Abstract
Spinal administration of nicotinic agonists can produce both hyperalgesic and analgesic effects in vivo. The cellular mechanisms underlying these behavioral phenomena are not understood. As a possible explanation for nicotinic hyperalgesia, we tested whether nicotinic acetylcholine receptors (nAChRs) could enhance excitatory transmission onto spinal cord dorsal horn neurons. Whole-cell patch–clamp recordings were performed in neonatal rat spinal cord slices. Activation of nAChRs enhanced glutamatergic synaptic transmission in 59% of dorsal horn neurons tested, and this effect was blocked by methyllycaconitine (10 nM), suggesting a key role for α7 nAChRs. Inhibition of acetylcholinesterase with methamidophos also enhanced transmission, demonstrating a similar effect of endogenous acetylcholine. nAChR activation also enhanced transmission by dorsal root entry zone stimulation, suggesting that α7 nAChRs on the central terminals of DRG afferents mediate this effect. Paired pre- and postsynaptic stimulation induced long-term potentiation of excitatory inputs to some of the dorsal horn neurons. Long-term potentiation induction was much more prevalent when nicotine was applied during stimulation. This effect also depended on both α7 nAChRs and N-methyl-d-aspartate glutamate receptors. Our findings demonstrate that α7 nAChRs can contribute to both short- and long-term enhancement of glutamatergic synaptic transmission in the spinal cord dorsal horn and provide a possible mechanism for nicotinic hyperalgesia.
Effective pain management is a major challenge for modern healthcare. Most pain medications fall under two classes of compounds, opioids and nonsteroidal antiinflammatory drugs. Recently, structural analogs of nicotine have also demonstrated strong analgesic potency (1, 2). Although these agents may provide alternate pain therapies, a number of issues remain to be resolved, such as addictive profiles and side effects, including paradoxical hyperalgesia. Considerable research has focused on the analgesic effects of nicotine and its analogs (2–7), yet the mechanisms underlying nicotinic hyperalgesia are not well understood.
Hyperalgesia and/or irritation have been observed after central injections of nicotinic agonists (8–10), systemic injections of subanalgesic doses (11), and intrathecal applications (12, 13). This intrathecal effect is associated with significant increases in excitatory transmitter release (14), and both nicotinic and glutamate receptor antagonists can decrease this hyperalgesia (15, 16). Although dorsal horn neurons are excited by nicotine (17), it is not known which subtypes of nicotinic acetylcholine receptors (nAChRs) are expressed by dorsal horn neurons or on presynaptic afferent terminals. For example, nicotinic enhancement of synaptic activity has been noted in the spinal cord dorsal horn (SCDH) in mice that lack the α4 nAChR subunit, but the nAChR subtype(s) that mediate this effect is unknown (5).
Neuronal nAChRs are pentameric ligand-gated ion channels, and molecular cloning has identified nine α and three β subunits (18, 19). These subunits assemble to form functional receptors in heteromeric combinations, such as α4β2, or as homopentamers, such as α7 (18– 22). Receptor subunit composition underlies the differences in functional properties, and there is considerable variation in subunit expression throughout the CNS. The diversity of nAChR subunits likely contributes to the dual analgesic and hyperalgesic effects of nicotinic agonists.
Our previous work demonstrated that dorsal root ganglion (DRG) neurons express multiple nAChR subtypes, and that α7 nAChRs are most prevalent (23). The α7 nAChR expressed on presynaptic terminals can enhance glutamate transmission in many areas of the CNS (24). In the SCDH, radiolabeled α-bungarotoxin binding decreases after dorsal root transection (25), suggesting that α7 nAChRs are expressed on the central terminals of DRG afferents and may modulate sensory transmission.
Using a spinal cord slice preparation from neonatal rats, we tested the role of α7 nAChRs in the modulation of excitatory glutamatergic synaptic transmission in the dorsal horn. These data address the role of nAChRs in synaptic plasticity between primary and secondary sensory neurons and may provide a model for nicotinic hyperalgesia.
Methods
Spinal Cord Slice Preparation. Whole-cell patch–clamp recordings were made from neurons in transverse slices of rat lumbar spinal cord from 6- to 14-day postnatal Sprague–Dawley rats. Animals were anesthetized with isoflurane, and the spinal cord was removed to ice-cold oxygenated artificial cerebrospinal fluid (aCSF) [in mM: 125 NaCl/2.5 KCl/25 NaHCO3/1 NaH2PO4/1 MgCl2(6H2O)/2.5 CaCl2/20 glucose, pH 7.4] bubbled with 95% O2/5% CO2. Dissection solutions also contained 1 mM ascorbic acid and 5 mM KCl. Five hundred micrometer transverse vibratome sections (Campden, Leicester, U.K.) were incubated for >1 h at 37°C before recording. The external solution for recordings was aCSF with 1 μM tetrodotoxin, except where noted. Cells were visualized by using IR illumination on an Axioskop 2 microscope (Zeiss). An Axopatch 200B Patch Clamp (Axon Instruments, Union City, CA) with a Digidata 1320A (Axon) were used to acquire data to a Pentium computer (Dell, Austin, TX) with PCLAMP 8 software (Axon). Recordings were digitized at 5 KHz and filtered at 1 KHz. The pipette solution contained (in mM): 154 K-gluconate, 1 KCl, 1 EGTA, 10 Hepes, 10 glucose, 5 K-ATP, and 100 μM GTP, pH 7.4. Chloride currents were outward at Vm = -70 mV, which was important because strychnine inhibits α7 nAChRs and could not be used to block glycine receptors (26). Drug applications for slice recordings were controlled by bath perfusion (2 ml/min; ALA, Westbury, NY). Experiments were performed at room temperature.
