Abstract
Kainate receptors (KA-Rs) are members of the glutamate-gated family of ionotropic receptors, which also includes N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptors. KA-Rs are important modulators of interneuron excitability in the CA1 region of the hippocampus. Activation of these receptors enhances interneuron firing, which robustly increases spontaneous inhibitory postsynaptic currents in pyramidal neurons. We report here that ethanol (EtOH) potently inhibits this KA-R-mediated effect at concentrations as low as those that can be achieved in blood after the ingestion of just 1–2 drinks (5–10 mM). Pressure application of kainate, in the presence of AMPA and NMDA receptor antagonists, evoked depolarizing responses in interneurons that triggered repetitive action potential firing. EtOH potently inhibited these responses to a degree that was sufficient to abolish action potential firing. This effect appears to be specific for KA-Rs, as EtOH did not affect action potential firing triggered by AMPA receptor-mediated depolarizing responses. Importantly, EtOH inhibited interneuron action potential firing in response to KA-R activation by synaptically released glutamate, suggesting that our findings are physiologically relevant. KA-R-dependent modulation of glutamate release onto pyramidal neurons was not affected by EtOH. Thus, EtOH increases excitability of pyramidal neurons indirectly by inhibiting the KA-R-dependent drive of γ-aminobutyric acid (GABA)ergic interneurons. We postulate that this effect may explain, in part, some of the paradoxical excitatory actions of this widely abused substance. The excitatory actions of EtOH may be perceived as positive by some individuals, which could contribute to the development of alcoholism.
Kainate receptors (KA-Rs) are glutamate-gated ion channels that play important roles in the regulation of hippocampal excitability. In mossy fiber-to-CA3 pyramidal neuron synapses, KA-Rs mediate synaptic currents and plasticity and modulate glutamate release presynaptically (reviewed in refs. 1 and 2). Although KA-Rs are also present in CA1 pyramidal neurons, these receptors are not activated synaptically (3, 4). In the CA1 region, however, KA-Rs inhibit glutamate release presynaptically (5, 6, 7) and also regulate action potential (AP)-dependent GABA release from interneurons; studies have shown that the frequency of AP-independent GABA release is either unaffected (8–10) or inhibited by KA-R activation (11). Activation of KA-Rs by micromolar concentrations of KA inhibits evoked γ-aminobutyric acid type A (GABAA) receptor (GABAA-R)-mediated inhibitory postsynaptic currents (eIPSCs) in CA1 pyramidal neurons (8, 9, 12–15). However, the precise mechanism and the physiological importance of this effect are a matter of controversy (reviewed in refs. 1 and 16). This effect was initially interpreted to indicate that activation of interneuronal KA-Rs exerts a disinhibitory effect on CA1 pyramidal neurons, but subsequent studies have challenged this interpretation. Studies from different laboratories have consistently shown that KA-Rs depolarize interneurons and induce repetitive AP firing, which results in a robust increase in the frequency of GABAA-R-mediated spontaneous IPSCs (sIPSCs) in CA1 pyramidal neurons (4, 8, 9). Importantly, activation of KA-Rs by synaptically released glutamate enhances axonal excitability in interneurons and increases sIPSC frequency in CA1 pyramidal neurons (10, 17). Therefore, the KA-R-mediated increase of interneuronal GABA release may function as an important homeostatic mechanism that prevents overexcitation of CA1 pyramidal neurons (1, 10, 18). Indeed, it was recently demonstrated that activation of GluR5-containing KA-Rs in interneurons inhibits CA1 pyramidal neurons and prevents seizure propagation in the neonatal hippocampus (19).
KA-Rs have emerged as important targets of alcohol's actions in the central nervous system. Ethanol (EtOH) inhibits recombinant KA-Rs in nonneuronal expression systems and native KA-Rs in cultured neurons at concentrations ≥25 mM (legal intoxication limit in the U.S. is 0.08 g/dl = 17 mM) (20–23). KA-R-mediated synaptic currents in CA3 pyramidal neurons in hippocampal slices are also inhibited by ≥20 mM EtOH (24). Moreover, it was recently discovered that 20–80 mM EtOH inhibits the KA-R-mediated inhibition of eIPSCs in CA1 pyramidal neurons (15), suggesting that KA-Rs in interneurons may also be sensitive to pharmacologically relevant concentrations of this drug of abuse.
Here, we provide direct evidence of a potent inhibitory effect of EtOH on interneuronal KA-Rs, which mediate a significant portion of the glutamatergic excitatory drive of these neurons (25). We demonstrate that interneuron firing in response to KA-R activation is inhibited by EtOH concentrations that can be achieved in blood after the ingestion of just one to two drinks (5–10 mM). We also show that this effect of EtOH results in a substantial decrease in the frequency of KA-R-driven sIPSCs in pyramidal neurons. We postulate that the effects of EtOH on interneuronal KA-Rs could contribute, at least in part, to some of the paradoxical excitatory actions that can be induced by low doses of this widely abused substance.
