Abstract
Long-chain fatty acid uptake, which provides a large part of myocardial energy, is impaired in human and murine hearts deficient in the membrane fatty acid translocase, FAT/CD36. We examined myocardial function in CD36-null mice using the working heart. Fatty acid oxidation and stores of glycogen, triglycerides, and ATP were reduced in CD36-deficient hearts and were restored to WT levels by rescue of myocyte CD36. Under normal perfusion conditions, CD36-null hearts had similar cardiac outputs and end-diastolic pressures as WT or transgenic hearts. After 6 min of ischemia, cardiac output decreased by 41% and end diastolic pressure tripled for CD36-null hearts, with no significant changes in WT or transgenic hearts. Null hearts also failed more frequently after ischemia as compared with WT or transgenics. To dissect out contribution of fatty acid uptake, a perfusate-lacking fatty acids was used. This decreased cardiac output after ischemia by 30% in WT hearts as compared with 50% for CD36-deficient hearts. End diastolic pressure, a negative index of myocardial performance, increased after ischemia in all heart types. Addition to the perfusate of a medium-chain fatty acid (caprylic acid) that does not require CD36 for uptake alleviated poor ischemic tolerance of CD36-null hearts. In summary, recovery from ischemia is compromised in CD36-deficient hearts and can be restored by CD36 rescue or by supplying medium-chain fatty acids. It would be important to determine whether the findings apply to the human situation where polymorphisms of the CD36 gene are relatively common.
Keywords: CD36 rescue, working heart, fatty acid oxidation
Fatty acid (FA) uptake consists of two components, passive diffusion and carrier-mediated transport specific for FA with >8–10 carbons (1). An 88-kDa glycoprotein, FAT (2), a homolog of human CD36 (3, 4) was implicated in FA transport by labeling with the transport inhibitor sulfo-N-succinimidyl oleate (1, 5). The role of FAT/CD36 in FA uptake was confirmed by studies of mice with CD36 deficiency or overexpression (6, 7). CD36 is abundant in the heart (2, 8), and its deficiency is associated with a 60–80% decrease in myocardial FA uptake (9, 10) and with a severalfold compensatory increase in glucose utilization (11).
There is strong evidence for a critical role of CD36-facilitated FA uptake during muscle contraction, which was shown to recruit the protein to the plasma membrane (12). Muscle-targeted overexpression of CD36 enhanced FA oxidation in response to contraction severalfold (7). In line with this, CD36-null mice (6) perform poorly on treadmill and swimming tests (A.I., unpublished observations). However the impact of CD36 deficiency on the performance of the heart, which relies on FA for energy, remains unknown. Such information may have clinical relevance because incidence of CD36 deficiency in humans ranges between 0.3% and 18.5% depending on the population (13). CD36-deficient humans have a defect in myocardial FA uptake (14, 15) that is similar in magnitude to that observed in the CD36-null mouse (16) and may be at risk for cardiomyopathy (17). In addition, CD36 deficiency, which increases susceptibility to insulin resistance from high glycemic diets (11, 18), could play a role in the etiology of diabetic cardiomyopathy (19). In this report, we have examined cardiac performance (output and end diastolic pressure) during ischemia–reperfusion in three mice models, with different levels of CD36 expression, and by using perfusions with and without FA.
Materials and Methods
Animal Protocols. All protocols were approved by Stony Brook University in accordance with New York State and Federal guidelines (Institutional Animal Care and Use Committee nos. 01-0514 and 01-0834).
Animal Models. Two mice models of altered CD36 expression were used: CD36-null mice (knockout, KO) were previously generated by Febbraio et al. (6) by targeted homologous recombination and backcrossed four times to C57BL/6 mice. CD36-null mice rescued for CD36 in heart and skeletal muscle (gene rescued, GR) were generated from the KO mice by using the muscle creatine kinase promoter as described (7). WT (C57BL/6) mice were used as controls.
Ischemia and Reperfusion of Working Hearts. Hearts were mounted on the perfusion apparatus as described (20). The perfusate consisted of bicarbonate buffer (KB) (135 mM/liter NaCl/25 mM/liter NaHCO3/4.7 mM/liter KCl/1.2 mM/liter MgSO4/1.2 mM/liter KH2PO4/2.5 mM/liter CaCl2, pH 7.4) containing 11 mM glucose. The buffer contained 1.2 mM palmitate or caprylic acid bound to 3% (0.44 mM) BSA except when FA was omitted, as indicated. The perfusate was oxygenated (95% O2/5% CO2) and maintained at a temperature of 38.5°C by using heated water jackets. The perfusion system could be used in two settings; retrograde (Langendorff mode) with perfusion via the aorta, and antegrade (working mode), with perfusion via the left atrium. Hearts were equilibrated for 5 min in the Langendorff mode and were then switched to the working mode against an afterload of 50 mmHg (1 mmHg = 133 Pa). A 24-gauge plastic cannula, connected to a pressure catheter probe (Millar Instruments, Houston), was placed in the left ventricular cavity via the apex to continuously measure end-systolic and end-diastolic pressures. Coronary flow was determined by collecting effluent from the heart and aortic flow by collecting aortic effluent from a height of 50 mmHg.
