Abstract
The ability to coordinate carbon (C) and nitrogen (N) metabolism enables plants to regulate development and metabolic responses to different environmental conditions. The regulator(s) or sensor(s) that monitor crosstalk between biosynthetic pathways and ultimately control the flow of C or N through them have remained elusive. We used an antisense strategy to demonstrate that the putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of C and N metabolism in Arabidopsis. Seeds from AtGLR1.1-deficient Arabidopsis (antiAtGLR1.1) lines did not germinate in the presence of an animal ionotropic glutamate receptor (iGLR) antagonist, but germination was restored upon coincubation with an iGLR agonist or the putative ligand glutamate. In antiAtGLR1.1 lines, endogenous abscisic acid (ABA) concentrations increased with iGLR antagonist treatments and decreased with coincubation with an iGLR agonist, suggesting that germination was controlled by ABA. antiAtGLR1.1 seedlings also exhibited sensitivity to increased levels of Ca2+ compared with wild type, and they exhibited a conditional phenotype that was sensitive to the C:N ratio. In the presence of C, specifically sucrose, but not glucose, mannitol, or sorbitol, antiAtGLR1.1 seeds did not germinate, but germination was restored upon coincubation with 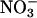 , but not
, but not 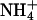 . Immunoblot, isoenzyme, and RT-PCR analyses indicate that AtGLR1.1 regulates the accumulation of distinct C- and N-metabolic enzymes, hexokinase 1 (HXK1) and zeaxanthin epoxidase (ABA1), by transcriptional control. We provide a model to describe the role of AtGLR1.1 in C/N metabolism and ABA biosynthesis, which in turn controls seed germination.
. Immunoblot, isoenzyme, and RT-PCR analyses indicate that AtGLR1.1 regulates the accumulation of distinct C- and N-metabolic enzymes, hexokinase 1 (HXK1) and zeaxanthin epoxidase (ABA1), by transcriptional control. We provide a model to describe the role of AtGLR1.1 in C/N metabolism and ABA biosynthesis, which in turn controls seed germination.
Other than hydrogen and oxygen as water, carbon (C), in the form of photosynthate, and nitrogen (N) are the two most important elements required for normal plant growth and development. Their efficient acquisition and utilization are associated with increased crop productivity and quality. To accommodate changing developmental and environmental demands on C and N resources, plants evolved highly sophisticated and complex sensory systems that “crosstalk” to regulate C or N assimilation, metabolism, and transport (1, 2). Higher plants have as many as four distinct N- and/or C-sensory systems that monitor the accumulation of different “check point” molecules, a list of which includes sucrose (Suc), glucose (Glc), 2-oxoglutarate, glutamine (Gln), glutamate (Glu), 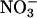 , and
, and 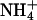 . Other than the identification of hexokinase as a Glc sensor (3) and a nuclear-encoded chloroplast N-regulatory PII-like element (4), many components of the C- and/or N-regulation and/or sensing systems have remained elusive.
. Other than the identification of hexokinase as a Glc sensor (3) and a nuclear-encoded chloroplast N-regulatory PII-like element (4), many components of the C- and/or N-regulation and/or sensing systems have remained elusive.
Members of a newly identified family of Arabidopsis genes (5) that encode proteins with high sequence similarity to the deduced amino acid sequences of the animal ionotropic glutamate receptors (iGLRs) have been designated as the putative glutamate receptor genes (AtGLRs; ref. 6). In invertebrates and vertebrates iGLRs function as ligand-gated ion channels that control signaling across neural synapses. Upon binding the ligand, Glu, a conformational change of the heterotetrameric complex opens a pore for the inward transport of Ca2+, Na+, or K+. Specificity of the ion depends on alternative splicing events in the pore-forming region of the peptide (7).