Materials and Methods
Unless indicated, all chemicals were from Sigma. Experiments were performed in coronal hippocampal slices that were prepared from 21- to 40-day-old male Sprague–Dawley rats. For recordings of sIPSCs from CA1 pyramidal neurons and all interneuron recordings, rats were anesthetized with ketamine 250 mg/kg, and 350- to 400-μM-thick slices were prepared with a vibratome as described (26). Artificial cerebrospinal fluid (ACSF) contained 126 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1 mM MgSO4, 26 mM NaHCO3, 2 mM CaCl2, 0.1 mM DL-AP5 (Tocris–Cookson, Bristol, U.K.), and 10 mM glucose, equilibrated with 95% O2/5% CO2. The α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptor (AMPAR) blocker, GYKI 53655 (30 μM; custom synthesized by Tocris–Cookson), was added to the ACSF to isolate KA-R-mediated responses. When indicated, bicuculline methiodide, 6,7-dinitroquinoxaline-2,3-dione (DNQX; Alexis, San Diego), SCH-50911 (Tocris–Cookson) or EtOH (AAPER Chemical, Shelbyville, KY) were added to the ACSF. After a recovery time of ≥80 min, slices were transferred to a chamber perfused with ACSF at a rate of 2–3 ml/min. Whole-cell patch-clamp electrophysiological recordings from CA1 pyramidal neurons and stratum radiatum–lacunosum moleculare interneurons were performed under infrared-differential interference contrast microscopy at 34°C with an Axopatch 200B amplifier (Axon Laboratories, Union City, CA). Interneurons were identified on the basis of their morphological characteristics, as described elsewhere (27); i.e., their somata appeared round or ovoid and they lacked discernible thick bifurcating apical dendritic processes. We confirmed that neurons with these characteristics corresponded to interneurons by passively filling some of them with 0.5% biocytin followed by fixation in 4% paraformaldehyde and staining with 0.1% streptavidin-cy3 (Jackson Immunolaboratories, West Grove, PA) as described elsewhere (28). Interneurons were visualized with a LSM-510 confocal microscope (Carl Zeiss, Thornwood, NY; University of New Mexico Cancer Center) and their neuronal arborizations were reconstructed from z axis projection images by using LSM5 image browser software. Microelectrodes had resistances of 3–5 MΩ. We recorded sIPSCs at a holding potential of -60 mV by using internal solution containing 140 mM CsCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM EGTA, 10 mM Hepes (pH 7.3), 2 mM Na2-ATP, and 2 mM QX-314 (Tocris–Cookson). The effect of KA-R activation on sIPSC frequency ran down after sequential applications of KA. Therefore, the effect of EtOH on the KA-R-mediated increase of sIPSC frequency was assessed on neurons from slices that had not been previously exposed to KA. Current-clamp experiments (Iholding = 0, unless indicated) were performed with an internal solution containing 135 mM K-gluconate, 10 mM MgCl2, 0.1 mM CaCl2, 1 mM EGTA, 10 mM Hepes (pH 7.3), and 2 mM Na2-ATP. Access resistances were between 20 and 35 MΩ; if access resistance changed >20%, the recording was discarded. A pneumatic picopump (World Precision Instruments, Sarasota, FL) was used to apply puffs of KA or AMPA (Tocris–Cookson). The puffing pipette was placed ≈200 μm from the cell, the pressure was varied between 4 and 7 psi, and the duration was between 2 and 10 sec. Trains of excitatory postsynaptic potentials (EPSPs) were evoked with a concentric bipolar electrode (FHC, Bowdoinham, ME) placed in the stratum radiatum. Data were acquired and analyzed with pClamp7 or 8 (Axon Laboratories); sIPSCs were analyzed with MINIS ANALYSIS program (Synaptosoft, Decatur, GA). For the experiments on the presynaptic actions of KA on glutamatergic terminals, slice preparation and recording methods were as described (24). Data are presented as mean ± SEM.
Results
We studied the modulation by KA of GABAergic tone in CA1 pyramidal neurons in rat hippocampal slices. We recorded IPSCs triggered in pyramidal neurons by the spontaneous AP-dependent release of GABA from interneurons; whole-cell voltage-clamp recordings were performed in the presence of GYKI 53655 (30 μM) and DL-AP5 (100 μM) to block AMPA and N-methyl-D-aspartate (NMDA) receptors (NMDAR), respectively. Spontaneous IPSCs were blocked by bicuculline methiodide, indicating that they were mediated by GABAARs (Fig. 1A; similar results seen in two additional neurons). Application of 0.5 μM KA induced a robust and reversible increase in sIPSC frequency (Figs. 1 B and C and 2). Ethanol potently inhibited this effect of KA (Figs. 1 D and E and 2). The KA-R-mediated increase in sIPSC frequency was blocked by the non-NMDA receptor antagonist DNQX (80 μM; n = 3; data not shown). Because GYKI 53655 was present, this finding confirms that the effect of KA was mediated by KA-Rs. Nonlinear regression analysis yielded an EtOH IC50 of 4.6 mM (95% confidence interval 2.1–10.1 mM; Fig. 2 Inset). Application of EtOH (2–25 mM) alone did not significantly affect basal sIPSC frequency (Figs. 1 D and E and 2); 50 mM EtOH induced a small but significant increase in sIPSC frequency (Fig. 2). The average sIPSC amplitudes in the presence of 2, 5, 10, 25, and 50 mM EtOH were 4.9 ± 3.7% (n = 10), 1.9 ± 3.2% (n = 8), 3.1 ± 3.5% (n = 7), -0.5 ± 6.9% (n = 7), and 0.9 ± 2.6% (n = 9) of control, respectively (data not shown; see Fig. 1D for an illustration of the lack of an effect of 10 mM EtOH alone on sIPSC amplitude).
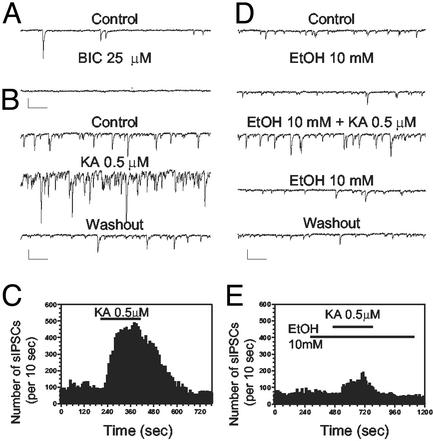
Fig. 1.