Preload was gradually increased from 5 to 25 mmHg (5-mmHg intervals) by raising the height of the preload reservoir, and data were acquired 30 s after changing preload pressure at each setting. The time at each preload was <90 s, and the total duration of the measurements averaged 8 min.
To examine ischemic tolerance, hearts were subjected to 6 min of global ischemia by occluding perfusate inflow and outflow, and this was followed by 8 min of reperfusion (5 min in the Langendorff mode and 3 min in the working mode). Then the same preload-dependent experiment was repeated, as described above. Hearts were not paced and were excluded from the study when they exhibited unstable pressure (>50% variation in pressure at steady state over 120 s).
Cardiomyocyte Isolation and Palmitate Oxidation. Cardiomyocytes, isolated from adult mouse hearts as described by Schaap et al. (21), were maintained in buffer (KH) consisting of 115 mM NaCl, 2.6 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1.0 mM CaCl2, 10 mMNaHCO3, and 10 mM Hepes, supplemented with 11 mM D-glucose and 2% BSA. FA oxidation was assayed by using 0.2 mM palmitate and 0.45 μCi/ml [1-14C]palmitate (ICN; 1 Ci = 37 GBq) bound to 2% BSA. Incubations were carried out at 37°C in glass vials (Kontes) under an atmosphere of 95% O2/5% CO2. At the end of the incubation, the medium was quickly transferred into vials equipped with a center well filled with 200 μl of ethanolamine/ethylene glycol (1:2, vol/vol). Perchloric acid was added through the cap to a concentration of 0.6 mM and the vials were incubated overnight with light shaking. The 14CO2 produced was determined by counting the ethanolamine/ethylene glycol mixture.
Measurement of Myocardial Glucose Uptake. Glucose uptake was evaluated in vivo (11) by injecting 12 μCi of 18F-fluorodeoxyglucose into the tail vein of mice fasted for 6 h. The mice were killed 2 h later, and the hearts were removed, weighed, and counted for radioactivity in a γ counter.
Triacylglycerol, Glycogen, and ATP Levels in Muscle. Mice hearts were quickly rinsed in saline, clamped between aluminum tongs precooled in liquid nitrogen, and stored at -80°C. Muscle ATP was measured after extraction in perchloric acid, enzymatically by using a kit from Sigma. Glycogen was measured as glucose after hydrolysis with KOH (30%) and HCL (0.6 N) (22). Triacylglycerol content was determined after lipid extraction as described (23). Tissue protein was determined according to Markwell et al. (24).
Immunostaining. Hearts isolated from anesthetized mice were rinsed in saline and immersed in formalin followed by sucrose (25). Cryosections on glass slides were washed and incubated with a primary polyclonal anti-CD36 antipeptide (F2-35) generated and characterized in our laboratory. A fluorescein (FITC)-conjugated goat anti-rat IgG (Jackson ImmunoResearch) was used as secondary antibody. The slides were examined by using a confocal microscope.
Results
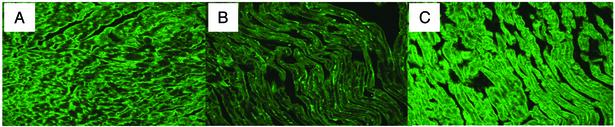
Reversal of the Metabolic Phenotype of CD36 Deficiency in Hearts Rescued for Myocyte CD36 Expression. Mice rescued for the CD36 gene (GR) in heart and muscle were generated from CD36 KO mice by using the muscle creatinine kinase promoter. Fig. 1 shows the levels of CD36 expression achieved in hearts of GR mice as compared with those from KO and WT hearts. Estimation based on Western blot analysis indicated that the level of CD36 protein in GR hearts was about double that of WT hearts (data not shown).
Fig. 1.
Cardiac expression of CD36 protein in different mouse models. Heart sections were prepared and incubated with polyclonal antibody against CD36 followed by a fluorescein-conjugated goat anti-rabbit IgG. Confocal images (equal exposure time) are shown. (A) WT. (B) CD36-null. (C) CD36 GR.
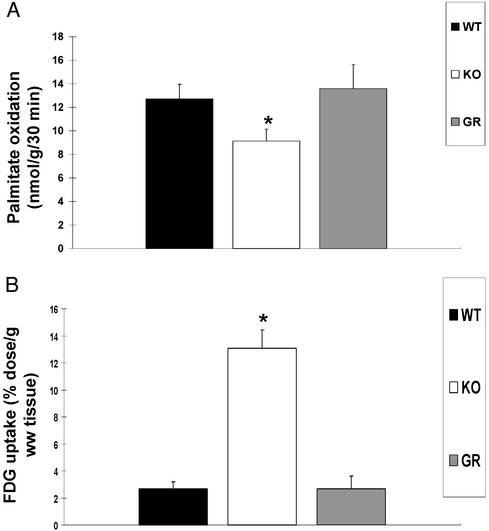
There was no difference in body and heart weights among WT, KO, and GR mice, but the heart/body weight ratio was significantly increased in CD36 KO mice (Table 1). Defective FA uptake has been demonstrated in hearts from CD36-deficient mice (9), rats (26), and humans (14, 15, 27) by using a FA derivative that can be esterified but that is poorly oxidized. To document the extent of impairment in FA oxidation consequent to the defect in FA uptake and to determine whether it was reversed by rescue of CD36 expression, palmitate oxidation and glucose uptake were examined. As shown in Fig. 2, CD36 deficiency caused a significant drop in palmitate oxidation, which was reversed by CD36 rescue. Also reversed was the large compensatory increase in glucose utilization observed in CD36 KO hearts.