Evoked Transmission and Synaptic Plasticity. The dorsal root entry zone (DREZ) (27) was electrically simulated at 0.067 Hz (A.M.P.I., Jerusalem), with bipolar tungsten electrodes (FHC, Bowdoinham, ME). The stimulus magnitude was set to the minimal level that evoked stable postsynaptic responses to limit problems due to direct depolarization of axon terminals (intensity range 1.9–8 V, 0.5 msec). Tetrodotoxin was excluded from the solutions, and bicuculline (20 μM) was added to block GABAergic inhibitory postsynaptic currents. Strychnine was not used due to effects on α7 nAChRs, but there was no monosynaptic glycinergic transmission. The criteria for monosynaptic excitatory postsynaptic currents (EPSCs) included brief latency (mean: 3.99 ± 0.33 msec, range: 1.3–7.8 msec) and stable response latencies with SD <1 msec, which did not change with stronger electrical stimulation. Also, high-frequency stimulation (50 Hz) caused a reduction in EPSC amplitude but no change in latency (28). Recordings were digitized at 20,000 Hz and filtered at 5,000 Hz. For long-term potentiation (LTP)/long-term depression (LTD) experiments, we used perforated patch recording with amphotericin B in the pipette (660 μg/ml in 2% DMSO) (29). The LTP/LTD induction paradigm consisted of 200 DREZ stimulations at 1 Hz with simultaneous 100-ms postsynaptic depolarizations to +10 mV. Long-term plasticity was assessed by comparing EPSC amplitudes during 10-min control with 30–40 min after induction (t test), and cells were grouped as LTP, LTD, or no change.
Cell Localization. Neuron position was documented immediately after recording. Biocytin (2 mg/ml) was included in the pipette for a subset of recordings to verify cell location. Slices were fixed with 4% paraformaldehyde in 0.1 M PBS overnight and washed in PBS. Cells were visualized on a Nikon Microphot EPI-FL microscope by using Cy3-streptavidin (Molecular Probes) or diaminobenzidine (Vector Laboratories).
Acutely Isolated Neurons. Isolation of dorsal horn neurons was as described (30). The dorsal third of a spinal cord slice was treated with 0.5 mg/ml pronase for 15 min (Calbiochem) and then 0.5 mg/ml protease-type X for 15 min. Tissue was incubated in a Ca2+-free artificial cerebrospinal fluid for 5 min and dissociated in Hepes-buffered saline. Cells were kept in a humidified chamber with 95% O2/5% CO2 for >1 h before recording. Isolation of sensory neurons was modified from a previous study (31). DRGs from 10–14 day postnatal rats were incubated for 20 min at 37°C in 1.4 mg/ml papain in L15 media (Life Technologies, Rockville, MD) followed by another 20 min in 1 mg/ml collagenase in L15. Cells were dissociated in L15 directly onto plastic culture dishes and incubated >1 h before recording. Whole-cell currents >10× baseline RMS noise were considered positive responses.
The external solution for acutely isolated neurons was (in mM): 140 NaCl/2.5 KCl/1 MgCl2/2 CaCl2/10 Hepes/10 glucose, pH 7.4. The pipette solution was (in mM): 145 K-gluconate/10 KCl/1 EGTA/10 Hepes/10 glucose/5 K-ATP/100 μM GTP, pH 7.4. An EPC7 patch clamp (HEKA Electronics, Lambrecht, Germany) and a Digidata 1200 interface to acquire data with PCLAMP 8 software (Axon). Electrode resistance was 2–5 MΩ. Neurons were visualized with an Axiovert 25 microscope (Zeiss). Piezo-controlled solution exchange (Piezo Systems, Cambridge, MA) provided rapid application of reagents (23).
Reagents. Methamidophos was from Chem Service (West Chester, PA), and tetrodotoxin was from Alomone Labs (Jerusalem). 6,7-Dinitroquinoxaline-2,3-dione and DL-2-amino-5 phosphonovaleric acid were from Tocris (Ballwin, MO). All other reagents were from Sigma/Research Biochemicals (Natick, MA).
Data Analysis and Statistics. The MINI ANALYSIS program (Synaptosoft, Decatur, GA) was used for the analysis of synaptic currents. Detection parameters were set to discriminate synaptic currents during that portion of the recording with the largest baseline noise (usually during nicotine application due to postsynaptic nAChRs). Each selected synaptic current was visually inspected to eliminate detection errors. SIGMAPLOT (SPSS, Chicago) provided statistical analysis and display. Cells with significant nAChR-induced changes in miniature EPSC (mEPSC) frequency (P < 0.05) were identified by using a Poisson regression analysis in STATA 6 (Stata, College Station, TX) (32). The mEPSC frequency data conform to a Poisson distribution using STATA 6. Cumulative amplitude distributions were compared by using the Kolmogorov–Smirnov test with SYSTAT software (SPSS). Data are presented as mean ± SEM.
