for 0, 2, 6, or 10 days. Survival assays at 24 h after a 2-h OGD exposure revealed that OPCs and later-stage precursors were highly vulnerable, whereas more mature OLs were relatively resistant to cell death (Fig. 1C). Because OPC cultures exhibited the greatest sensitivity to OGD toxicity, cultures of this stage were used for subsequent experiments detailed below.
Fig. 1.
Immunochemical characterization and response to OGD-induced toxicity of OL lineage cells. (A) Stage-specific cultures. Cultures were allowed to differentiate for 0, 2, 6, or 10 days and stained for the OL stage-specific markers A2B5, O4, O1, and MBP, as well as the astrocytic marker GFAP. Percent of immunolabeled cells in the total population and characteristic morphology for each stage are shown. (Scale bar = 50 μm.) (B) Effect of OGD duration on OPC death. Day 0 cultures were subjected to OGD for 0, 0.5, 1, 2, or 6 h, and cell survival was determined at 24 h. (C) Differential sensitivity of OL lineage cells at various maturational stages to OGD-induced death. Stage-specific cultures were exposed to OGD for 2 h, and cell survival was assayed at 24 h.
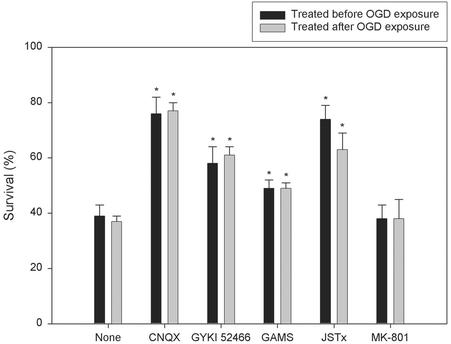
OPC Death Is Attenuated by AMPA-Preferring, Kainate-Preferring, and Ca2+-Permeable AMPA/Kainate Receptor Antagonists Administered Either During or After OGD. Previous studies established that OGD causes excitotoxic injury to OLs (11, 14). To validate our model, we first examined whether the AMPA/kainate receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX) could attenuate OGD-induced death in primary OPC cultures. NBQX dramatically attenuated OPC death induced by OGD (2 h) or kainate (300 μM), an excitotoxin that activates both AMPA and kainate receptors (24), in a dose-dependent manner (EC50 = 9.26 ± 1.6 and 12.3 ± 1.8 μM, respectively). NBQX (100 μM) achieved 80 ± 7% cell survival in OGD-induced death and completely blocked cell death induced by kainate (300 μM) that caused 90 ± 6% OPC death during 24 h (data not shown). We further examined the effect of the nonselective AMPA/kainate [6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) 100 μM], AMPA-preferring (GYKI 52466, 100 μM), kainate-preferring [γ-D-glutamylaminomethanesulfonic acid (GAMS), 500 μM], and Ca2+-permeable AMPA/kainate [joro spider toxin (JSTx) 1 μM] receptor antagonists on OGD-induced OPC death (Fig. 2). The NMDA receptor blocker MK-801 (10 μM) was used as a negative control. The receptor antagonists were applied either 10 min before or immediately after a 2-h OGD exposure and remained in culture medium until survival assays at 24 h. In both the pre- and post-OGD treatment paradigms, CNQX and JSTx markedly increased cell survival, whereas GYKI 52466 and GAMS each partially protected OPCs from OGD-induced cell death (Fig. 2).
Fig. 2.
Effect of glutamate receptor antagonists on OGD-induced OPC death. Cultures were either not treated (None) or given CNQX (100 μM), GYKI 52466 (100 μM), GAMS (500 μM), JSTx (1 μM), or MK-801 (10 μM) either 10 min before (dark bar) or immediately after (light bar) exposure to OGD for 2 h. *, P < 0.05 vs. the OGD-only control (None).