Table 1. Body and heart weights for WT, CD36-null (KO), and CD36 rescued (GR) mice.
| Group (n) | Body weight, g | Heart weight, g | Heart/body (×100) |
|---|---|---|---|
| WT (15) | 26.6 ± 0.8 | 0.24 ± 0.01 | 0.91 ± 0.02 |
| KO (15) | 25.4 ± 0.7 | 0.26 ± 0.01 | 1.01 ± 0.03* |
| GR (10) | 26.0 ± 0.9 | 0.24 ± 0.02 | 0.94 ± 0.04 |
Data are means (±SEM).
P < 0.05; WT vs. KO.
Fig. 2.
Palmitate oxidation and glucose uptake before and after transgenic rescue of CD36. (A) Palmitate oxidation rates of isolated cardiomyocytes from WT, CD36-null (KO), and CD36 rescued (GR) mice were determined at a FA/BSA ratio of 0.67. Data are means ± SEM (n = 6 per group). *, Significantly different from WT and GR (P < 0.01). (B) 18F-2-FDG uptake in WT, CD36-null, and GR hearts. Mice were injected with 12 μCi of 18F-2-FDG in a lateral tail vein. Hearts were removed, weighed, and counted for 18F-2-FDG radioactivity 120 minutes after injection; uptake rate is expressed per gram of wet tissue. Data are means ± SEM (n = 3 per group). *, Significantly different from WT and GR (P < 0.02).
The impaired ability of the CD36-deficient myocardium to metabolize FA was associated with reduced energy stores as shown in Table 2. Levels of glycogen, triglycerides, and ATP were significantly lower in hearts from CD36-null mice as compared with either WT or CD36 transgenic mice. These data documented that rescuing myocyte CD36 expression is associated with a reversal of the metabolic phenotype of myocardial CD36 deficiency.
Table 2. Glycogen, triglycerides, and ATP contents in hearts from WT, CD36-null (KO), and CD36 rescued (GR) mice.
| Group (n) | Glycogen, μg glucose/mg of protein | Triglycerides, μg/mg of protein | ATP, μmol/g of tissue |
|---|---|---|---|
| WT (4) | 1.43 ± 0.21 | 28.52 ± 4.82 | 5.35 ± 0.43 |
| KO (4) | 0.78 ± 0.10* | 20.81 ± 2.13* | 3.32 ± 0.47* |
| GR (4) | 1.01 ± 0.11 | 30.65 ± 5.03 | 6.56 ± 0.75 |
Data are means ± SEM.
P < 0.01, WT and GR versus KO.
Optimal Period of Ischemic Insult. Tolerance to ischemia has not been well studied in the murine heart, and as a result, there is little information related to what periods of ischemia are appropriate for this model. Although long periods of global ischemia (30–50 min) were used with mice hearts in the Langendorff mode (28–30), they were inadequate for the working preparation where severe hemodynamic dysfunction was observed with ischemic periods longer than 10 min (31).
In our hands, an ischemic insult of 12 min caused 100% functional failure of WT hearts, and 8 min was still associated with a significant failure rate. Decreasing the ischemic episode to 6 min allowed recovery of the majority of hearts tested and consequently was used for all experiments.
CD36 Deficiency and Heart Function Before and After Ischemia. The impact of CD36 deficiency on heart function was evaluated by measuring cardiac output and end-diastolic pressure before and after 6 min of global ischemia in working hearts isolated from KO, WT, and GR mice. To determine the effect of FA metabolism more specifically, FA were omitted from the perfusate when indicated.
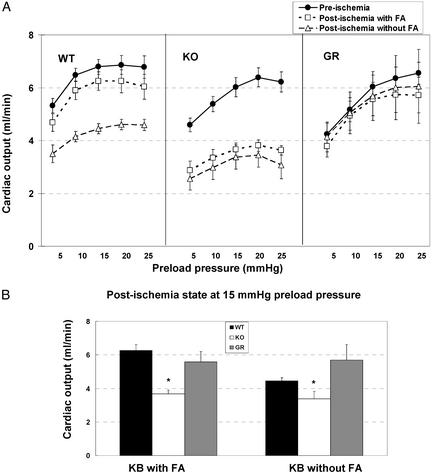
Cardiac Output. Fig. 3A shows cardiac output as a function of preload pressure. Under normal perfusion conditions, the cardiac output curves for hearts from KO, WT, or GR mice were similar. FA omission from the perfusate did not significantly alter cardiac output so for clarity only one curve is shown for plus and minus FA in the preischemic period.
Fig. 3.
Cardiac output pre- and postischemia in hearts with or without CD36-Cardiac output of WT, CD36-null (KO), and CD36 rescued (GR) mice are shown in A as a function of increasing preload pressures (mmHg). Hearts, perfused with bicarbonate buffer (KB) with and without FA were compared before and after 6 min of global ischemia and 8 min of reperfusion. (B) A comparison of cardiac output across groups at 15 mmHg of preload pressure is shown. *, Significantly different from WT and GR (P < 0.01).