EtOH inhibits the KA-R-mediated increase of sIPSC frequency in CA1 pyramidal neurons. (A) sIPSCs are fully blocked by the GABAA-R antagonist, bicuculline (BIC; 25 μM). (B and C) Bath application of KA (0.5 μM) for 3.5 min, in the presence of GYKI 53665 (30 μM) and DL-AP5 (100 μM), induced a robust and reversible sIPSC frequency increase. (D and E) EtOH (10 mM) did not affect either the basal amplitude or frequency of sIPSCs, but reduced the increase in frequency induced by KA-R-activation. (Scale bars: 100 pA and 250 ms.)
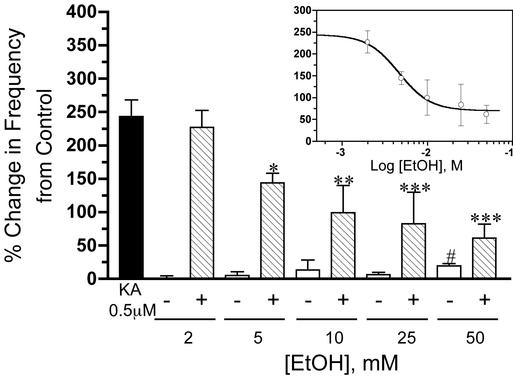
Fig. 2.
EtOH inhibits the KA-R-mediated effect on sIPSC frequency in a concentration-dependent manner. The filled bar represents the average percent change induced by KA (0.5 μM) on sIPSC frequency. Striped bars represent the effect of increasing concentrations of EtOH on this KA-R-dependent effect. Open bars represent the percent change in basal sIPSC frequency induced by EtOH alone. (Inset) A nonlinear regression fit of the inhibitory effect of EtOH on the KA-R-induced increase of sIPSC frequency (IC50 = 4.6 mM). Each bar or symbol represents the mean ± SEM of 7–28 neurons. *, P < 0.05; **, P < 0.01; ***, P < 0.001, by one-way ANOVA followed by Bonferroni's multiple comparison test; #, P < 0.05, by one sample t test versus theoretical mean of zero.
We also recorded directly from interneurons under whole-cell current-clamp conditions. We studied interneurons located in the stratum radiatum near the stratum lacunosum moleculare, such as the one illustrated in Fig. 3. These interneurons extended neurites mainly into the stratum radiatum, stratum pyramidale, and/or stratum oriens. Pressure application of KA (5 μM in the micropipette located ≈200 μm from the soma) in the presence of GYKI 53655 (30 μM) and DL-AP5 (100 μM), caused a reversible depolarization (14 ± 2 mV; n = 8) and repetitive AP firing (42 ± 7 APs per evoked response; n = 8) in these neurons (Fig. 4). Pressure application of 5 μM AMPA in the absence of GYKI, reversibly depolarized the interneurons to a similar extent (21 ± 3 mV; n = 7) and also caused them to fire repetitive APs (95 ± 15 APs per evoked response; n = 7). Injection of depolarizing current pulses (35 pA; 200 msec; Vm = -70 mV) induced a 13 ± 3 mV depolarization in these type of interneurons and repetitive AP firing (6 ± 1 APs per evoked response; n = 5; Fig. 4). Bath application of EtOH (10 mM) significantly reduced the amplitude of KA-R-mediated evoked potentials sufficiently to abolish AP firing (Fig. 4). In contrast, EtOH did not significantly affect the amplitude of AMPAR-mediated evoked potentials or the AP firing in response to AMPA (Fig. 4). Moreover, it did not affect the amplitude of responses evoked by depolarizing current injection or AP firing in response to this depolarization (Fig. 4). Application of 10 mM EtOH alone did not significantly affect either the interneuronal resting membrane potential (control = -68 ± 3 mV and EtOH = -69 ± 3 mV; n = 20) or the membrane resistance (control = 302 ± 17 MΩ and EtOH = 277 ± 15 MΩ; n = 5).
Fig. 3.
Anatomical reconstruction of a stratum radiatum-stratum lacunosum moleculare interneuron. Shown is a single z axis projection of 20 confocal microscopy sections (4 μm) of one of the interneurons that were studied electrophysiologically. See Materials and Methods for details of histochemical procedures. Similar results were obtained with six additional neurons (data not shown). (Scale bar: 100 μm.) L-M, stratum lacunosum moleculare; SR, stratum radiatum; SP, stratum pyramidale; SO, stratum oriens.
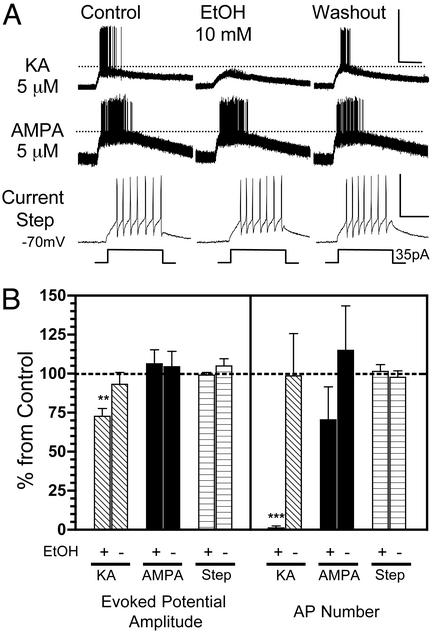
Fig. 4.