Results
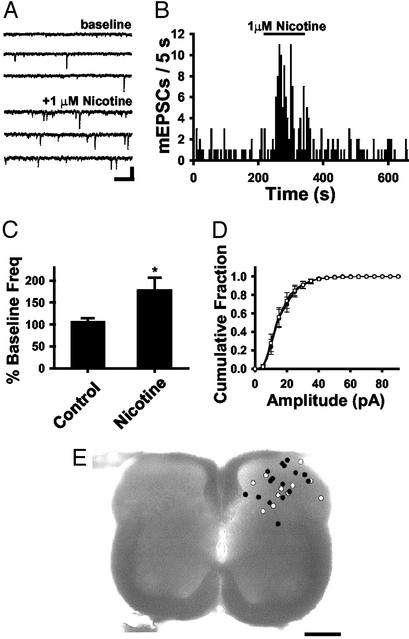
α7 nAChR Activation Enhances Excitatory Synaptic Transmission in the SCDH. To test the effects of nicotine on glutamatergic transmission in the spinal cord, whole-cell patch–clamp recordings were carried out in transverse slices of neonatal rat spinal cord. Neurons were recorded throughout the dorsal third of the spinal cord. Action potentials were blocked by tetrodotoxin in the external solutions. All neurons had mEPSCs (Fig. 1A), which were blocked with 6,7-dinitroquinoxaline-2,3-dione (15 μM; n = 6), indicating glutamate transmission via non-N-methyl-D-aspartate (NMDA) receptors. No evidence for cholinergic mEPSCs was observed. Bath application of 1 μM nicotine increased the frequency of glutamatergic mEPSCs in 59% of the neurons (n = 16/27; Fig. 1 A and B), with an average increase to 222.4 ± 45.7% of baseline (P < 0.05). Eight more neurons responded with an increase in mEPSC frequency that was not statistically significant. The average nicotine-induced change in mEPSC frequency was 177.9 ± 28.8% of control (Fig. 1C), whereas control experiments without nicotine had no change in mEPSC frequency (106.5 ± 7.5%). Nicotine did not induce a change in mEPSC amplitude (P > 0.05; Fig. 1D), which is consistent with a presynaptic modulation of neurotransmitter release. Thus the nAChRs that modify transmission are likely located on the terminals, near the sites of glutamate release. In some recordings, biocytin-filled neurons were labeled with Cy3 or diaminobenzidine to verify position. Fig. 1E shows the positions of nicotine-responsive (filled circles) and nonresponsive neurons (open circles). These data suggest that nicotine can modulate excitatory inputs to neurons of nociceptive and nonnociceptive modalities.
Fig. 1.
Nicotine enhances glutamatergic transmission in the SCDH. (A) Voltage-clamp recording from a SCDH neuron from control and with 1 μM nicotine (Vh = -70 mV). (Bar = 20 pA, 250 ms.) (B) Frequency histogram showing an increase in glutamatergic mEPSCs with 1 μM nicotine. (C) The average normalized frequency data from all neurons (n = 27; *, P < 0.05) compared with control without nicotine (n = 10). (D) Cumulative distributions illustrate no difference in average mEPSC amplitudes during baseline and nicotine treatment (P > 0.05). (E) The locations of recorded neurons presented as nicotine responders (n = 16, filled circles) or nonresponders (n = 11, open circles). (Bar = 250 μm.)
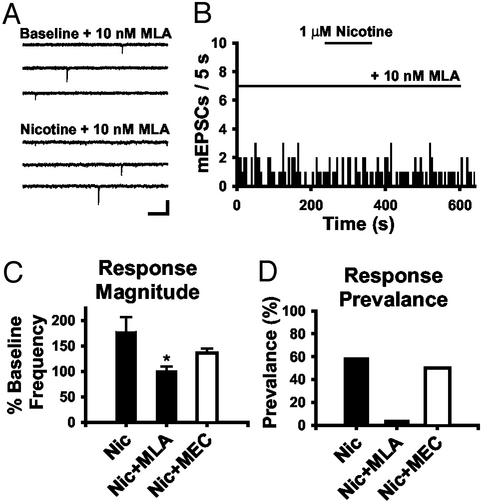
Nicotinic agonists and antagonists were then used to assess the contribution of specific nAChR subtypes to the presynaptic enhancement of glutamate release. In the presence of 10 nM methyllycaconitine (MLA), an α7-selective antagonist, nicotine induced an increase in mEPSC frequency in only one of 23 neurons, whereas two of 23 showed a decrease, and the majority (87%) showed no change (Fig. 2 A and B). The average mEPSC frequency during nicotine application with MLA was 101.2 ± 8.5% of baseline (Fig. 2C). Mecamylamine (MEC) is selective for non-α7 nAChRs at low concentrations (1 μM) (33), and treatment with this antagonist did not affect nicotine-induced increases in mEPSC frequency (166.5 ± 11.8% of baseline; n = 20; P > 0.05; Fig. 2C). Response prevalence was decreased by MLA, but not MEC, treatment (Fig. 2D). Together, these data support a role for presynaptic α7 nAChRs in the enhancement of glutamate release in dorsal horn.
Fig. 2.
Enhancement of synaptic transmission is due to α7 nAChR activation. (A) Raw current traces during baseline and with 1 μM nicotine + 10 nM MLA in the external solutions. (Bar = 20 pA, 250 ms.) (B) mEPSC histogram showing the lack of response to 1 μM nicotine with 10 nM MLA. (C) Summary histogram of the magnitude of nicotine-induced changes in mEPSC frequency in control (177.9 ± 28.8%; n = 27) and with 10 nM MLA (101.2 ± 8.5%; n = 23; *, P < 0.05) or 1 μM mecamylamine (135.9 ± 8.5%; n = 20; P > 0.05). (D) Response prevalence under these three conditions.
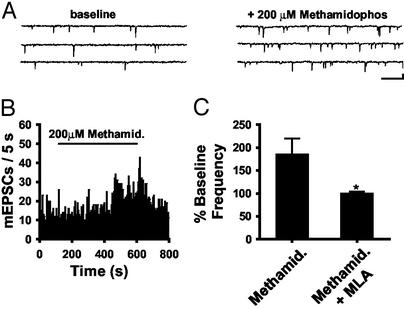
Endogenous ACh Activates Presynaptic α7 nAChRs. Cholinergic neurons within the spinal cord project to the dorsal horn (34, 35). To assess the interaction of endogenous ACh with presynaptic α7 nAChRs in the dorsal horn, we blocked acetycholinesterase while monitoring excitatory synaptic transmission. Atropine (1 μM) was included in the external solutions to block muscarinic effects. Methamidophos, an organophosphate acetylcholinesterase inhibitor (36), enhanced the frequency of mEPSCs in the dorsal horn (Fig. 3 A and B). The slow onset of the effect is consistent with a slow accumulation of ACh in the slice. Methamidophos increased mEPSC frequency in four of five neurons to 206.9 ± 33.5% of baseline on average. This effect was blocked by 10 nM MLA (100.4 ± 3.2% baseline; n = 6; Fig. 3C). Neostigmine (10 μM), another acetycholinesterase inhibitor, also increased the mEPSC frequency in six of 13 cells tested to 188.5 ± 30.0% the baseline (data not shown).