The biochemical and physiological function(s) of the AtGLRs are not well defined. Results from whole plant assays with the iGLR antagonist 6,7-dinitroquinoxaline-2,3-(1H,4H)-dione (DNQX) (5) or the agonist S(+)-β-methyl-α,β-diaminopropionic acid (BMAA) (8) suggest that the AtGLRs may mediate the transmission of light signals, because plants treated to these compounds exhibit growth characteristics indicative of exposure to different wavelengths of light or decreased light intensity. These characteristics include elongated hypocotyls, a decreased angular opening between the cotyledons, and decreased amounts of chlorophyll. However, in both instances a link between the observed physiological effects of the iGLR antagonist or agonist on plant development and any AtGLR were implied and not directly tested. In addition, there is evidence for a relationship between the AtGLRs and Ca2+. Constitutive overexpression of AtGLR3.2 (designated AtGLR2 in the original publication) resulted in symptoms of Ca2+ deprivation that were reversed by the application of exogenous Ca2+ (9). Electrophysiological experiments on intact roots from WT Arabidopsis demonstrated a synchronous increase in cytosolic Ca2+ with a Glu-mediated voltage potential across the plasma membrane (10). Despite implications that Glu may function as a signaling molecule to regulate plant growth and development (1), there are no clear data to indicate that the AtGLRs are involved in these processes.
In this study we developed antiAtGLR1.1 lines to examine the physiological, biochemical, and molecular effects of decreased amounts of the AtGLR1.1 peptide in Arabidopsis. Our results from vertical growth assays, which compared the germination of antiAtGLR1.1 versus WT seeds in the absence or presence of an iGLR antagonist, iGLR agonist, ligand, or different cations, suggest that AtGLR1.1 has characteristics of a peptide that functions in a manner similar to an iGLR, i.e., a ligand-gated ion channel. We demonstrate that antiAtGLR1.1 lines have elevated abscisic acid (ABA) titers compared with those of WT. ABA titers increase in antiAtGLR1.1 lines treated with the iGLR antagonist DNQX, which inhibited seed germination. Coincubation of antiAtGLR1.1 seeds in DNQX and the iGLR agonist BMAA decreases ABA titers and restores germination. Immunoblot, isoenzyme, and RT-PCR analyses demonstrate that decreased accumulation of AtGLR1.1 in antiAtGLR1.1 lines was associated with a decrease in (i) key C- and N-metabolic isoenzymes and their corresponding transcripts and (ii) the hexokinase 1 (HXK1) transcript. However, there was an increase in the transcript of ABA1, a gene known to control ABA biosynthesis, in the antiAtGLR1.1 lines. Combined, our findings suggest that AtGLR1.1 regulates C and N metabolism and controls seed germination by means of changes in ABA.
Materials and Methods
Vertical Growth Assay and Maintenance of Plants. Seedlings were maintained in the vertical position on square Petri plates on complete Murashige and Skoog (MS) medium or MS minus inorganic nitrogen medium (Caisson Laboratories, Sugar City, ID) supplemented with different C or N compounds, amino acids, or DNQX and/or BMAA and 0.8% (wt/vol) Phytagar (Life Technologies) for 7 days. Growth conditions were maintained at 20–21°C, with 60–70% relative humidity, under cool white fluorescent lights (140 μmol of photons per m2 per s) with a 16-h light/8-h dark cycle. Seeds from transgenic line 2 (Fig. 1A) were used in all of the vertical growth experiments. All experiments described below were conducted in duplicate or triplicate.
Fig. 1.