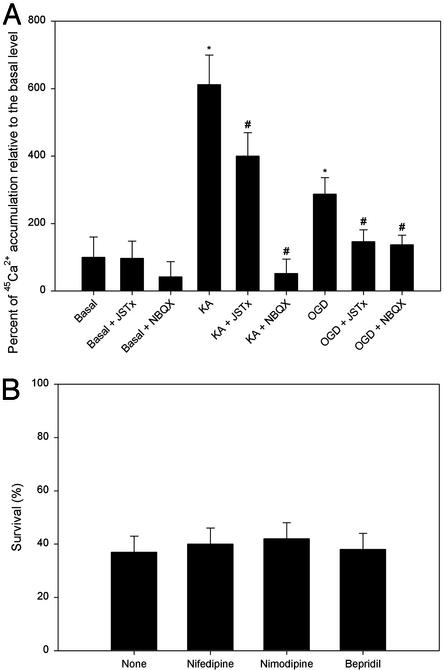
Exposure of OPCs to OGD Causes Ca2+ Influx in a JSTx-Sensitive Manner. The protective effect of JSTx implicates Ca2+-permeable AMPA/kainate receptors in OGD-induced OPC death. We then determined Ca2+ influx by directly measuring 45Ca2+ uptake. Exposure of OPCs to kainate (300 μM) for 10 min or OGD for 2 h resulted in robust Ca2+ entry compared with the basal level (Fig. 3A), where no cell loss was yet apparent (data not shown). NBQX fully blocked and JSTx partially prevented kainate-induced Ca2+ influx, whereas both JSTx and NBQX markedly prevented OGD-induced Ca2+ uptake (Fig. 3A). NBQX (100 μM) and JSTx (1 μM) applied together did not significantly differ from NBQX (100 μM) alone (data not shown).
Fig. 3.
OGD or kainate elicits Ca2+ entry into OPCs via Ca2+-permeable AMPA/kainate receptors on these cells. (A) Effect of JSTx and NBQX on kainate- or OGD-evoked Ca2+ influx in OPCs. Cultures were either not treated or given JSTx (1 μM) or NBQX (100 μM), together with 45CaCl2 (8 μCi/ml), before adding kainate (KA, 300 μM) or immediately after exposure to OGD for 2 h. 45Ca2+ uptake was measured in 10 min. *, P < 0.001 vs. the basal level; ♯, P < 0.001 vs. the absence of JSTx or NBQX. (B) Effect of nifedipine, nimodipine, and bepridil on OGD-induced OPC death. Cultures were either not treated (None) or given nifedipine (50 μM), nimodipine (10 μM), or bepridil (100 μM) before exposure to OGD for 2 h. No significant difference was detected between the treated and untreated groups (None) (P > 0.05).
In addition to glutamate receptors, other potential routes of Ca2+ entry include voltage-gated Ca2+ channels and the Na+–Ca2+ exchanger on the cell membrane (25). However, neither voltage-sensitive Ca2+ channel blockers nifedipine (50 μM) and nimodipine (10 μM) nor an inhibitor for the Na+–Ca2+ exchange bepridil (100 μM) protected against OGD-induced OPC toxicity (Fig. 3B).
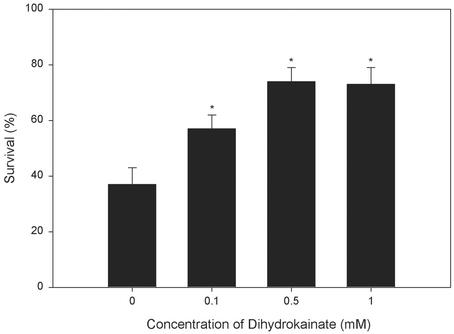
Dihydrokainate (DKA) Protects OPCs from OGD-Induced Death. Glutamate release by the reversal of glutamate transporters can result in rapid ischemic death of immature OLs (11). Similarly, DKA (0.1–1 mM), a specific inhibitor of glutamate transport (26), markedly prevented cell death elicited by OGD (Fig. 4). These concentrations of DKA did not significantly affect kainate-evoked Ca2+ influx in OPCs as measured by 45Ca2+ uptake (data not shown), excluding the possibility that DKA might directly block glutamate receptors.
Fig. 4.