EtOH inhibits evoked potentials and AP firing triggered by pressure application of KA onto interneurons. (A) Sample traces of current–clamp recordings (I = 0) from CA1 stratum radiatum-stratum lacunosum moleculare interneurons. (Top) In the presence of DL-AP5 (100 μM) and GYKI 53655 (30 μM), pressure application of KA (5 μM in micropipette located ≈200 μm from the soma) induced reproducible evoked potentials and bursts of AP firing. Bath application of EtOH (10 mM) induced a reversible decrease of the KA-R-mediated evoked potentials and also abolished firing. (Middle) The same experiment was performed by pressure-delivering 5 μM AMPA to another interneuron in the presence of DL-AP5 only. EtOH did not inhibit AMPAR-mediated evoked potential amplitude and AP firing. (Bottom) Lack of an effect of EtOH on depolarization-induced AP firing in another interneuron. (Scale bars for Top and Middle: 50 mV and 5 sec; for Bottom: 50 mV and 100 msec.) (B) Summary of the effects of EtOH on KA-R-dependent and AMPAR-dependent evoked potential amplitude and AP number. Also shown is the summary of the effect of EtOH on the amplitude of depolarization induced by current injection and AP number in response to this current injection. Data were normalized with respect to control responses (represented by the dashed line). +, the effect of 10 mM EtOH; -, the effect of the washout. Each bar represents the mean ± SEM of five to eight neurons. **, P < 0.01, ***, P < 0.001, by one sample t test versus theoretical mean of 100.
We next assessed the effect of EtOH on KA-R-mediated interneuron EPSPs evoked by synaptic glutamate release from Schaffer collateral axonal terminals. These experiments were also performed in the whole-cell current-clamp mode. EPSPs were evoked by trains of five stimuli at 20 Hz to maximize the induction of AP firing. In the presence of blockers of NMDA (DL-AP5; 100 μM), GABAA (bicuculline methiodide; 25 μM) and GABAB (SCH-50911; 20 μM) receptors, repetitive stimulation of the Schaffer collateral reproducibly induced non-NMDA receptor-mediated EPSPs (peak amplitude: 29 ± 2 mV; n = 4) that triggered AP firing (Fig. 5A). In agreement with a recent report (29), GYKI 53655 (30 μM) reduced the peak amplitude of the first non-NMDA EPSP by 63 ± 5% (n = 4) and abolished AP firing (Fig. 5 A–C). In the continuous presence of GYKI, an increase in stimulation intensity enhanced EPSP amplitude and restored AP firing (Fig. 5 A–C). A subsequent application of EtOH (10 mM) significantly reduced the peak amplitude of the KA-R-mediated compound EPSPs and reduced AP firing (Fig. 5). These events were abolished by DNQX (Fig. 5 A–C). Paired-pulse facilitation of KA-R-mediated EPSPs was not affected by EtOH; the ratio of the amplitude of the first to the second EPSP were 2 ± 0.4 (n = 4) and 2.5 ± 0.8 (n = 4) in the absence and presence of EtOH, respectively.
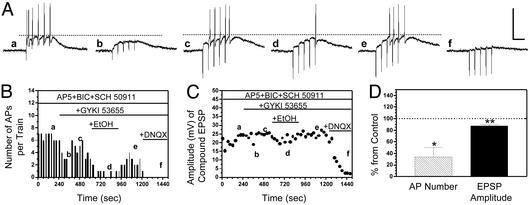
Fig. 5.
EtOH inhibits KA-R-mediated interneuron EPSPs and AP firing evoked by stimulation of the Schaffer collaterals. (A) Sample traces of current–clamp recordings (I = 0) from a CA1 stratum radiatum-stratum lacunosum moleculare interneuron. (a) In the presence of DL-AP5 (100 μM), bicuculline (25 μM), and SCH-50911 (20 μM), a train of five stimuli (20 Hz) delivered in the stratum radiatum induced non-NMDA EPSPs that triggered a burst of APs. (b) GYKI 53665 (30 μM) blocked the AMPA component of the EPSPs and eliminated firing. (c) An elevation in the stimulation intensity increased the amplitude of the KA-R-mediated EPSPs and restored AP firing. (d and e) Bath application of EtOH (10 mM) reversibly decreased the peak amplitude of the KA-R-mediated compound EPSPs and reduced AP number. (f) The KA-R-mediated EPSPs were fully blocked by DNQX (80 μM). (Scale bars: 50 mV and 100 msec.) (B and C) Time courses illustrating the effects of the sequential application of GYKI, EtOH, and DNQX on EPSP amplitude and AP number in the same cell illustrated in A. (D) Summary of the effect of EtOH (10 mM) on AP number and EPSP amplitude. Each bar represents the mean ± SEM of five neurons. *, P < 0.05; **, P < 0.01, by one sample t test versus theoretical mean of 100. Data were normalized with respect to control responses (represented by the dashed line).