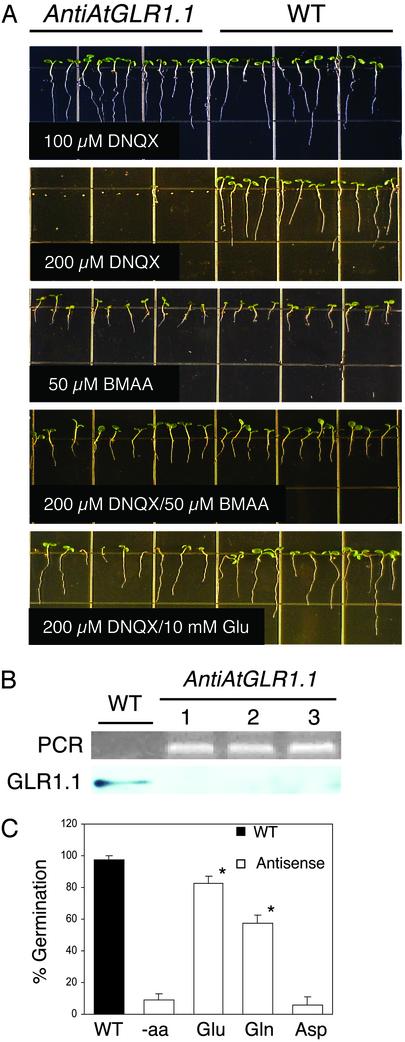
Effects of the putative ligand, Glu, and iGLR antagonist and agonist on WT and antiAtGLR1.1 lines. (A) Seeds/seedlings from WT and antiAtGLR1.1 (transgenic line 2 from B) lines were maintained on MS medium containing 3% (wt/vol) Suc and 0.8% (wt/vol) Phytagar for 7 days in the presence of DNQX, BMAA, DNQX/BMAA, or DNQX/Glu. Each grid is 13 × 13 mm. (B) The presence of the antiAtGLR1.1 gene construct was confirmed by PCR, and accumulation of the AtGLR1.1 peptide was determined by immunoblot analysis. WT and the three independent antiAtGLR1.1 lines (1, 2, 3) are indicated. (C) Restoration of seed germination from DNQX-mediated inhibition by different amino acids, Glu, Gln, or aspartate (Asp). The germination of WT and transgenic (line 2 in B) seeds with 200 μM DNQX was scored after a 7-day incubation in the presence of 10 mM Glu, Gln, or Asp or in the absence (-aa) of the indicated amino acid. Each treatment consists of 10–12 seedlings (n = 4; *, P < 0.005). Small bars represent the SE.
Antisense Construct. A 654-bp BamHI fragment from the Arabidopsis EST clone 107M14T7 (GenBank accession no. T22862) obtained from The Arabidopsis Biological Resource Center (Ohio State University, Columbus), was cloned into a BamHI-digested modified binary pBI221 vector (CLONTECH), pPV1, in the inverse orientation. Arabidopsis thaliana (L.) Heynh. ecotype Wassilewskija was transformed by vacuum infiltration (11).
Antibody Production. The C terminus, 88 aa, of AtGLR1.1 was expressed in a GST-fusion system. PCR was performed with the gene-specific primers 5′trunAtGLR1.1oxEco (5′-GCCCGAATTCGAAATTATCCGAATGATT-3′) and 3′trunAtGLR1. 1oxEco (5′-GCCCGAATTCTAACCGCGCAACTCACGGAA-3′) and the EST clone as the template. All primers were commercially synthesized (Bio-Synthesis, Lewisville, TX). The amplification reaction, cloning, purification of the truncated AtGLR1.1 protein, and antibody production were performed as described by Turano et al. (12).
Immunoblot Analysis and Enzyme Activity Stains. Immunoblot and isoenzyme stains were performed on crude protein preparations from leaves of 30-day-old plants. Immunoblot analysis of Gln synthetase (GS) and ferredoxin-dependent Glu synthase (Fd-GOGAT, glutamate–2-oxoglutarate aminotransferase) were performed with antibodies and protocols described by Turano and Muhitch (13). Antibodies for asparate aminotransferase (AAT)2 (14) and AAT3 (15) were obtained from Ben Matthews (U.S. Department of Agriculture/Agricultural Research Service, Beltsville, MD). Antiserum to NADP(H)-dependent glutamate dehydrogenase (GDH) was prepared to a portion of the deduced amino acid sequence (RDIKSQQRSLRDYSKTYARAKYFDELKPWNERC) by following the protocols developed by Animal Pharm Services (Healdsburg, CA). Enzyme stains for NAD-dependent GDH were conducted as described by Turano et al. (16), and the other stains were conducted as described by Vallejos (17) except that DL-isocitrate was added to a final concentration of 40 mM. Samples were standardized by equal protein loading (20 μg per lane) on the MiniPROTEAN 3 system (Bio-Rad) and 3 μg per lane on the 8–25 gradient PhastGel System (Amersham Pharmacia Biosciences) for 6-phosphogluconate dehydrogenase (6PGDH) activity. Immunoblots and stained gels were photographed and densitometrically analyzed with the Kodak Digital Science 1D Image Analysis Software package (version 3.0).