After 6 min of global ischemia, cardiac output decreased in WT hearts only slightly when FA was included (empty squares) and significantly (by 30% on average) when FA was omitted (empty triangles). Cardiac output was not affected by the 6-min ischemia in CD36-rescued hearts (GR). In contrast, ischemia decreased output of hearts from CD36-deficient mice by 41% and 50% when the perfusate contained or lacked FA. An across-groups comparison of cardiac output after ischemia at 15 mmHg of preload pressure is shown in Fig. 3B. Cardiac output was significantly lower for KO mice as compared with both WT and GR, regardless of whether the perfusate was with or without FA. The differences observed, in the absence of FA, between GR and WT hearts can be attributed to the higher expression of CD36 in GR mice.
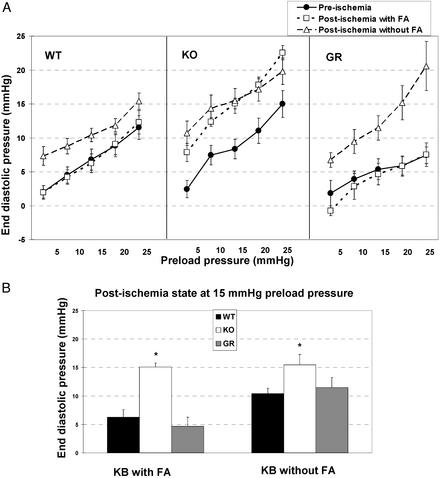
End-Diastolic Pressure. End-diastolic pressure is an index of myocardial efficiency to which it is inversely related. Before ischemia (Fig. 4A), hearts from KO, WT, and GR mice had similar end-diastolic pressure versus preload pressure curves. After ischemia, end-diastolic pressures only increased significantly in hearts deficient in CD36. However, when palmitate was omitted from the perfusate (empty triangles), end diastolic pressure increased after ischemia for all hearts, regardless of CD36 expression. The increases were more pronounced in KO and GR hearts as compared with WT hearts.
Fig. 4.
End-diastolic pressure pre- and postischemia in hearts with or without CD36. End-diastolic pressure of hearts from WT, CD36-null (KO), and GR mice were evaluated at increasing preload pressure (A). Hearts, perfused with bicarbonate buffer (KB) with and without FA, were compared before and after 6 min of global ischemia and 8 min of reperfusion. (B) A comparison across groups is shown, with end-diastolic pressure at 15 mmHg of preload pressure. *, Significantly different from WT and GR (P < 0.01).
A comparison across groups, of postischemia end diastolic pressure at 15 mmHg of preload pressure is shown in Fig. 4B. End-diastolic pressure was significantly higher in KO hearts as compared with WT or GR hearts whether FA was present or absent from the perfusate.
The above findings show that, under nonischemic conditions, CD36-deficient hearts have normal cardiac output and end-diastolic pressure. CD36 deficiency significantly reduces tolerance to ischemia, and this is reversed by rescue of CD36 expression in cardiomyocytes. Omission of FA from the perfusate decreases cardiac output and increases end-diastolic pressure in hearts from WT mice, producing changes that are similar though not equivalent to those observed in hearts from KO mice.
Effect of CD36 Deficiency on Myocardial Survival from Ischemia. A fraction of the hearts exhibited ventricular fibrillation or tachychardia after the ischemia and failed to functionally recover from the insult. As shown in Table 3, survival of hearts from KO mice was consistently lower than that of WT hearts and this was not affected by omitting FA from the perfusate. A 6-min ischemic insult was associated with 53% survival of KO hearts as compared with 80% of WT and GR hearts. Thus, the CD36-null heart is at significantly higher risk of functional failure from ischemia as compared with the heart expressing functional CD36.
Table 3. Heart survival after global ischemia.
| 6 min, %
|
|||
|---|---|---|---|
| Ischemia time | Without FA | With FA | 12 min, % |
| WT | 73 (14) | 78 (10) | 0 (5) |
| KO | 55 (22)* | 53 (15)* | 0 (5) |
| GR | 80 (10) | 80 (8) | 0 (5) |
Hearts were subjected to the indicated periods of ischemia, and the percentage of hearts that recovered is shown. The hearts were considered failing when they had ventricular arrhythmia (persistent ventricular fibrillations) or absence of aortic flow (against 55 mmHg after load pressure, the left ventricle was incapable of producing any forward pressure). The number of cases is between parentheses. Data are means ± SEM.
P < 0.01, WT and GR versus KO.