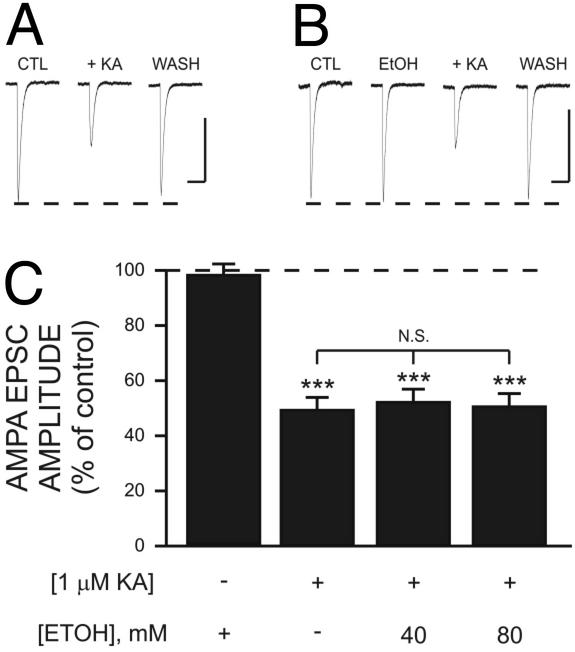
Finally, we determined the effect of EtOH on presynaptic KA-R-dependent modulation of glutamate release in Schaffer collateral-to-CA1 pyramidal neuron synapses. We recorded AMPAR-mediated excitatory postsynaptic currents (EPSCs) that were evoked by electrical stimulation of the stratum radiatum in the presence of D-AP5 (50 μM) and bicuculline methiodide (20 μM). In agreement with previous reports (5, 6, 7), we found that 1 μM KA inhibited the amplitude of AMPA EPSCs (Fig. 6 A and C). EtOH (40 and 80 mM) alone had no effect on the amplitude of AMPA EPSCs and it did not significantly affect the KA-induced inhibition of AMPAR-mediated EPSCs (Fig. 6 B and C). A comparable result was obtained for NMDAR-mediated EPSCs recorded in the presence of bicuculline methiodide (20 μM) and NBQX (1 μM). NMDAR-dependent EPSCs were inhibited by 46 ± 4% (n = 17) and 53 ± 4% (n = 11) by 1 μM KA in the absence and presence of 80 mM EtOH, respectively (data not shown).
Fig. 6.
EtOH has no effect on presynaptic KA receptor-dependent inhibition of AMPA EPSCs in CA1 pyramidal neurons. Traces are averages of five to nine AMPA EPSCs recorded in the presence of bicuculline (20 μM) and D-AP5 (50 μM). Representative traces illustrating the inhibitory effect of 1 μM KA on AMPAR-mediated EPSCs in the absence (A) and presence (B) of 80 mM EtOH. Note that EtOH alone has no effect on the amplitude of AMPA EPSCs or KA inhibition of AMPA EPSCs. (Scale bars: 100 pA and 200 msec.) (C) Summary of the effect of EtOH on KA inhibition of AMPA EPSCs. ***, P < 0.001 with respect to responses obtained in the presence of EtOH alone by one-way ANOVA; each bar represents the mean ± SEM of 7–27 neurons. Data were normalized with respect to control responses (represented by the dashed line).
Discussion
We demonstrate here that EtOH potently inhibits KA-R function in CA1 hippocampal interneurons. We initially tested the effect of EtOH on KA-R-mediated modulation of GABAergic tone in this region. In agreement with previous reports, we found that bath application of KA, in the presence of AMPAR and NMDAR blockers, enhances spontaneous AP-dependent GABA release from interneurons, resulting in a massive increase in sIPSC frequency in pyramidal neurons (8, 9, 18). Importantly, we found that EtOH inhibits this KA-R-dependent effect with an IC50 of 4.6 mM. By comparison, a number of studies have reported that EtOH concentrations ≥10 mM are required to significantly inhibit NMDAR-dependent population EPSPs in the CA1 region of hippocampal slices (30–32). Moreover, significant inhibition of AMPAR-mediated population EPSPs was only observed with 100 mM EtOH in this hippocampal region (30). This result is in agreement with our finding that AMPAR-mediated responses in CA1 pyramidal neurons and interneurons are not significantly affected by EtOH. Consequently, interneuronal KA-Rs appear to be the most EtOH sensitive glutamatergic ionotropic receptor subtype in the CA1 region of the rat hippocampus. This conclusion can be extended to the CA3 hippocampal region, where we found that KA-Rs are the only members of the ionotropic glutamate receptor family that are significantly inhibited by 20–40 mM EtOH (24).
Our study also demonstrates that 2–50 mM EtOH does not affect the amplitude of basal GABAA-mediated sIPSCs. This result is in general agreement with several reports that concentrations of EtOH ≥40 mM are required to significantly potentiate GABAA-R-mediated postsynaptic responses in CA1 pyramidal neurons (refs. 33 and 34; reviewed in ref. 35). Moreover, we found that EtOH, at concentrations ≤25 mM, does not significantly affect basal sIPSC frequency, indicating that it does not directly modulate GABA release in response to spontaneous AP firing in CA1 interneurons. It also suggests that spontaneous GABAergic transmission is not under the tonic control of KA-Rs under our recording conditions. If this had been the case, we would have expected to observe inhibition of basal sIPSC frequency in the presence of EtOH. This finding is not completely unexpected given that glutamate is just one of the many neurotransmitters that regulate spontaneous firing in CA1 hippocampal interneurons (36). Indeed, several studies have demonstrated that blockade of glutamate receptors does not significantly inhibit basal sIPSC frequency in hippocampal pyramidal neurons (37, 38). It should be noted that KA-Rs may control spontaneous CA1 interneuronal firing under some conditions; it was recently established that KA-R-mediated spontaneous EPSCs can contribute ≈50% of the glutamate-induced current in CA1 interneurons in slices from immature rats (25).