RT-PCR Analysis. Total RNA was extracted from leaves of 30-day-old plants with the RNeasy Plant Mini Kit (Qiagen, Valencia, CA). Total RNA (1 μg) served as the template for RT-PCR using the RT-PCR beads System (Amersham Pharmacia Biosciences) with 25 pmol of each transcript-specific primer. All primers were commercially synthesized (Bio-Synthesis). The RT-PCR was conducted as follows: 42°C for 15 min, 95°C for 5 min, followed by 25 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 2 min, and a 72°C extension reaction for 2 min. The primers used and the corresponding accession numbers are available on request. RNA integrity and concentration, PCR controls and fragment identifications were conducted and analyzed as previously described (12).
ABA Determinations. To quantify the ABA content of antiAtGLR1.1 and WT seeds and seedlings, 0.1 g of frozen homogenized tissue was suspended in 1.5 ml of extraction buffer (100 mg/liter butylated hydroxytoluene/0.5 g/liter citric acid monohydrate in 80% methanol). The suspension was rotated overnight at 4°C and centrifuged at 1,000 × g for 20 min. The supernatant was collected and transferred to a new tube and vacuum dried. The dried residue was dissolved with 100 μl of methanol plus TBS (50 mM Tris/0.1 mM MgCl2/0.15 M NaCl, pH 7.8), and ABA was quantified with the Phytodetek-ABA-Kit as described by the manufacturer (Idetek, Sunnyvale, CA).
Results and Discussion
AtGLR1.1 Has iGLR-Like Characteristics. To determine the mechanistic action of AtGLR1.1, we tested antiAtGLR1.1 lines for altered sensitivity to the iGLR antagonist DNQX and/or agonist BMAA (Fig. 1A). Previous pharmacological studies in plants determined the bioactive concentrations of these chemicals to be 200 μM DNQX, which resulted in the inhibition of hypocotyl shortening and leaf greening (5), and 50 μM BMAA, which increased hypocotyl elongation and decreased cotyledon opening (8). antiAtGLR1.1 seeds maintained on MS medium with 3% Suc supplemented with low concentrations (100 μM) of DNQX (Fig. 1A) or without DNQX (data not shown) germinated and seedlings developed similarly to WT. However, the germination of antiAtGLR1.1 seeds was inhibited (91 ± 0.3%, n = 40) in the presence of 200 μM DNQX. PCR analysis confirmed the existence of the antiAtGLR1.1 construct, and decreased accumulation of the AtGLR1.1 peptide was confirmed by immunoblot analysis (Fig. 1B). This screen was used to identify three representative transgenic lines with a detectable reduction in the accumulation of AtGLR1.1 peptide compared with that of WT. Our results show an inverse relationship between the accumulation of the AtGLR1.1 peptide and antagonist sensitivity. DNQX hypersensitivity in antiAtGLR1.1 lines is consistent with the hypothesis that these lines have functional GLRs, but the GLRs contain fewer AtGLR1.1 subunits and are thus more readily saturated by the inhibitory effects of the antagonist compared with WT. Conversely, in WT plants there are more AtGLR1.1-containing receptors, and at the same concentration of DNQX that adversely affects the antiAtGLR1.1 lines the antagonist is not saturating and is therefore not inhibitory. Seed germination and seedling development were similar among antiAtGLR1.1 and WT lines treated with 50 μM BMAA (Fig. 1A). The DNQX-mediated inhibition of germination and development of antiAtGLR1.1 seedlings was reversed by simultaneous incubation with 50 μM BMAA or 10 mM Glu. The 50 μM BMAA treatment was more effective and less variable for the reversal of the inhibitory effects of DNQX than 10 mM Glu. BMAA and Glu reversal of DNQX-mediated inhibition of germination is consistent with presumption that these compounds compete for similar binding sites and act as an antagonist or agonist to AtGLR1.1 in a manner similar to the iGLRs. At 10 mM, Glu reversed the DNQX-mediated inhibition