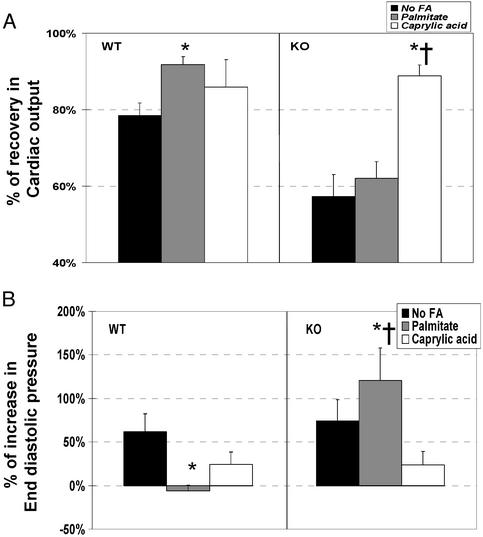
Effect of Medium-Chain FA. Long-chain FA did not significantly improve ischemic tolerance of the CD36-deficient myocardium (Figs. 3 and 4), so we examined whether medium-chain FA, which do not require CD36 for uptake, would be effective. Fig. 5 shows that addition of caprylic acid (8C) to the perfusate improved ischemic recovery of KO hearts, whereas palmitic acid did not. In contrast, in WT hearts, caprylic and palmitic acids were equally effective in improving postischemic cardiac output, but palmitic acid was more effective in reversing the postischemic increase in end-diastolic pressure.
Fig. 5.
Effect of caprylic acid in the perfusate on cardiac tolerance to ischemia. Hearts from WT and CD36-null (KO) were perfused with KB buffer containing palmitate or caprylic acid. Recovery from ischemia was assessed and compared with hearts that were perfused with bicarbonate buffer without FA. Cardiac function is shown as the ratio of postischemic to preischemic values, with 100% representing the preischemic values. (A) Cardiac output. (B) End-diastolic pressure. *, Significantly different from “No FA” condition (P < 0.01). †, Significantly different from “Palmitate” condition (P < 0.01).
Discussion
The data presented in this paper illustrate how CD36-facilitated FA transport impacts cardiac performance and tolerance to ischemia. CD36 plays an important role in determining heart FA oxidation (Fig. 2) and energy production (Table 2). CD36 expression levels correlate well with relative rates of FA utilization (32, 33) and a rate-limiting role of CD36-facilitated FA uptake in FA metabolism has been documented both in transgenic mice overexpressing CD36 (7) and in CD36-null mice (6, 9). Muscle contraction (rats and mice) increases membrane expression of CD36, which enhances FA transport and consequently FA utilization and ATP production (7, 32, 33). The working heart must produce a constant and abundant supply of ATP, and under normal conditions this is largely contributed (50–80%) by uptake and utilization of long-chain FAs (34, 35). The CD36-null heart, which cannot take up FA, exhibits a large compensatory increase in glucose utilization (11). However, this increase is apparently not sufficient for maintaining optimal levels of cellular energy stores, and our data show that this makes the CD36-null heart more vulnerable to insult from ischemic episodes. The low content of energy stores in CD36-deficient hearts at the start of the ischemic insult and the impaired ability to replenish these stores during the reperfusion were likely important contributors to the functional abnormalities observed post ischemia. As shown in Table 2, preischemic levels of glycogen, triglycerides, and ATP were reduced with CD36 deficiency. In addition, omission of FA from the perfusate, which was associated with a drop in tissue ATP levels (data not shown), produced significant malfunction of WT and GR hearts after ischemia. In line with this, perfusion of CD36-deficient hearts with buffer containing caprylic acid, a medium-chain FA that does not require CD36 for uptake, was associated with significant improvements in functional recovery from ischemia indicating that the dysfunction was reversible and consequent to metabolic abnormalities.
For hearts expressing a functional CD36, presence of palmitate in the perfusate accelerated restoration of normal energy levels as documented by the increase in tissue ATP and facilitated recovery of the heart from the ischemic insult. This finding is in line with the report that mechanical recovery of the injured heart after ischemia depends on the amount of substrate metabolized on reperfusion (36). The slight improvement of ischemic recovery observed in CD36-deficient hearts in presence of palmitate may reflect activity of FA transporters other than CD36 (37–39) or FA entering myocytes passively. As documented by Coburn et al. (9), CD36 mediates ≈60% of myocardial FA uptake.
In contrast to our findings, Liu et al. (40), with the working rat heart preparation, showed that high levels of FA delay recovery of cardiac efficiency after ischemia. This different outcome may reflect differences in FA metabolism by rat versus murine hearts (26, 41). A more likely explanation may be the different reperfusion times used by Liu et al. (40) versus this study of 40 and 8 min, respectively. Because tissue insult is promoted by extending reperfusion time (42–44), the detrimental effect of FA most likely is linked to a declining FA oxidative ability of the myocardium as reperfusion is prolonged. This would also be consistent with findings in the failing heart where function can be improved by treatment with FA oxidation inhibitors (45). The failing heart has limited capability to oxidize FA because it has reverted to a fetal metabolic gene profile and depends on glucose utilization for energy (46, 47).