Crowder et al. (15) recently found that EtOH, at concentrations ≥20 mM, significantly inhibits the KA-R-dependent inhibition of eIPSCs in CA1 pyramidal neurons. In contrast, the results of the present study indicate that EtOH concentrations as low as 5 mM are sufficient to inhibit the KA-R-mediated increase in sIPSC frequency in these neurons. The apparent differential EtOH sensitivity of the KA-R-mediated effects described by Crowder et al. (15) and those presented here may simply reflect that assessing the effect of EtOH on the KA-R-dependent increase in sIPSC frequency is a more sensitive assay. Alternatively, this discrepancy could be caused by the fact that two different populations of KA-Rs may mediate the inhibition of eIPSCs and the potentiation of sIPSC frequency (11). For instance, the KA-induced inhibition of monosynaptic IPSCs, but not the potentiation of sIPSCs, has been shown to depend on the activation of Gi/o proteins, phospholipase C, and protein kinase C in the presynaptic terminal (11). However, the KA-R-induced increase in the success rate of unitary IPSCs is not affected by protein kinase C inhibition (10). In addition, activation of KA-Rs with low concentrations of glutamate causes a significant reduction in eIPSC amplitude without affecting sIPSC frequency (11). Thus, it is possible that KA-Rs that inhibit eIPSCs have lower sensitivity to EtOH than those that potentiate sIPSCs, which could be due to differences in their subunit composition, posttranslational regulation, or association with other proteins. The latter possibility seems more likely given that recombinant KA-Rs with specific differences in their subunit composition are all similarly sensitive to acute EtOH exposure (21) and that changes in phosphorylation of homomeric recombinant GluR6 receptors do not affect acute sensitivity to EtOH (39). However, it must be kept in mind that the role of subunit composition and phosphorylation in determining the sensitivity of native KA-Rs to EtOH has yet to be investigated. For instance, studies with knockout mice lacking GluR5 and GluR6 subunits indicate that KA-Rs in interneurons of the stratum radiatum are heteromers containing both of these subunits (18) and their coassembly may increase sensitivity to acute EtOH. As for KA-Rs in GABAergic interneurons, differences in subunit composition, association with other proteins or posttranslational regulation could also explain the lack of sensitivity to EtOH of KA-Rs in CA1 glutamatergic terminals. For instance, KA-Rs in CA1 glutamatergic terminals modulate excitatory synaptic transmission via a presynaptic G protein-dependent mechanism (7).
Taken together, the results of the study of Crowder et al. (15) and those reported here indicate that EtOH can exert apparently opposite actions on the excitability of pyramidal neurons via its interactions with interneuronal KA-Rs. On one hand, it reduces the KA-R-dependent excitatory drive to interneurons, which decreases tonic (i.e., sIPSCs) GABAergic transmission in pyramidal neurons (i.e., disinhibits). On the other, EtOH decreases the KA-R-dependent inhibition of phasic (i.e., eIPSCs) GABAergic transmission in these neurons (i.e., increases inhibition). Thus, the findings of these studies raise the question as to which of these effects would be more physiologically relevant in vivo. Given that pyramidal neurons in the intact hippocampus should receive GABAergic input in the form of phasic activity superimposed on tonic activity, we believe that both effects are important. The EtOH-induced decrease in the KA-R-driven GABAergic inhibitory tone will reduce responsiveness of pyramidal neurons to excitatory input. Our data suggest that the dominant effect at very low EtOH concentrations (5–10 mM) will be to depress this inhibitory tone, thereby increasing neuronal excitability. On top of this effect, EtOH will also decrease the KA-R-dependent inhibition of eIPSCs at higher concentrations (i.e., ≥20 mM). Assuming that the physiological counterpart of an eIPSC corresponds to the phasic activity driven by the coordinated firing of interneuronal ensembles, then the EtOH-induced attenuation of the KA-R-mediated inhibition of eIPSCs will likely have an impact on network activity in the CA1 region (40, 41). Indeed, it has been demonstrated that an enhancement in the glutamatergic excitatory drive to pools of hippocampal interneurons increases the frequency of their oscillatory activity (42). However, whether KA-Rs participate in the regulation of this type of activity remains an open question for future research. It should be emphasized, however, that at concentrations ≥20 mM, EtOH will also affect (i) KA-Rs in CA3 pyramidal neurons (24) and (ii) other neuronal proteins (for example, GABAA-Rs and NMDARs). The end result of its combined actions on all of these proteins is believed to cause neuronal depression (reviewed in ref. 35).
A key finding of our study is that the mechanism of action of EtOH involves an all-or-none effect on interneuronal firing. We found that EtOH inhibited KA-R-mediated evoked potentials to a degree that is sufficient to abolish interneuron firing in response to activation of these receptors. This finding explains the relatively steep relationship between EtOH concentration and inhibition of the KA-induced increase in sIPSC frequency. This steep concentration dependence likely reflects the EtOH-induced progressive inhibition of KA-R-evoked firing in the different interneurons that synapse onto the particular pyramidal neuron under study. Interestingly, 10 mM EtOH inhibited responses evoked by pressure application of KA by only ≈25%; however, the KA-induced increase in sIPSC frequency was reduced by ≈60% by this concentration of EtOH. These findings suggest that a relatively modest effect of ethanol on interneuronal KA-Rs becomes nonlinearly amplified into a more substantial decrease in tonic inhibition of principal neurons. This suggestion is important given that pharmacologically relevant concentrations of EtOH have generally been found to produce relatively small effects on the function of neurotransmitter-gated and voltage-gated ion channels (reviewed in ref. 35). Thus, our results clearly illustrate that effects of EtOH, which may seem minimal when examined in isolated neurons, could have a dramatic impact on the excitability of neurons when studied in the context of a circuit such as the one studied here.