Fig. 3.
Enhancement of mEPSC frequency by endogenous ACh. (A) Raw current traces during baseline and with 200 μM methamidophos. (Bar = 20 pA, 250 ms.) (B) mEPSC histogram during control and 200 μM methamidophos exposure. (C) Average change in mEPSC frequency after methamidophos (185.9 ± 3.3%; n = 5) and with 10 nM MLA (100.4 ± 3.2%; n = 6; *, P < 0.05). All data were averaged for responders and nonresponders.
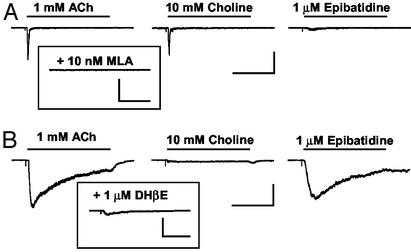
Acutely Isolated Dorsal Horn and DRG Neurons Express α7 nAChRs. Because dorsal horn neurons receive glutamatergic input from both local spinal cord neurons and DRG afferents, we tested which cell types expressed functional nAChRs. Acutely isolated DRG and dorsal horn neurons were tested for responses to nicotinic agonists. Each neuron was first tested for sensitivity to ACh and the α7-selective agonist choline. Rapid responses with choline sensitivity were classified as α7 (24% of dorsal horn neurons, n = 11/46), and these were blocked with the α7-selective antagonist MLA (Fig. 4A). ACh currents with slow decay kinetics were insensitive to choline and were classified as non-α7 (20% of SCDH neurons, n = 9/46). These currents were also activated by the potent nicotinic analgesic epibatidine and were blocked by dihydro-β-erythroidine (Fig. 4B) but not MLA. Unexpectedly, we found limited overlap of α7 and non-α7 expression in SCDH neurons. When one current type was expressed, the other was always <5% of the magnitude of the dominant nAChR class.
Fig. 4.
Expression of nAChRs on acutely isolated dorsal horn neurons. (A) ACh or choline induced a rapid inward current in an acutely isolated dorsal horn neuron that was insensitive to epibatidine. These α7 currents could be inhibited by MLA (Inset; n = 4) and were seen in 24% of recordings (n = 46). [Bar = 400 pA, 500 ms (Left); 400 pA, 100 ms (Inset).] (B) In a different neuron, ACh or epibatidine induced a current with slower inactivation kinetics that was insensitive to choline. This non-α7 current was see in 20% of recordings (n = 46) and could be inhibited by dihydro-β-erythroidine (Inset; n = 4; Bar = 400 pA, 400 ms) but not MLA (not shown; n = 2).
Acutely isolated DRG neurons were also tested for functional nAChR expression. ACh or choline induced MLA-sensitive currents in 67% of the neurons (n = 8/12; not shown). ACh also activated MLA-insensitive non-α7 nAChRs currents in 6/12 neurons. Previous data from cultured DRG neurons indicate at least two other nAChR subtypes in these neurons, but with much lower prevalence (23). Thus, DRG neurons have a higher prevalence of α7 nAChR expression than dorsal horn neurons. Although the transmitter phenotype was not determined in the isolated dorsal horn neurons, glutamatergic dorsal horn neurons may also express α7 channels.
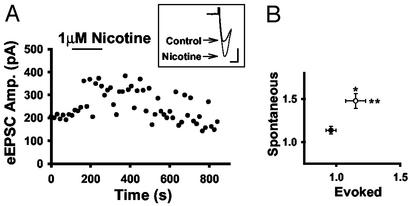
α7 nAChRs Are Expressed on the Central Terminals of DRG Afferents. Because both dorsal horn and DRG neurons express α7 nAChRs, we tested the effect of nicotine on evoked EPSC (eEPSCs) stimulated at the DREZ (Fig. 5A). The DREZ was stimulated at 0.067 Hz, with 20 μM bicuculline in the external solutions. Spontaneous EPSC (sEPSC) frequency was also monitored to categorize cells as responders and nonresponders to nicotine. Of the 18 neurons tested for changes in both sEPSCs and eEPSCs, eight had a nicotine-induced increase in sEPSC frequency to 147.8 ± 8.7% of baseline and were classified as responders. Of these eight responders, five cells also had increases in eEPSC amplitudes. Fig. 5A shows a responder with a significant increase in eEPSC amplitude. The average eEPSC amplitude was 115 ± 7.9% of control for responders, and 95.8 ± 4.0% of control for nonresponders. Fig. 5B shows the average change in sEPSC frequency and eEPSC amplitude for the responders (n = 8; open circle) versus nonresponders (n = 10; closed circle). This modulation could reflect either an increase in the probability of glutamate release or the recruitment of additional afferent inputs by nicotine-induced depolarization. In either scenario, α7 nAChRs on DRG afferents enhance glutamate transmission in dorsal horn.
Fig. 5.
α7 nAChR expression on the central terminals of DRG afferents. (A) Evoked synaptic current amplitudes from one neuron before and after nicotine treatment. (Inset) Currents are individual traces. (Bar = 100 pA, 4 ms.) (B) Averages of evoked current amplitudes and sEPSC frequency for responders (n = 8; open circle) and nonresponders (n = 10; filled circle) (* and **, P < 0.05).