The pivotal role of efficient long-chain FA utilization for myocardial integrity and function has been documented in multiple studies. Pharmacological inhibition of long-chain FA oxidation was associated with cardiomyopathy (48, 49). Mice deficient in the mitochondrial trifunctional protein crucial for β oxidation died soon after birth as a result of cardiac lesions (50). Mice lacking the enzyme of FA oxidation long-chain acyl-CoA dehydrogenase appeared normal, but hypoglycemia and cardiac and hepatic lipidosis were noted with fasting, and sudden death was observed in ≈5% of the cases (51). The CD36-deficient mice have impaired FA oxidation that is secondary to a defect in membrane FA uptake. They exhibit heart hypertrophy (Table 1) with dilation of the left ventricle (52), hypoglycemia, and accumulation of triglycerides in the liver with fasting (11). In vitro, CD36-deficient hearts had poor functional recovery and a higher risk of ventricular fibrillation after ischemia. This finding would suggest that a compromised myocardial response to stressors also occurs in vivo, but this remains to be determined. Finally, it is worth noting that the opposite metabolic phenotype of CD36 deficiency, meaning conditions associated with highly increased FA uptake, also leads to myocardial pathology if the oxidative pathway is overwhelmed by FA supply (53, 54).
The relevance of the findings in this study to the CD36-deficient human remains to be established. However, there are important similarities between the metabolic phenotype of myocardial CD36 deficiency in mice (9, 26) and humans (16). The magnitude of the defect in FA uptake, evaluated in both cases by using the FA analog, BMIPP, is similar and >60% (9, 26). Consistent with this observation, a large compensatory increase in myocardial glucose utilization has been measured in both species (16). In addition, the CD36-deficient murine heart exhibits signs of hypertrophy (Table 1), and association of CD36 deficiency with hypertrophic cardiomyopathy has been reported in humans (15, 17, 27, 55).
Clinically, the incidence of CD36 deficiency is high in some subpopulations such as Japanese, Africans, and African-Americans, where it ranges from 3% to 18.5% (56–58). The present study suggests that it would be important to determine whether humans with CD36 deficiency are more susceptible to heart dysfunction and failure after ischemic episodes and whether this can be reversed by metabolic interventions.
Finally, it has been recently documented that CD36 deficiency increases susceptibility to insulin resistance from diets high in simple sugars (11). Such diets have been shown to contribute to the pathogenesis of the metabolic syndrome in humans (59), a condition highly associated with cardiomyopathy.
Acknowledgments
This study was supported by American Heart Association Grant 0030345T (to A.I.) and National Institute of Diabetes and Digestive and Kidney Diseases Grant 33301 (to N.A.A.). J.F.F.B. was supported by Netherlands Heart Foundation Grant 97.149.
Abbreviations: FA, fatty acids; KO, knockout; GR, gene-rescued.
References
- 1.Abumrad, N., Harmon, C. & Ibrahimi, A. (1998) J. Lipid Res. 39, 2309-2318. [PubMed] [Google Scholar]
- 2.Abumrad, N. A., el-Maghrabi, M. R., Amri, E. Z., Lopez, E. & Grimaldi, P. A. (1993) J. Biol. Chem. 268, 17665-17668. [PubMed] [Google Scholar]
- 3.Tandon, N. N., Lipsky, R. H., Burgess, W. H. & Jamieson, G. A. (1989) J. Biol. Chem. 264, 7570-7575. [PubMed] [Google Scholar]
- 4.Oquendo, P., Hundt, E., Lawler, J. & Seed, B. (1989) Cell 58, 95-101. [DOI] [PubMed] [Google Scholar]
- 5.Harmon, C. M. & Abumrad, N. A. (1993) J. Membr. Biol. 133, 43-49. [DOI] [PubMed] [Google Scholar]
- 6.Febbraio, M., Abumrad, N. A., Hajjar, D. P., Sharma, K., Cheng, W., Pearce, S. F. & Silverstein, R. L. (1999) J. Biol. Chem. 274, 19055-19062. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahimi, A., Bonen, A., Blinn, W. D., Hajri, T., Li, X., Zhong, K., Cameron, R. & Abumrad, N. A. (1999) J. Biol. Chem. 274, 26761-26766. [DOI] [PubMed] [Google Scholar]