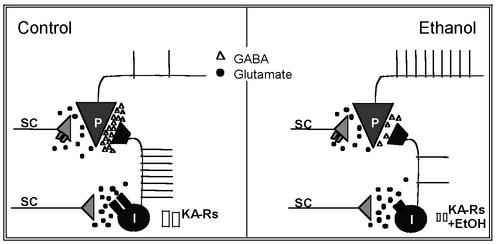
Our study also shows that EtOH inhibits interneuron firing in response to KA-R activation by synaptically released glutamate. This finding suggests that the results obtained with exogenous application of KA have physiological relevance. We postulate that the effects of EtOH on interneuronal KA-Rs are likely to have profound effects on the excitability of pyramidal neurons as illustrated by the simplified model shown in Fig. 7. Numerous studies have established that at concentrations produced by the ingestion of one to two drinks (5–10 mM), EtOH causes paradoxical excitatory effects, including memory facilitation (43, 44, 45). Importantly, an increase in the spontaneous firing rate of single units in the dorsal hippocampus has been demonstrated after injection of low doses of EtOH into rats (46). However, mechanisms explaining how a powerful central nervous system depressant such as EtOH can produce these paradoxical excitatory effects have remained elusive. The results of our study suggest that inhibition of the KA-R-dependent excitatory drive of inhibitory interneurons could explain, at least in part, some of these stimulatory actions of EtOH, which may be perceived as positive by some individuals and contribute to the development of alcoholism.
Fig. 7.
Simplified model of the EtOH-induced inhibition of the KA-R-mediated excitatory drive of interneurons. (Left) Under control conditions, glutamate (circles) released from the Schaffer collaterals (SC) activates interneuronal KA-Rs, which depolarize interneurons (I), and induces AP firing. This effect triggers sIPSCs onto CA1 pyramidal neurons (P); GABA is represented by triangles. Presynaptic KA-Rs in Schaffer collateral glutamatergic terminals that synapse onto CA1 pyramidal neurons are depicted in gray. Activation of these receptors inhibits glutamate release. The net effect of KA-Rs activation is to increase tonic inhibition of CA1 pyramidal neuron's excitability. AMPARs and axonal KA-Rs are not depicted for clarity. (Right) In the presence of low concentrations of EtOH (5–10 mM), KA-Rs in interneurons are inhibited, which reduces the excitatory drive to these neurons, reduces GABAA-R-mediated sIPSCs, and increases the excitability of pyramidal neurons. KA-Rs in glutamatergic terminals are unaffected by EtOH. The effects of higher concentrations of EtOH (≥20 mM) are not depicted for clarity (see Discussion); at these concentrations, EtOH will (i) reduce the inhibitory effect of KA-R activation on eIPSCs (15), (ii) inhibit KA-R-mediated synaptic currents in CA3 pyramidal neurons (24), and (iii) modulate the function of other neuronal proteins, including GABAA-Rs and NMDARs.
Acknowledgments
This paper is dedicated to the memory of Dr. Tom Dunwiddie. We thank D. Partridge for critically reading the manuscript. This work was supported by U.S. Army Grant DAMD17-00-1-0579, the Alcoholic Beverage Medical Foundation, and the Wake Forest University Center for the Neurobehavioral Study of Alcohol (supported by National Institutes of Health Grant AA11997).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACSF, artificial cerebrospinal fluid; AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionate; AMPAR, AMPA receptor; AP, action potential; DNQX, 6,7-dinitroquinoxaline-2,3-dione; EPSC, excitatory postsynaptic current; EPSP, excitatory postsynaptic potential; EtOH, ethanol; GABA, γ-aminobutyric acid; IPSC, inhibitory postsynaptic current; sIPSC, spontaneous IPSC; eIPSC, evoked IPSC; KA, kainate; KA-R, KA receptor; NMDA, N-methyl-D-aspartate; NMDAR, NMDA receptor.
References
- 1.Kullmann, D. M. (2001) Neuron 32, 561-564. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz, D., Mellor, J., Frerking, M. & Nicoll, R. A. (2001) Proc. Natl. Acad. Sci. USA 98, 11003-11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castillo, P. E., Malenka, R. C. & Nicoll, R. A. (1997) Nature 388, 182-186. [DOI] [PubMed] [Google Scholar]
- 4.Bureau, I., Bischoff, S., Heinemann, S. F. & Mulle, C. (1999) J. Neurosci. 19, 653-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamiya, H. & Ozawa, S. (1998) J. Physiol. (London) 509, 833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vignes, M., Clarke, V. R., Parry, M. J., Bleakman, D., Lodge, D., Ornstein, P. L. & Collingridge, G. L. (1998) Neuropharmacology 37, 1269-1277. [DOI] [PubMed] [Google Scholar]
- 7.Frerking, M., Schmitz, D., Zhou, Q., Johansen, J. & Nicoll, R. A. (2001) J. Neurosci. 21, 2958-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cossart, R., Esclapez, M., Hirsch, J. C., Bernard, C. & Ben-Ari, Y. (1998) Nat. Neurosci. 1, 470-478. [DOI] [PubMed] [Google Scholar]
- 9.Frerking, M., Malenka, R. C. & Nicoll, R. A. (1998) Nat. Neurosci. 1, 479-486. [DOI] [PubMed] [Google Scholar]