Paired-Pulse Depression in the Dorsal Horn. We evaluated glutamate release probability in the dorsal horn by using paired-pulse stimulation. Two DREZ stimulations (P1 and P2) separated by 25–30 ms resulted in depression of transmission in the majority of recordings (15/21). Only one of 21 neurons showed paired-pulse facilitation, and five showed no difference between P1 and P2 (not shown). In the presence of nicotine, only 17% (three of 18) neurons showed an enhancement of P2/P1. These data are consistent with a high release probability at excitatory synapses in the dorsal spinal cord.
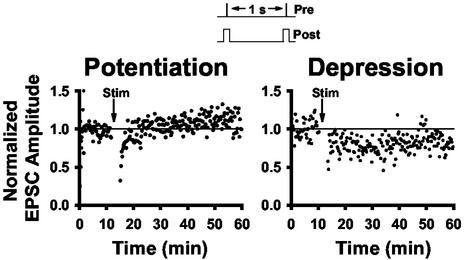
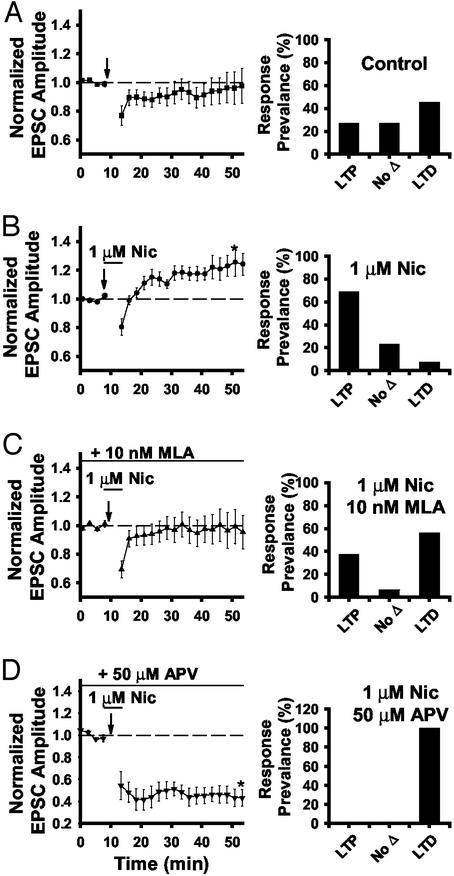
α7 nAChR Activation Increases the Prevalence of LTP. Because nicotine has been shown to affect long-term synaptic plasticity of other synapses (29, 37), we tested nicotinic modulation of LTP induction in the dorsal horn during DREZ stimulation by using perforated-patch recording. Neurons with a stable postsynaptic response to DREZ stimulation (0.5 ms, 0.067 Hz) for >10 min were subjected to 200 stimulations at 1 Hz with 100-ms postsynaptic depolarizations to +10 mV (Fig. 6 Inset). This strong pairing protocol has been shown to reliably induce LTP in other regions of the CNS. In our recordings, LTP was observed in only 27% of cells (n = 3 of 11; Fig. 6), no long-term change was seen in 27% of cells (n = 3 of 11), and LTD was induced in 45% of cells (n = 5 of 11; Fig. 6). Fig. 7A illustrates the average normalized EPSC amplitudes for all of the control cells, as well as the prevalence of response types. The high prevalence of LTD may reflect a predominance of potentiated synapses in the SCDH. We then tested the effects of nicotine on this long-term synaptic modulation.
Fig. 6.
LTP and LTD induction in the dorsal horn. The protocol used to induce long-term changes in eEPSC amplitudes consisted of 200 DREZ stimuli at 1Hz (0.5 ms) during simultaneous postsynaptic depolarizations to +10 mV (100 ms). Shown are examples of potentiation and depression induced by this protocol in different neurons.
Fig. 7.
Nicotine enhances LTP induction in the dorsal horn. (A) Average normalized eEPSC amplitudes before and after the stimulation protocol (n = 11). Bar graphs (A–D Right) illustrate the prevalence of LTP, LTD, and no change under each condition. (B) Average normalized eEPSC amplitudes with 1 μM nicotine applied 2 min before and during the simulation paradigm (n = 13). (C) Average normalized eEPSC amplitudes with 1 μM nicotine in the presence of 10 nM MLA (n = 16). (D) Average normalized eEPSC amplitudes with 1 μM nicotine in the presence of 50 μM dl--2-amino-5 phosphonovaleric acid (n = 9).
Nicotine application 2 min before and during the stimulation paradigm resulted in a much higher prevalence of LTP, as illustrated by the average normalized EPSC amplitudes (Fig. 7B). LTP was induced in 69% of neurons (n = 9 of 13), and only 8% of the nicotine-treated neurons expressed LTD (n = 1 of 13). This nicotine-induced enhancement of LTP was reduced after exposure to 10 nM MLA (n = 16; Fig. 7C). These data support the idea that α7 nAChRs mediate the nicotine-induced enhancement of LTP induction. Enhancement of LTP induction by nicotine was also eliminated in the presence of 50 μM DL-2-amino-5 phosphonovaleric acid, but the prevalence of LTD was increased (n = 10; Fig. 7D), indicating NMDA receptor dependence of LTP but not LTD under these conditions.
Discussion
These results demonstrate that activation of presynaptic α7 nAChRs in the SCDH can enhance glutamatergic transmission. This enhancement can also be induced by endogenous ACh. A subpopulation of both dorsal horn and DRG neurons express α7 nAChRs, and our findings support the idea that α7 nAChRs expressed on the central terminals of DRG afferents enhance excitatory transmission in the spinal cord. Activation of these receptors can push the balance of synaptic modulation in the dorsal horn toward LTP. These results do not exclude the possibility that nAChRs expressed by dorsal horn neurons also contribute to modulation. In sum, our findings support a model of nicotinic hyperalgesia that involves the short- and long-term enhancement of glutamatergic transmission.