- 8.Van Nieuwenhoven, F. A., Verstijnen, C. P., Abumrad, N. A., Willemsen, P. H., Van Eys, G. J., Van der Vusse, G. J. & Glatz, J. F. (1995) Biochem. Biophys. Res. Commun. 207, 747-752. [DOI] [PubMed] [Google Scholar]
- 9.Coburn, C. T., Knapp, F. F., Jr., Febbraio, M., Beets, A. L., Silverstein, R. L. & Abumrad, N. A. (2000) J. Biol. Chem. 275, 32523-32529. [DOI] [PubMed] [Google Scholar]
- 10.Luiken, J. J., Willems, J., van Der Vusse, G. J. & Glatz, J. F. (2001) Am. J. Physiol. 281, E704-E712. [DOI] [PubMed] [Google Scholar]
- 11.Hajri, T., Han, X. X., Bonen, A. & Abumrad, N. A. (2002) J. Clin. Invest. 109, 1381-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonen, A., Luiken, J. J., Arumugam, Y., Glatz, J. F. & Tandon, N. N. (2000) J. Biol. Chem. 275, 14501-14508. [DOI] [PubMed] [Google Scholar]
- 13.Aitman, T. J., Cooper, L. D., Norsworthy, P. J., Wahid, F. N., Gray, J. K., Curtis, B. R., McKeigue, P. M., Kwiatkowski, D., Greenwood, B. M., Snow, R. W., et al. (2000) Nature 405, 1015-1016. [DOI] [PubMed] [Google Scholar]
- 14.Nozaki, S., Tanaka, T., Yamashita, S., Sohmiya, K., Yoshizumi, T., Okamoto, F., Kitaura, Y., Kotake, C., Nishida, H., Nakata, A., et al. (1999) Mol. Cell. Biochem. 192, 129-135. [PubMed] [Google Scholar]
- 15.Tanaka, T., Nakata, T., Oka, T., Ogawa, T., Okamoto, F., Kusaka, Y., Sohmiya, K., Shimamoto, K. & Itakura, K. (2001) J. Lipid Res. 42, 751-759. [PubMed] [Google Scholar]
- 16.Fukuchi, K., Nozaki, S., Yoshizumi, T., Hasegawa, S., Uehara, T., Nakagawa, T., Kobayashi, T., Tomiyama, Y., Yamashita, S., Matsuzawa, Y. & Nishimura, T. (1999) J. Nucl. Med. 40, 239-243. [PubMed] [Google Scholar]
- 17.Tanaka, T., Sohmiya, K. & Kawamura, K. (1997) J. Mol. Cell. Cardiol. 29, 121-127. [DOI] [PubMed] [Google Scholar]
- 18.Aitman, T. J. (2001) Lancet 357, 651-652. [DOI] [PubMed] [Google Scholar]
- 19.Brinkmann, J. F., Abumrad, N. A., Ibrahimi, A., van der Vusse, G. J. & Glatz, J. F. (2002) Biochem. J. 367, 561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuyama, N., Leavens, J. E., McKinnon, D., Gaudette, G. R., Aksehirli, T. O. & Krukenkamp, I. B. (2000) Circulation 102, III312-II318. [DOI] [PubMed] [Google Scholar]
- 21.Schaap, F. G., Binas, B., Danneberg, H., van der Vusse, G. J. & Glatz, J. F. (1999) Circ. Res. 85, 329-337. [DOI] [PubMed] [Google Scholar]
- 22.Passonneau, J. V. & Lauderdale, V. R. (1974) Anal. Biochem. 60, 405-412. [DOI] [PubMed] [Google Scholar]
- 23.Folch, J., Lees, M. & Sloane Stanley, G. H. (1957) J. Biol. Chem. 226, 497-509. [PubMed] [Google Scholar]
- 24.Markwell, M. A., Haas, S. M., Bieber, L. L. & Tolbert, N. E. (1978) Anal. Biochem. 87, 206-210. [DOI] [PubMed] [Google Scholar]
- 25.Yang, Y., Min, J. Y., Rana, J. S., Ke, Q., Cai, J., Chen, Y., Morgan, J. P. & Xiao, Y. F. (2002) J. Appl. Physiol. 93, 1140-1151. [DOI] [PubMed] [Google Scholar]
- 26.Hajri, T., Ibrahimi, A., Coburn, C. T., Knapp, F. F., Jr., Kurtz, T., Pravenec, M. & Abumrad, N. A. (2001) J. Biol. Chem. 276, 23661-23666. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka, T., Okamoto, F., Sohmiya, K. & Kawamura, K. (1997) Jpn. Circ. J. 61, 724-725. [DOI] [PubMed] [Google Scholar]
- 28.Plumier, J. C., Ross, B. M., Currie, R. W., Angelidis, C. E., Kazlaris, H., Kollias, G. & Pagoulatos, G. N. (1995) J. Clin. Invest. 95, 1854-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matherne, G. P., Linden, J., Byford, A. M., Gauthier, N. S. & Headrick, J. P. (1997) Proc. Natl. Acad. Sci. USA 94, 6541-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, G., Chen, Y., Saari, J. T. & Kang, Y. J. (1997) Am. J. Physiol. 273, H1090-H1095. [DOI] [PubMed] [Google Scholar]
- 31.De Windt, L. J., Willems, J., Roemen, T. H., Coumans, W. A., Reneman, R. S., Van Der Vusse, G. J. & Van Bilsen, M. (2001) Am. J. Physiol. 280, H2572-H2580. [DOI] [PubMed] [Google Scholar]
- 32.Bonen, A., Dyck, D. J., Ibrahimi, A. & Abumrad, N. A. (1999) Am. J. Physiol. 276, E642-E649. [DOI] [PubMed] [Google Scholar]
- 33.Luiken, J. J., Turcotte, L. P. & Bonen, A. (1999) J. Lipid Res. 40, 1007-1016. [PubMed] [Google Scholar]