- 10.Jiang, L., Xu, J., Nedergaard, M. & Kang, J. (2001) Neuron 30, 503-513. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Moreno, A. & Lerma, J. (1998) Neuron 20, 1211-1218. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Moreno, A., Herreras, O. & Lerma, J. (1997) Neuron 19, 893-901. [DOI] [PubMed] [Google Scholar]
- 13.Clarke, V. R., Ballyk, B. A., Hoo, K. H., Mandelzys, A., Pellizzari, A., Bath, C. P., Thomas, J., Sharpe, E. F., Davies, C. H., Ornstein, P. L., et al. (1997) Nature 389, 599-603. [DOI] [PubMed] [Google Scholar]
- 14.Min, M. Y., Melyan, Z. & Kullmann, D. M. (1999) Proc. Natl. Acad. Sci. USA 96, 9932-9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowder, T. L., Ariwodola, O. J. & Weiner, J. L. (2002) J. Pharmacol. Exp. Ther. 303, 937-944. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Ari, Y. & Cossart, R. (2000) Trends Neurosci. 23, 580-587. [DOI] [PubMed] [Google Scholar]
- 17.Semyanov, A. & Kullmann, D. M. (2001) Nat. Neurosci. 4, 718-723. [DOI] [PubMed] [Google Scholar]
- 18.Mulle, C., Sailer, A., Swanson, G. T., Brana, C., O'Gorman, S., Bettler, B. & Heinemann, S. F. (2000) Neuron 28, 475-484. [DOI] [PubMed] [Google Scholar]
- 19.Khalilov, I., Hirsch, J., Cossart, R. & Ben-Ari, Y. (2002) J. Neurophysiol. 88, 523-527. [DOI] [PubMed] [Google Scholar]
- 20.Dildy-Mayfield, J. E. & Harris, R. A. (1995) J. Neurosci. 15, 3162-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valenzuela, C. F. & Cardoso, R. A. (1999) J. Pharmacol. Exp. Ther. 288, 1199-1206. [PubMed] [Google Scholar]
- 22.Valenzuela, C. F., Bhave, S., Hoffman, P. & Harris, R. A. (1998) J. Neurochem. 71, 1777-1780. [DOI] [PubMed] [Google Scholar]
- 23.Costa, E. T., Soto, E. E., Cardoso, R. A., Olivera, D. S. & Valenzuela, C. F. (2000) Alcohol Clin. Exp. Res. 24, 220-225. [PubMed] [Google Scholar]
- 24.Weiner, J. L., Dunwiddie, T. V. & Valenzuela, C. F. (1999) Mol. Pharmacol. 56, 85-90. [DOI] [PubMed] [Google Scholar]
- 25.Cossart, R., Epsztein, J., Tyzio, R., Becq, H., Hirsch, J., Ben-Ari, Y. & Crepel, V. (2002) Neuron 35, 147-159. [DOI] [PubMed] [Google Scholar]
- 26.Shuttleworth, C. W. & Connor, J. A. (2001) J. Neurosci. 21, 4225-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christie, B. R., Franks, K. M., Seamans, J. K., Saga, K. & Sejnowski, T. J. (2000) Hippocampus 10, 673-683. [DOI] [PubMed] [Google Scholar]
- 28.Buhler, A. V. & Dunwiddie, T. V. (2002) J. Neurophysiol. 87, 548-557. [DOI] [PubMed] [Google Scholar]
- 29.Frerking, M. & Ohliger-Frerking, P. (2002) J. Neurosci. 22, 7434-7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovinger, D. M., White, G. & Weight, F. F. (1990) J. Neurosci. 10, 1372-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrisett, R. A., Martin, D., Oetting, T. A., Lewis, D. V., Wilson, W. A. & Swartzwelder, H. S. (1991) Neuropharmacology 30, 1173-1178. [DOI] [PubMed] [Google Scholar]
- 32.Swartzwelder, H. S., Wilson, W. A. & Tayyeb, M. I. (1995) Alcohol Clin. Exp. Res. 19, 1480-1485. [DOI] [PubMed] [Google Scholar]
- 33.Weiner, J. L., Gu, C. & Dunwiddie, T. V. (1997) J. Neurophysiol. 77, 1306-1312. [DOI] [PubMed] [Google Scholar]
- 34.Weiner, J. L., Valenzuela, C. F., Watson, P. L., Frazier, C. J. & Dunwiddie, T. V. (1997) J. Neurochem. 68, 1949-1959. [DOI] [PubMed] [Google Scholar]
- 35.Little, H. J. (1999) Pharmacol. Ther. 84, 333-353. [DOI] [PubMed] [Google Scholar]
- 36.Parra, P., Gulyas, A. I. & Miles, R. (1998) Neuron 20, 983-993. [DOI] [PubMed] [Google Scholar]
- 37.Bergles, D. E., Doze, V. A., Madison, D. V. & Smith, S. J. (1996) J. Neurosci. 16, 572-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McBain, C. J., Eaton, J. V., Brown, T. & Dingledine, R. (1992) Brain Res. 592, 255-260. [DOI] [PubMed] [Google Scholar]
- 39.Valenzuela, C. F., Cardoso, R. A., Lickteig, R., Browning, M. D. & Nixon, K. M. (1998) Alcohol Clin. Exp. Res. 22, 1292-1299. [PubMed] [Google Scholar]
- 40.Buzsaki, G. (2001) Neurochem. Res. 26, 899-905. [DOI] [PubMed] [Google Scholar]
- 41.Jefferys, J. G., Traub, R. D. & Whittington, M. A. (1996) Trends Neurosci. 19, 202-208. [DOI] [PubMed] [Google Scholar]
- 42.Traub, R. D., Whittington, M. A., Colling, S. B., Buzsaki, G. & Jefferys, J. G. (1996) J. Physiol. 493, 471-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips, T. J. & Shen, E. H. (1996) Int. Rev. Neurobiol. 39, 243-282. [DOI] [PubMed] [Google Scholar]
- 44.Rossetti, Z. L., Carboni, S., Stancampiano, R., Sori, P., Pepeu, G. & Fadda, F. (2002) Alcohol Clin. Exp. Res. 26, 181-185. [PubMed] [Google Scholar]
- 45.Parker, E. S., Morihisa, J. M., Wyatt, R. J., Schwartz, B. L., Weingartner, H. & Stillman, R. C. (1981) Psychopharmacology (Berlin) 74, 88-92. [DOI] [PubMed] [Google Scholar]
- 46.Grupp, L. A. (1980) Psychopharmacology (Berlin) 70, 95-103. [DOI] [PubMed] [Google Scholar]