Histological and functional evidence supports α7 nAChR expression on the central terminals of DRG afferents. α-bungarotoxin binding in the dorsal horn is greatly reduced after dorsal rhizotomy (25), suggesting that α7 nAChRs are expressed predominantly on sensory afferents. We have previously shown that multiple nAChR subtypes are expressed by DRG neurons, and the α7 nAChR is the most prevalent, with expression on 77% of large-diameter neurons and 32% of small-diameter neurons (23). We hypothesized that α7 nAChR expression on the central terminals of DRG afferents would enhance glutamate transmission. Choline acetyltransferase (ChAT), the synthesizing enzyme for ACh, is expressed in axonal varicosities that synapse onto the central terminals of sensory afferents (38), providing an endogenous source of ACh. These ChAT-positive axonal varicosities in the dorsal horn likely originate from intrinsic spinal neurons (34, 35, 39). Interestingly, dorsal horn cholinergic neurons are believed to provide ACh for muscarinic enhancement of GABA release (40), and spinal muscarinic analgesia has been reported (41). ACh may therefore regulate both analgesic and hyperalgesic mechanisms in the spinal cord by modulating both excitatory and inhibitory neurotransmitter systems.
Nicotinic enhancement of glutamatergic transmission has been noted in other regions of the CNS and has been implicated in diverse processes such as addiction and memory (29, 42, 43). α7 nAChR-mediated enhancement can occur at lower concentrations of nicotine than are often required for activation of postsynaptic receptors, which may explain the hyperalgesia seen at subanalgesic doses of nicotinic agonists (11, 44). Intrathecal nicotinic agonist application has been associated with release of the excitatory amino acids glutamate and aspartate (14), and applications of DL-2-amino-5 phosphonovaleric acid or MK-801 can block components of the nicotinic irritation and hyperalgesia (15, 16).
The response to paired-pulse stimulation suggests that sensory inputs to dorsal horn neurons have a high release probability. Nicotinic enhancement of evoked inputs in the dorsal horn was of a lower magnitude than other synapses, i.e., 115 ± 7.9% in spinal cord vs. 141 ± 7% in the ventral tegmental area (29), 160% in the prefrontal cortex (45), and 165 ± 14% in the hippocampus (46). This effect could be mediated by a decreased threshold for presynaptic action potentials, allowing recruitment of additional inputs, as seen at other synapses (32). Alternatively, although the probability of release is generally high, the range of paired-pulse ratios suggests that release probability is not saturated for every cell. Thus, nicotine may increase evoked transmission by enhancing the probability of glutamate release, as shown in the mEPSC experiments.
In addition to nicotinic enhancement of mEPSC frequency and eEPSC amplitude, nicotine also enhanced LTP induction in the dorsal horn. Numerous other reports have demonstrated LTD and LTP expression in dorsal horn. Low-frequency stimulation of nociceptors induces LTD, which depends on NMDA and metabotropic glutamate receptor activity (47, 48). Robust stimulation of dorsal roots can induce either LTP or LTD in dorsal horn, and LTP but not LTD induction is NMDA-receptor dependent under these conditions (49). LTP of dorsal horn field potentials has been demonstrated by noxious heat, mechanical, and chemosensitive stimuli but only in spinalized rats, suggesting that supraspinal inputs regulate its induction (50). Our results show that α7 nAChR activation can enhance LTP induction in the dorsal horn.
Our stimulation protocol, which induces LTP in many different CNS preparations (51), induced more depression than potentiation in the dorsal horn. These data suggest that the sensory inputs to the dorsal spinal cord may already be in a potentiated state, allowing only LTD after stimulation. This idea is also supported by the high prevalence of paired-pulse depression in the dorsal horn. Nicotine treatment increased the prevalence and magnitude of LTP at these synapses, suggesting that additional potentiation is possible when nAChRs are activated. It is intriguing that, in the presence of nicotine, inhibition of NMDA receptors eliminated LTP but not LTD. A current model of LTD suggests that relatively small increases in postsynaptic calcium activate the signaling cascade, leading to sequestration of AMPA receptors. Thus, the postsynaptic depolarizations in the induction protocol apparently provided sufficient stimulus for calcium influx to support LTD, independent of NMDA receptors. This observation is consistent with a previous report that strong stimulation of sensory inputs to dorsal horn induces LTD in the presence of NMDA antagonists (49).
There is precedent for nicotinic enhancement of LTP induction in other CNS areas. For example, nicotine acts via presynaptic α7 nAChRs in the ventral tegmental area to promote LTP (29). In the hippocampus, nicotine enhances LTP induction (52), and focal ACh application enhances LTP primarily through activation of postsynaptic nAChRs (37). Our data show that α7 nAChRs in the spinal cord may play both pre- and postsynaptic roles in long-term synaptic plasticity.
The enhancement of glutamatergic synaptic transmission in the superficial dorsal horn is consistent with nicotinic hyperalgesia. This “hyperalgesia” is likely a complex phenomenon, because nicotine enhances synaptic transmission throughout the dorsal horn (Fig. 1E) and may affect nonnociceptive sensory modalities. Furthermore, there is behavioral evidence that α7 nAChR activation in the spinal cord might also contribute to choline-induced analgesia, although the pharmacology implicates both nicotinic and muscarinic receptors in this phenomenon (53).