- 34.Lopaschuk, G. D., Belke, D. D., Gamble, J., Itoi, T. & Schonekess, B. O. (1994) Biochim. Biophys. Acta 1213, 263-276. [DOI] [PubMed] [Google Scholar]
- 35.Belke, D. D., Larsen, T. S., Lopaschuk, G. D. & Severson, D. L. (1999) Am. J. Physiol. 277, R1210-R1217. [DOI] [PubMed] [Google Scholar]
- 36.Saddik, M. & Lopaschuk, G. D. (1992) J. Biol. Chem. 267, 3825-3831. [PubMed] [Google Scholar]
- 37.Berk, P. D. & Stump, D. D. (1999) Mol. Cell. Biochem. 192, 17-31. [PubMed] [Google Scholar]
- 38.Gimeno, R. E., Ortegon, A. M., Patel, S., Punreddy, S., Ge, P., Sun, Y., Lodish, H. F. & Stahl, A. (2003) J. Biol. Chem. 278, 16039-16044. [DOI] [PubMed] [Google Scholar]
- 39.Richards, M. R., Listenberger, L. L., Kelly, A. A., Lewis, S. E., Ory, D. S. & Schaffer, J. E. (2003) J. Biol. Chem. 278, 10477-10483. [DOI] [PubMed] [Google Scholar]
- 40.Liu, Q., Docherty, J. C., Rendell, J. C., Clanachan, A. S. & Lopaschuk, G. D. (2002) J. Am. Coll. Cardiol. 39, 718-725. [DOI] [PubMed] [Google Scholar]
- 41.Christe, M. E. & Rodgers, R. L. (1994) J. Mol. Cell. Cardiol. 26, 1371-1375. [DOI] [PubMed] [Google Scholar]
- 42.Schrijvers, A. H., de Groot, M. J., Heijnen, V. V., van der Vusse, G. J., Frederik, P. M. & Reneman, R. S. (1990) J. Mol. Cell. Cardiol. 22, 653-665. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell, M. B., Winter, C. B., Banerjee, A. & Harken, A. H. (1993) Surg. Gynecol. Obstet. 177, 97-114. [PubMed] [Google Scholar]
- 44.Reffelmann, T. (2002) Am. J. Physiol. 282, H766-H772. [DOI] [PubMed] [Google Scholar]
- 45.Stanley, W. C. & Chandler, M. P. (2002) Heart Fail. Rev. 7, 115-130. [DOI] [PubMed] [Google Scholar]
- 46.Sack, M. N., Rader, T. A., Park, S., Bastin, J., McCune, S. A. & Kelly, D. P. (1996) Circulation 94, 2837-2842. [DOI] [PubMed] [Google Scholar]
- 47.Razeghi, P., Young, M. E., Alcorn, J. L., Moravec, C. S., Frazier, O. H. & Taegtmeyer, H. (2001) Circulation 104, 2923-2931. [DOI] [PubMed] [Google Scholar]
- 48.Schwab, K. O., Ensenauer, R., Matern, D., Uyanik, G., Schnieders, B., Wanders, R. A. & Lehnert, W. (2003) Eur. J. Pediatr. 162, 90-95. [DOI] [PubMed] [Google Scholar]
- 49.de las Fuentes, L., Herrero, P., Peterson, L. R., Kelly, D. P., Gropler, R. J. & Davila-Roman, V. G. (2003) Hypertension 41, 83-87. [DOI] [PubMed] [Google Scholar]
- 50.Ibdah, J. A., Paul, H., Zhao, Y., Binford, S., Salleng, K., Cline, M., Matern, D., Bennett, M. J., Rinaldo, P. & Strauss, A. W. (2001) J. Clin. Invest. 107, 1403-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurtz, D. M., Rinaldo, P., Rhead, W. J., Tian, L., Millington, D. S., Vockley, J., Hamm, D. A., Brix, A. E., Lindsey, J. R., Pinkert, C. A., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 15592-15597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicholson, A. C., Febbraio, M., Han, J., Silverstein, R. L. & Hajjar, D. P. (2000) Ann. N.Y. Acad. Sci. 902, 128-131, and discussion 131-133. [PubMed] [Google Scholar]
- 53.Chiu, H. C., Kovacs, A., Ford, D. A., Hsu, F. F., Garcia, R., Herrero, P., Saffitz, J. E. & Schaffer, J. E. (2001) J. Clin. Invest. 107, 813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finck, B. N., Han, X., Courtois, M., Aimond, F., Nerbonne, J. M., Kovacs, A., Gross, R. W. & Kelly, D. P. (2003) Proc. Natl. Acad. Sci. USA 100, 1226-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kashiwagi, H., Tomiyama, Y., Nozaki, S., Kiyoi, T., Tadokoro, S., Matsumoto, K., Honda, S., Kosugi, S., Kurata, Y. & Matsuzawa, Y. (2001) Hum. Genet. 108, 459-466. [DOI] [PubMed] [Google Scholar]
- 56.Takeishi, Y., Chiba, J., Abe, S., Tonooka, I., Komatani, A. & Tomoike, H. (1992) Eur. J. Nucl. Med. 19, 775-782. [DOI] [PubMed] [Google Scholar]
- 57.Lee, K., Godeau, B., Fromont, P., Plonquet, A., Debili, N., Bachir, D., Reviron, D., Gourin, J., Fernandez, E., Galacteros, F. & Bierling, P. (1999) Transfusion 39, 873-879. [DOI] [PubMed] [Google Scholar]
- 58.Hirooka, K., Yasumura, Y., Ishida, Y., Komamura, K., Hanatani, A., Nakatani, S., Yamagishi, M. & Miyatake, K. (2000) Jpn. Circ. J. 64, 731-735. [DOI] [PubMed] [Google Scholar]
- 59.Liu, S. & Manson, J. E. (2001) Curr. Opin. Lipidol. 12, 395-404. [DOI] [PubMed] [Google Scholar]