Effect of dihydrokainate on OGD-induced OPC death. Cultures were either not treated or given dihydrokainic acid (0.1, 0.5, or 1 mM) before exposure to OGD for 2 h. *, P < 0.05 vs. the OGD-only control (zero concentration).
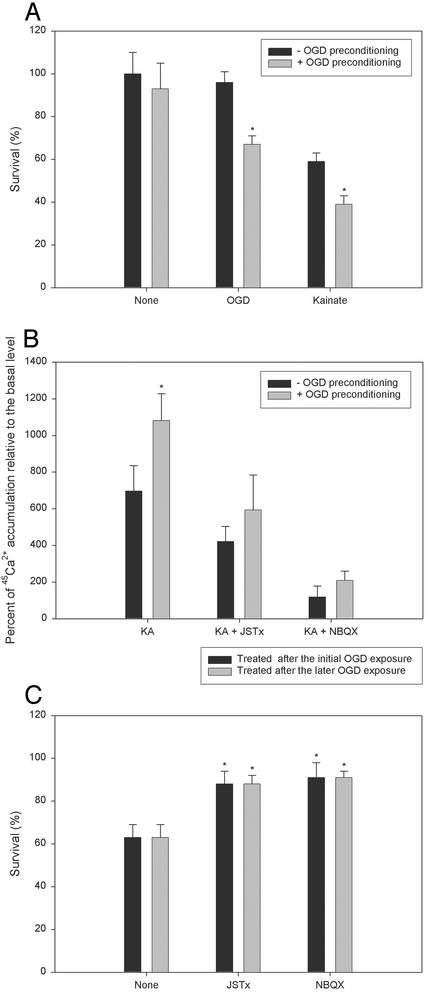
Pre- or Posttreatment with NBQX or JSTx Blocks Increased OPC Vulnerability to Subsequent Ca2+-Mediated Excitotoxicity After Sublethal OGD Preconditioning. A model of sublethal OGD preconditioning of OPCs was used to simulate repeated hypoxia-ischemia. Cultures were exposed to 1-h OGD preconditioning and 24 h later were re-exposed to either OGD for 1 h or kainate (50 μM). OGD for 1 h alone did not result in apparent OPC loss at 24 h (Fig. 1B). However, OPCs subjected to this sublethal OGD preconditioning exposure were significantly more vulnerable to subsequent OGD- or kainate-induced cell death than were naïve cultures (Fig. 5A).
Fig. 5.
Sublethal preexposure of OPCs to OGD enhances the tendency for cell death in subsequent OGD or excitotoxic exposure. (A) Increased vulnerability of OPCs after sublethal OGD preconditioning to subsequent OGD- or kainate-induced death. Cultures were either not treated (dark bar) or subjected to 1-h OGD preconditioning exposure (light bar), and 24 h later reexposed to OGD (1 h) or treated with kainate (50 μM), with survival assessed after 24 h. *, P < 0.001 between the presence and absence of OGD preconditioning. (B) Effect of sublethal OGD preconditioning on kainate (KA)-evoked Ca2+ influx in OPCs. Cultures were either not treated (dark bar) or subjected to 1-h OGD preconditioning exposure (light bar), and 24 h later were either remained untreated or were given JSTx (1 μM) or NBQX (100 μM), together with 45CaCl2 (8 μCi/ml), before adding kainate (300 μM). 45Ca2+ uptake was measured in 10 min. *, P < 0.001 between the presence and absence of OGD preconditioning. (C) Effect of JSTx and NBQX on sublethal OGD preconditioning-promoted OPC death. Cultures were subjected to 1-h OGD preconditioning exposure, and 24 h later were reexposed to OGD for 1 h. JSTx (1 μM) or NBQX (100 μM) was added into the culture medium either after the initial OGD exposure for 22 h and was removed 2 h before the later OGD exposure (dark bar) or after the later OGD exposure and was remained in the medium (light bar). *, P < 0.001 vs. the OGD preconditioned culture absent of JSTx or NBQX (None).