of seed germination (Fig. 1A). To test the specificity of Glu, the ability of other amino acids (Gln and Asp) to reverse the DNQX-mediated inhibition of seed germination was tested (Fig. 1C). Neither Asp nor Gln was as effective as Glu (85%) in the restoration of germination to DNQX-treated antiAtGLR1.1 lines. Interestingly, at 10 mM, Gln did increase germination to 59%; this increase may be due to the rapid conversion of Gln to Glu, the putative ligand. Similar observations and explanations for a Gln response have been reported for WT lines coincubated with an iGLR agonist (8). In animals, iGLRs function as an inward-rectifying K+, Na+, or Ca2+ ligand-gated ion channel. If AtGLR1.1 functions in a manner similar to the iGLRs, then antiAtGLR1.1 lines would be expected to have altered sensitivity to at least one of these ions. Results from vertical growth assays revealed that antiAtGLR1.1 lines were as sensitive as WT to K+ or Na+, as measured by inhibition of root growth (data not shown). However, the antiAtGLR1.1 lines were more sensitive to increased levels of supplemented Ca2+ than WT. These findings suggest a relationship between altered levels of AtGLR1.1 and Ca2+ sensitivity. Genetic and physiological studies of another AtGLR (AtGLR3.2) also observed changes in sensitivity to Ca2+, and not to K+ or Na+ (9). The results from both physiological studies are supported by extensive phylogenetic analysis of the pore-forming region (ion-permeability region) of the AtGLRs and corresponding regions of other ligand-gated ion channels and K+ channels that demonstrated the AtGLRs are not similar to the K+ channels (18).
ABA Concentrations in the DNQX- or BMAA-Treated Plants. It has been established that ABA plays an important role in seed germination (19). As shown in Fig. 1A, the germination of seeds from antiAtGLR1.1 lines was inhibited on MS medium with 3% Suc supplemented with 200 μM DNQX. We hypothesized that decreased germination of the antiAtGLR1.1 seeds by DNQX was due to elevated ABA titers. To test this hypothesis, endogenous ABA concentrations were determined in 3-day-old WT and antiAtGLR1.1 seedlings incubated in liquid culture containing MS medium with 3% Suc, in the absence or presence of 200 μM DNQX and/or 50 μM BMAA (Table 1). In the absence of either agonist or antagonist, the antiAtGLR1.1 seedlings contained 8 times more ABA than did WT. When the medium was supplemented with 200 μM DNQX the ABA content in WT seedlings was similar to that of the nontreated WT controls. Coincubation of WT seedlings with 200 μM DNQX and 50 μM BMAA resulted in a 425% or 460% increase in ABA content compared with nontreated or DNQX-treated WT seedlings, respectively. When the medium was supplemented with 200 μM DNQX, the ABA content in antiAtGLR1.1 seedlings increased 160% to that of the nontreated antiAtGLR1.1 controls. Coincubation of antiAtGLR1.1 seedlings with 200 μM DNQX and 50 μM BMAA resulted in a 37% decrease in ABA compared with the DNQX-treated antiAtGLR1.1 seedlings. However, the ABA content in antiAtGLR1.1 seedlings treated with 200 μM DNQX and 50 μM BMAA was nearly 80% higher than that of the antiAtGLR1.1 seedlings control. Compared with WT seedlings treated with 200 μM DNQX and 50 μM BMAA, similarly treated antiAtGLR1.1 seedlings had a 240% increase in ABA. Combined, these findings suggest that decreased AtGLR1.1 “activity,” caused either by less protein (antisense) or by inhibition of the GLRs by the antagonist, results in increased ABA. The inhibitory effects of the two approaches are cumulative, because the antiAtGLR1.1 seedlings were more sensitive to effects of the antagonist, which resulted in the highest levels of ABA observed in these experiments. Furthermore, these findings strongly suggest that endogenous ABA levels must reach a threshold to inhibit seed germination.