Recent reports indicate that nicotine can also enhance inhibitory glycinergic and GABAergic synaptic transmission in SCDH (54, 55). The nAChR subtypes underlying this effect are activated by nicotinic analgesics such as epibatidine and ABT594. nAChR activation in the spinal cord can also affect tonic serotonin release (7). Thus, it is likely that modulation of other neurotransmitter systems contributes to the effects of nicotine on sensory transduction. Some of the enhancement of glutamate inputs to the dorsal horn may occur at synapses onto inhibitory interneurons, thus contributing to analgesic effects of nAChR activity. Understanding these mechanisms in greater detail may lead to the development of more specific and efficacious nicotinic analgesics.
Acknowledgments
This work was supported by National Institutes of Health Grants NS35090 and DA015918 (to D.M.) and 1F30DA06033 and T32HD07009 (to J.G.), and by the Brain Research Foundation.
Preliminary results of this work have been published [Genzen, J. R. & McGehee, D. S. (1999) Soc. Neurosci. Abstr. 25, 394.16].
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACh, acetylcholine; nAChR, nicotinic ACh receptor; DRG, dorsal root ganglion; MLA, methyllycaconitine; EPSC, excitatory postsynaptic current; mEPSC, miniature EPSC; sEPSC, spontaneous EPSC; eEPSC, evoked EPSC; DREZ, dorsal root entry zone; LTP, long-term potentiation; LTD, long-term depression; NMDA, N-methyl-D-aspartate; SCDH, spinal cord dorsal horn.
References
- 1.Spande, T. F., Garraffo, H. M., Edwards, M. W., Yeh, H. J. C., Pannell, L. & Daly, J. W. (1992) J. Am. Chem. Soc. 114, 3475-3478. [Google Scholar]
- 2.Bannon, A. W., Decker, M. W., Holladay, M. W., Curzon, P., Donnelly-Roberts, D., Puttfarcken, P. S., Bitner, R. S., Diaz, A., Dickenson, A. H., Porsolt, R. D., et al. (1998) Science 279, 77-81. [DOI] [PubMed] [Google Scholar]
- 3.Flores, C. M. (2000) Pain 88, 1-6. [DOI] [PubMed] [Google Scholar]
- 4.Decker, M. W. & Meyer, M. D. (1999) Biochem. Pharmacol. 58, 917-923. [DOI] [PubMed] [Google Scholar]
- 5.Marubio, L. M., del Mar Arroyo-Jimenez, M., Cordero-Erausquin, M., Lena, C., Le Novere, N., de Kerchove d'Exaerde, A., Huchet, M., Damaj, M. I. & Changeux, J. P. (1999) Nature 398, 805-810. [DOI] [PubMed] [Google Scholar]
- 6.Damaj, M. I., Fei-Yin, M., Dukat, M., Glassco, W., Glennon, R. A. & Martin, B. R. (1998) J. Pharmacol. Exp. Ther. 284, 1058-1065. [PubMed] [Google Scholar]
- 7.Cordero-Erausquin, M. & Changeux, J. P. (2001) Proc. Natl. Acad. Sci. USA 98, 2803-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahley, T. L. & Berntson, G. G. (1979) Psychopharmacology (Berlin) 65, 279-283. [DOI] [PubMed] [Google Scholar]
- 9.Parvini, S., Hamann, S. R. & Martin, W. R. (1993) J. Pharmacol. Exp. Ther. 265, 286-293. [PubMed] [Google Scholar]
- 10.Hamann, S. R. & Martin, W. R. (1992) J. Pharmacol. Exp. Ther. 261, 707-715. [PubMed] [Google Scholar]
- 11.Masner, R. (1972) Ann. Med. Exp. Biol. Fenn. 50, 205-212. [PubMed] [Google Scholar]
- 12.Khan, I. M., Taylor, P. & Yaksh, T. L. (1994) J. Pharmacol. Exp. Ther. 270, 150-158. [PubMed] [Google Scholar]
- 13.Rueter, L. E., Meyer, M. D. & Decker, M. W. (2000) Brain Res. 872, 93-101. [DOI] [PubMed] [Google Scholar]
- 14.Khan, I. M., Marsala, M., Printz, M. P., Taylor, P. & Yaksh, T. L. (1996) J. Pharmacol. Exp. Ther. 278, 97-106. [PubMed] [Google Scholar]
- 15.Khan, I. M., Taylor, P. & Yaksh, T. L. (1994) J. Pharmacol. Exp. Ther. 271, 1550-1557. [PubMed] [Google Scholar]
- 16.Khan, I. M., Stanislaus, S., Zhang, L., Taylor, P. & Yaksh, T. L. (2001) J. Pharmacol. Exp. Ther. 297, 230-239. [PubMed] [Google Scholar]
- 17.Urban, L., Willetts, J., Murase, K. & Randic, M. (1989) Brain Res. 500, 12-20. [DOI] [PubMed] [Google Scholar]
- 18.McGehee, D. S. (1999) in Molecular and Functional Diversity of Ion Channels and Receptors, ed. Seeburg, P. (N.Y. Acad. Sci., New York), Vol. 868. [PubMed]
- 19.Lindstrom, J. (1996) Ion Channels 4, 377-450. [DOI] [PubMed] [Google Scholar]
- 20.Sargent, P. B. (1993) Annu. Rev. Neurosci. 16, 403-443. [DOI] [PubMed] [Google Scholar]
- 21.McGehee, D. S. & Role, L. W. (1995) Annu. Rev. Physiol. 57, 521-546. [DOI] [PubMed] [Google Scholar]