Kainate-evoked Ca2+ uptake was measured 24 h following OGD (1 h) preconditioning to determine whether the enhanced vulnerability of OPCs by preconditioning was associated with an increase in Ca2+ influx. Ca2+ entry was significantly greater in preconditioned cultures compared with controls, and this enhanced Ca2+ influx was attenuated by JSTx or NBQX (Fig. 5B).
To determine whether the priming effect of sublethal OGD was mediated by AMPA/kainate receptors, we applied JSTx (1 μM) or NBQX (100 μM) (i) after the initial sublethal OGD exposure for 22 h but removed the agents 2 h before the second OGD exposure, or (ii) after the second OGD exposure. In both of the experimental paradigms, JSTx or NBQX markedly protected the cells at 24 h after the second OGD exposure (Fig. 5C). To control for a possible effect of residual antagonist remaining after wash before the second OGD insult in the first paradigm, JSTx (1 μM) or NBQX (100 μM) application was delayed to 20 h after the initial OGD exposure for 2 h and then removed 2 h before the later OGD exposure, and no protective effect against the sublethal OGD-promoted death was observed (data not shown). Additionally, sister cultures were treated with JSTx (1 μM) or NBQX (100 μM) for 22 h and then washed away 2 h before exposure of the cells to OGD for 2 h. Such prior exposure of cultures to JSTx or NBQX followed by a 2 h wash had no protective effect on OGD (2 h)-induced cell death (data not shown). These data indicated that the protective effect of AMPA/kainate receptor blockade by JSTx and NBQX in the first paradigm was caused by the abolishment of the sublethal OGD priming effect rather than the residual effect of the antagonists on the second OGD exposure.
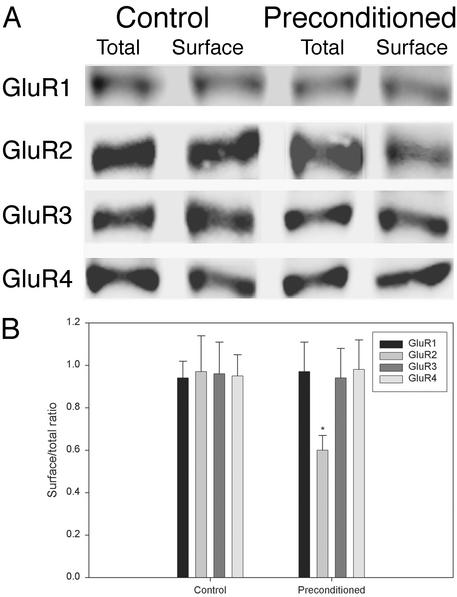
Sublethal OGD Preconditioning of OPCs Down-Regulates the GluR2 Subunit of AMPA Receptors on Cell Surface. AMPA receptors are heteromeric and formed by GluR1–4 subunits, and the abundance of GluR2 inversely correlates with Ca2+ permeability of the receptors (25, 27). To determine whether the priming effect was associated with a change of the receptor composition, we measured GluR1–4 subunit protein expression in control versus preconditioned (1 h) cultures at 24 h after OGD. Immunoblots (Fig. 6A) and quantification of surface/total receptors (Fig. 6B) revealed that surface GluR2 was significantly decreased in preconditioned cultures compared with nonconditioned controls.
Fig. 6.
Sublethal preexposure of OPCs to OGD down-regulates the GluR2 subunit of AMPA receptors on cell surface. (A) Representative immunoblots of total versus surface expressed GluR1–4 in control and preconditioned (1 h) cultures at 24 h. (B) Surface/total ratios calculated from densitometric quantification of the immunoreactive bands. *, P < 0.001 vs. control.