- 22.Elgoyhen, A. B., Vetter, D. E., Katz, E., Rothlin, C. V., Heinemann, S. F. & Boulter, J. (2001) Proc. Natl. Acad. Sci. USA 98, 3501-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genzen, J. R., Van Cleve, W. & McGehee, D. S. (2001) J. Neurophysiol. 86, 1773-1782. [DOI] [PubMed] [Google Scholar]
- 24.Broide, R. S. & Leslie, F. M. (1999) Mol. Neurobiol. 20, 1-16. [DOI] [PubMed] [Google Scholar]
- 25.Ninkovic, M. & Hunt, S. P. (1983) Brain Res. 272, 57-69. [DOI] [PubMed] [Google Scholar]
- 26.Matsubayashi, H., Alkondon, M., Pereira, E. F., Swanson, K. L. & Albuquerque, E. X. (1998) J. Pharmacol. Exp. Ther. 284, 904-913. [PubMed] [Google Scholar]
- 27.Li, P., Kerchner, G. A., Sala, C., Wei, F., Huettner, J. E., Sheng, M. & Zhuo, M. (1999) Nat. Neurosci. 2, 972-977. [DOI] [PubMed] [Google Scholar]
- 28.Hori, Y., Endo, K. & Takahashi, T. (1996) J. Physiol. 492, 867-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansvelder, H. D. & McGehee, D. S. (2000) Neuron 27, 349-357. [DOI] [PubMed] [Google Scholar]
- 30.Murase, K., Ryu, P. D. & Randic, M. (1989) Neurosci. Lett. 103, 56-63. [DOI] [PubMed] [Google Scholar]
- 31.Eckert, S. P., Taddese, A. & McCleskey, E. W. (1997) J. Neurosci. Methods 77, 183-190. [DOI] [PubMed] [Google Scholar]
- 32.Mansvelder, H. D., Keath, J. R. & McGehee, D. S. (2002) Neuron 33, 905-919. [DOI] [PubMed] [Google Scholar]
- 33.Alkondon, M. & Albuquerque, E. X. (1993) J. Pharmacol. Exp. Ther. 265, 1455-1473. [PubMed] [Google Scholar]
- 34.Barber, R. P., Phelps, P. E., Houser, C. R., Crawford, G. D., Salvaterra, P. M. & Vaughn, J. E. (1984) J. Comp. Neurol. 229, 329-346. [DOI] [PubMed] [Google Scholar]
- 35.Borges, L. F. & Iversen, S. D. (1986) Brain Res. 362, 140-148. [DOI] [PubMed] [Google Scholar]
- 36.Camara, A. L., Braga, M. F., Rocha, E. S., Santos, M. D., Cortes, W. S., Cintra, W. M., Aracava, Y., Maelicke, A. & Albuquerque, E. X. (1997) Neurotoxicology 18, 589-602. [PubMed] [Google Scholar]
- 37.Ji, D., Lape, R. & Dani, J. A. (2001) Neuron 31, 131-141. [DOI] [PubMed] [Google Scholar]
- 38.Ribeiro-da-Silva, A. & Cuello, A. C. (1990) J. Comp. Neurol. 295, 370-384. [DOI] [PubMed] [Google Scholar]
- 39.Todd, A. J. (1991) Neuroscience 44, 741-746. [DOI] [PubMed] [Google Scholar]
- 40.Baba, H., Kohno, T., Okamoto, M., Goldstein, P. A., Shimoji, K. & Yoshimura, M. (1998) J. Physiol. (London) 508, 83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaksh, T. L., Dirksen, R. & Harty, G. J. (1985) Eur. J. Pharmacol. 117, 81-88. [DOI] [PubMed] [Google Scholar]
- 42.Gray, R., Rajan, A. S., Radcliffe, K. A., Yakehiro, M. & Dani, J. A. (1996) Nature 383, 713-716. [DOI] [PubMed] [Google Scholar]
- 43.McGehee, D. S., Heath, M. J., Gelber, S., Devay, P. & Role, L. W. (1995) Science 269, 1692-1696. [DOI] [PubMed] [Google Scholar]
- 44.Khan, I. M., Buerkle, H., Taylor, P. & Yaksh, T. L. (1998) Neuropharmacology 37, 1515-1525. [DOI] [PubMed] [Google Scholar]
- 45.Gioanni, Y., Rougeot, C., Clarke, P. B., Lepouse, C., Thierry, A. M. & Vidal, C. (1999) Eur. J. Neurosci. 11, 18-30. [DOI] [PubMed] [Google Scholar]
- 46.Fisher, J. L. & Dani, J. A. (2000) Neuropharmacology 39, 2756-2769. [DOI] [PubMed] [Google Scholar]
- 47.Chen, J. & Sandkuhler, J. (2000) Neuroscience 98, 141-148. [DOI] [PubMed] [Google Scholar]
- 48.Sandkuhler, J., Chen, J. G., Cheng, G. & Randic, M. (1997) J. Neurosci. 17, 6483-6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Randic, M., Jiang, M. C. & Cerne, R. (1993) J. Neurosci. 13, 5228-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandkuhler, J. & Liu, X. (1998) Eur. J. Neurosci. 10, 2476-2480. [DOI] [PubMed] [Google Scholar]
- 51.Colino, A. & Malenka, R. C. (1993) J. Neurophysiol. 69, 1150-1159. [DOI] [PubMed] [Google Scholar]
- 52.Fujii, S., Ji, Z., Morita, N. & Sumikawa, K. (1999) Brain Res. 846, 137-143. [DOI] [PubMed] [Google Scholar]
- 53.Damaj, M. I., Meyer, E. M. & Martin, B. R. (2000) Neuropharmacology 39, 2785-2791. [DOI] [PubMed] [Google Scholar]
- 54.Kiyosawa, A., Katsurabayashi, S., Akaike, N. & Pang, Z. P. (2001) J. Physiol. 536, 101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeda, D., Nakatsuda, T., Papke, R. & Gu, J. G. (2003) Pain 101, 13-23. [DOI] [PubMed] [Google Scholar]