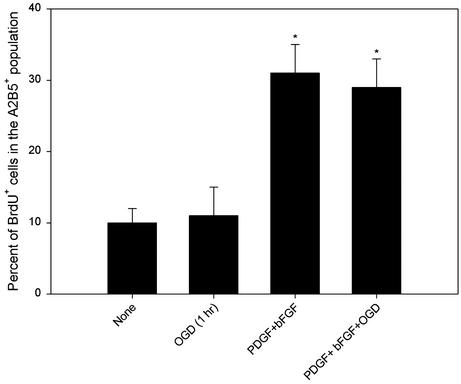
Sublethal OGD Preconditioning Does Not Affect OPC Proliferation and Differentiation. Because altered proliferation or differentiation of OPCs may confound survival assays, we evaluated whether the priming effect of exposure to sublethal OGD on subsequent susceptibility to excitotoxicity involved an effect on cell proliferation or differentiation. First, cells were double-immunostained with anti-BrdUrd and the OL precursor marker A2B5, and the percent of BrdUrd+ cells in A2B5+ populations was determined. OPCs had a very limited cell-intrinsic proliferative capability in the absence of appropriate mitogens, whereas the growth factors, PDGF and bFGF (each at 10 ng/ml), dramatically enhanced cell proliferation by ≈3-fold (Fig. 7). Sublethal OGD exposure did not alter either the basal or growth factor-stimulated BrdUrd incorporation (Fig. 7). Nevertheless, the survival assays in the present study were performed in the basal defined medium without supplemental growth factors.
Fig. 7.
BrdUrd incorporation in OPCs. Cultures were either not treated, given PDGF+bFGF (each at 10 ng/ml), and/or subjected to 1-h sublethal OGD preconditioning, and 22 h later BrdUrd (10 μM) was added to the culture medium 2 h before termination of experiment. Quantitative analysis was performed by determining the percent of BrdUrd+ cells in the A2B5+ population. *, P < 0.001 vs. control (None).
Second, we assessed the differentiation potential of the cultures with or without sublethal OGD exposure. No differences were seen between the two conditions when cell morphology and the sequential emergence of a panel of stage-specific OL markers, A2B5, O4, O1, and MBP, were measured after 0, 2, 6, and 10 days of cellular maturation (data not shown). Thus, neither the proliferation nor the differentiation of the cultures following sublethal OGD exposure differed from nonconditioned controls. In addition, none of the pharmacological agents used in the present study affected cell proliferation or differentiation under the cell survival assay conditions used in this study (data not shown). We thus concluded that neither cell proliferation nor differentiation was likely to be a confounding variable in the survival assays performed in the present study.
Discussion
Oligodendroglia express functional AMPA/kainate receptors and are highly vulnerable to excitotoxic or hypoxic–ischemic injury mediated by excesive activation of these receptors (8, 9, 11–15, 19, 28, 29, 31), but not NMDA receptors that are important in neuronal excitotoxicity (7). The present study implicates a direct role for JSTx-sensitive Ca2+-permeable AMPA/kainate receptors in hypoxic–ischemic OPC injury. The efficacy of posttreatment with AMPA/kainate receptor antagonists, including JSTx, suggests that later injury results from delayed events downstream from Ca2+-permeable AMPA/kainate receptors. Another novel finding of this study is that sublethal OGD preconditioning of OPC cultures enhances vulnerability to subsequent excitotoxic Ca2+ influx and cell injury, and this priming effect can be prevented by AMPA/kainate receptor antagonists applied either during or after sublethal OGD. Furthermore, the priming effect appears to be mediated at least in part by a down-regulation of cell-surface GluR2 in preconditioned cultures that could increase AMPA receptor Ca2+ permeability after sublethal OGD. Thus, intervention of Ca2+permeable AMPA/kainate receptors and downstream signaling events may represent a therapeutic strategy with clinical potential for acute and recurrent hypoxic–ischemic injury to OPCs in disorders such as PVL.
Maturational Dependency of Oligodendroglial Susceptibility to OGD. Our experiments using defined, stage-specific cultures demonstrate that the vulnerability of OL lineage cells to OGD-induced death is maturation-dependent and that precursor cells are highly vulnerable. Immature OLs are significantly more vulnerable to OGD (11), oxidative stress (20), and glutamate excitotoxicity (19) in vitro than are MBP-expressing mature OLs. The enhanced susceptibility of these cells compared with more mature OLs may be due in part to a developmental lack of antioxidant enzymes to control oxidative damage (32), an overexpression of non-NMDA glutamate receptors that mediate the excitotoxicity on developing OLs (19), and a transiently increased Ca2+ signaling during OL development (33). In addition, we have previously demonstrated a relative up-regulation of glutamate receptors in developing OLs in vivo during the age window of white matter vulnerability as compared with younger or older ages (15). Importantly, precursors and immature OLs populate the developing white matter during the window of greatest vulnerability to in vivo intracerebral AMPA injections or hypoxia-ischemia (15) and to PVL in premature infants (1, 2). Thus, glutamate receptor-mediated toxicity to OPCs may play a role in the age-dependent hypoxic–ischemic white matter injury and PVL.
Role of Specific Subtypes of AMPA/Kainate Receptors in OGD-Induced OPC Death. Pre- or post-OGD glutamate receptor blockade with either the AMPA-preferring antagonist GYKI 52488 or the kainate-preferring antagonist GAMS was protective in the OGD model, suggesting a role for both AMPA and kainate receptor subtypes in OGD-induced OPC death. Furthermore, JSTx prevented toxicity or Ca2+ influx induced by OGD to a degree similar to that of CNQX or NBQX. The partial blockade of kainate-evoked Ca2+ influx by JSTx implies that kainate can also elicit Ca2+ influx through JSTx-insensitive receptors (34). However, JSTx-sensitive receptors are likely to be a predominant mode of OGD-induced Ca2+ entry, as specific blockers of voltage-gated Ca2+ channels and Na+-Ca2+ exchangers were not protective against OGD-induced OPC death. Ca2+-permeable AMPA/kainate receptors have previously been implicated in neuronal excitotoxicity (35, 36, 37). This study suggests that excitotoxicity mediated by these receptors may be a major factor in OGD-induced OPC death.
Posttreatment Efficacy of Selective AMPA/Kainate Receptor Antagonists in the Acute OGD Paradigm. Pharmacological blockade of AMPA/kainate receptors was effective when administered after OGD, suggesting persistent activation of Ca2+-permeable AMPA/kainate receptors by extracellular glutamate after the insult. Additionally, endogenous glutamate release by the reversal of glutamate transporters during OGD may contribute to non-NMDA receptor-mediated excitotoxicity in neighboring cells or in the same cells via a cell-autologous feedback loop in immature OLs (11). Indeed, the glutamate transporter inhibitor DKA prevented OGD-induced OPC death, suggesting that reversed glutamate transport may occur during hypoxic–ischemic injury and may be relevant to PVL.
NBQX fully blocked kainate-induced cytotoxicity, but did not completely abolish OGD-induced death, suggesting that mechanisms independent of AMPA/kainate receptors may also contribute to the OGD-induced toxicity. In addition to receptor-dependent mechanisms, glutamate toxicity to immature OLs may involve nonreceptor-mediated oxidative stress (38).
The Priming Effect of Sublethal OGD Preconditioning Exposure. Sublethal exposure to OGD is relevant to the specific clinical setting of the premature infant in which multiple ischemic events are common (1). Sublethal OGD preconditioning enhances OPC vulnerability to subsequent OGD- or kainate-induced Ca2+ influx and cell death, and this enhancement can be abolished by NBQX or JSTx. The priming effect of sublethal OGD preconditioning is not related to a disturbance of proliferation or maturation of OPCs. Interestingly, cerebral ischemic preconditioning has a protective effect on neuronal excitotoxicity (16, 39). In neurons, ischemic tolerance is mediated largely by activation of NMDA receptors (17, 40). In contrast, AMPA receptor-mediated excitotoxicity is actually enhanced after sublethal OGD preconditioning of hippocampal neurons (41). The latter results are consistent with our observation that OPCs, which only express non-NMDA receptors, can be primed via AMPA/kainate receptors by sublethal OGD. Preconditioning mediated by AMPA/kainate receptors appears to promote subsequent excitotoxicity in both glia and neurons, in contrast to preconditioning mediated by NMDA receptor, which results in neuroprotection. The toxicity-enhancing effect of AMPA/kainate receptor-mediated preconditioning depends on JSTx-sensitive Ca2+-permeable receptors. The higher Ca2+ permeability of AMPA receptors after sublethal OGD is at least in part due to a decrease in cell-surface GluR2, justifying future investigation of the role of Ca2+-mediated downstream events underlying the priming effect of sublethal OGD.
Supplementary Material
Acknowledgments
We thank Amanda Greene for assistance with the preparation of OL cultures, and Dr. Pamela L. Follett for her suggestions. This research was supported by National Institute of Neurological Disorders and Stroke Grants T32 AG00222–11 (to W.D.), R01 NS31718 (to F.E.J.), and P01 NS38475 (to J.J.V., P.A.R., and F.E.J.), a grant from the United Cerebral Palsy Foundation (to F.E.J.), and Mental Retardation Research Center Grant P30 HD18655 from the National Institute of Child Health and Development.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; OL, oligodendrocyte; JSTx, joro spider toxin; OGD, oxygen–glucose deprivation; OPC, oligodendroglial precursor cell; PVL, periventricular leukomalacia; NBQX, 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; NMDA, N-methyl-D-aspartate; GAMS, γ-D-glutamylaminomethanesulfonic acid.
References
- 1.Volpe, J. J. (2001) in Neurology of the Newborn (Saunders, Philadelphia), 4th Ed., pp. 217-276.
- 2.Back, S. A., Luo, N. L., Borenstein, N. S., Levine, J. M., Volpe, J. J. & Kinney, H. C. (2001) J. Neurosci. 21, 1302-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raff, M. C., Miller, R. H. & Noble, M. (1983) Nature 303, 390-396. [DOI] [PubMed] [Google Scholar]
- 4.Pfeiffer, S., Warrington, A. E. & Bansal, R. (1993) Trends Cell Biol. 3, 191-197. [DOI] [PubMed] [Google Scholar]
- 5.Baumann, N. & Pham-Dinh, D. (2001) Physiol. Rev. 81, 871-927. [DOI] [PubMed] [Google Scholar]
- 6.Levine, J. M., Reynolds, R. & Fawcett, J. W. (2001) Trends Neurosci. 24, 39-47. [DOI] [PubMed] [Google Scholar]
- 7.Patneau, D. K., Wright, P. W., Winters, C., Mayer, M. L. & Gallo, V. (1994) Neuron 12, 357-371. [DOI] [PubMed] [Google Scholar]
- 8.Matute, C., Sanchez-Gomez, M. V., Martinez-Millan, L. & Miledi, R. (1997) Proc. Natl. Acad. Sci. USA 94, 8830-8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald, J. W., Althomsons, S. P., Hyrc, K. L., Choi, D. W. & Goldberg, M. P. (1998) Nat. Med. 4, 291-297. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Gomez, M. V. & Matute, C. (1999) Neurobiol. Dis. 6, 475-485. [DOI] [PubMed] [Google Scholar]
- 11.Fern, R. & Möller, T. (2000) J. Neurosci. 20, 34-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshioka, A., Hardy, M., Younkin, D. P., Grinspan, J. B., Stern, J. L. & Pleasure, D. (1995) J. Neurochem. 64, 2442-2448. [DOI] [PubMed] [Google Scholar]
- 13.Yoshioka, A., Bacskai, B. & Pleasure, D. (1996) J. Neurosci. Res. 46, 427-437. [DOI] [PubMed] [Google Scholar]
- 14.Yoshioka, A., Yamaya, Y., Saiki, S., Kanemoto, M., Hirose, G., Beesley, J. & Pleasure, D. (2000) Brain Res. 854, 207-215. [DOI] [PubMed] [Google Scholar]
- 15.Follett, P. L., Rosenberg, P. A., Volpe, J. J. & Jensen, F. E. (2000) J. Neurosci. 20, 9235-9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Zulueta, M., Feldman, A. B., Klesse, L. J., Kalb, R. G., Dillman, J. F., Parada, L. F., Dawson, T. M. & Dawson, V. L. (2000) Proc. Natl. Acad. Sci. USA 97, 436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabb, M. C. & Choi, D. W. (1999) J. Neurosci. 19, 1657-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarthy, K. D. & de Vellis, J. (1980) J. Cell Biol. 85, 890-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg, P. A., Dai, W., Gan, X. D., Ali, S., Fu, J., Back, S. A., Sanchez, R. M., Segal, M. M., Follett, P. L., Jensen, F. E. & Volpe, J. J. (2003) J. Neurosci. Res. 71, 237-245. [DOI] [PubMed] [Google Scholar]
- 20.Back, S. A., Gan, X., Li, Y., Rosenberg, P. A. & Volpe, J. J. (1998) J. Neurosci. 18, 6241-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogler, O., Wren, D., Barnett, S. C., Land, H. & Noble, M. (1990) Proc. Natl. Acad. Sci. USA 87, 6368-6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry, S. W., Epstein, L. G. & Gelbard, H. A. (1997) BioTechniques 22, 1020-1022. [DOI] [PubMed] [Google Scholar]
- 23.Chung, H. J., Xia, J., Scannevin, R. H., Zhang, X. & Huganir, R. L. (2000) J. Neurosci. 20, 7258-7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollmann, M. & Heinemann, S. (1994) Annu. Rev. Neurosci. 17, 31-108. [DOI] [PubMed] [Google Scholar]
- 25.Sattler, R. & Tymianski, M. (2000) J. Mol. Med. 78, 3-13. [DOI] [PubMed] [Google Scholar]
- 26.Li, S., Mealing, G. A. R., Morley, P. & Stys, P. K. (1999) J. Neurosci. 19, RC16 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pellegrini-Giampietro, D. E., Gorter, J. A., Bennett, M. V. & Zukin, R. S. (1997) Trends Neurosci. 20, 464-470. [DOI] [PubMed] [Google Scholar]
- 28.Matute, C. (1998) Proc. Natl. Acad. Sci. USA 95, 10229-10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, S. & Stys, P. K. (2000) J. Neurosci. 20, 1190-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu, H., Barks, J. D., Liu, Y. Q. & Silverstein, F. S. (2001) Brain Res. Dev. Brain Res. 132, 175-178. [DOI] [PubMed] [Google Scholar]
- 31.Tekkok, S. B. & Goldberg, M. P. (2001) J. Neurosci. 21, 4237-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, J., Lado, E., Mayer-Proschel, M. & Noble, M. (2000) Proc. Natl. Acad. Sci. USA 97, 10032-10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itoh, T., Beesley, J., Itoh, A., Cohen, A. S., Kavanaugh, B., Coulter, D. A., Grinspan, J. B. & Pleasure, D. (2002) J. Neurochem. 81, 390-402. [DOI] [PubMed] [Google Scholar]
- 34.Meucci, O. & Miller, R. J. (1998) Neuropharmacology 37, 1431-1443. [DOI] [PubMed] [Google Scholar]
- 35.Perkinton, M. S., Sihra, T. S. & Williams, R. J. (1999) J. Neurosci. 19, 5861-5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka, H., Grooms, S. Y., Bennett, M. V. & Zukin, R. S. (2000) Brain Res. 886, 190-207. [DOI] [PubMed] [Google Scholar]
- 37.Weiss, J. H. & Sensi, S. L. (2000) Trends Neurosci. 23, 365-371. [DOI] [PubMed] [Google Scholar]
- 38.Oka, A., Belliveau, M. J., Rosenberg, P. A. & Volpe, J. J. (1993) J. Neurosci. 13, 1441-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glazier, S. S., O'Rourke, D. M., Graham, D. I. & Welsh, F. A. (1994) J. Cereb. Blood Flow Metab. 14, 545-553. [DOI] [PubMed] [Google Scholar]
- 40.Aizenman, E., Sinor, J. D., Brimecombe, J. C. & Herin, G. A. (2000) J. Pharmacol. Exp. Ther. 295, 572-577. [PubMed] [Google Scholar]
- 41.Ying, H. S., Weishaupt, J. H., Grabb, M., Canzoniero, L. M., Sensi, S. L., Sheline, C. T., Monyer, H. & Choi, D. W. (1997) J. Neurosci. 17, 9536-9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.