Table 1. ABA content in 3-day-old WT and antiGLR1.1 seedlings maintained on MS plus 3% Suc medium supplemented with an iGLR agonist or antagonist.
| ABA, μg/g
|
||
|---|---|---|
| Treatment | WT | antiAtGLR1.1 |
| Control (MS + 3% Suc) | 0.174 ± 0.002 | 1.393 ± 0.050 |
| 200 μM DNQX | 0.159 ± 0.004 | 3.759 ± 0.435 |
| 200 μM DNQX + 50 μM BMAA | 0.739 ± 0.083 | 2.520 ± 0.121 |
Results are expressed as μg of ABA per g of frozen tissue. Data are means ± SD (n = 3).
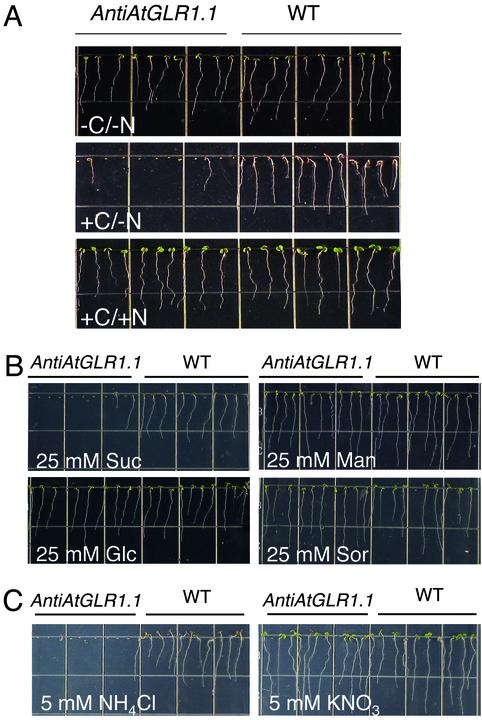
AtGLR1.1 Alters C/N Sensitivity. Because Glu is a potential ligand, and Glu metabolism and plant development are affected by different C:N ratios, we subjected plants to different C:N regimes to determine the effect on seedling development. AntiAtGLR1.1 plants exhibited a conditional phenotype that was sensitive to different C:N treatments (Fig. 2A). Seeds from antiAtGLR1.1 and WT lines germinated and seedlings developed similarly on MS nutrient plates in the absence of inorganic N or supplemental C (-C/-N). When supplemental C (3% Suc) was provided to the medium, germination of the antiAtGLR1.1 lines was inhibited (87 ± 0.5%, n = 44) compared with WT (+C/-N). However, the addition of N (5 mM NH4NO3 and 5 mM KNO3) to the C-containing medium restored the antiAtGLR1.1 seeds and seedlings to WT germination rates and growth, respectively (+C/+N). These results suggest that differences in C and N availability alter germination and development, and high C:N ratios inhibit germination.
Fig. 2.
Effect of C and/or N on germination. (A) Seeds from WT or antiAtGLR1.1 lines were sown on 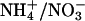 -free MS medium containing 0.8% (wt/vol) Phytagar supplemented with no C or inorganic N (-C/-N), with C (3.0% Suc) and no inorganic N (+C/-N), or with C (3.0% Suc) and inorganic N (5 mM
-free MS medium containing 0.8% (wt/vol) Phytagar supplemented with no C or inorganic N (-C/-N), with C (3.0% Suc) and no inorganic N (+C/-N), or with C (3.0% Suc) and inorganic N (5 mM 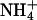 and 10 mM
and 10 mM 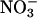 )(+C/+N). (B)WTor antiAtGLR1.1 seeds were sown on
)(+C/+N). (B)WTor antiAtGLR1.1 seeds were sown on  -free MS supplemented with 25 mM Suc, Glc, mannitol (Man), or sorbitol (Sor). (C) Seeds from WT or antiAtGLR1.1 lines were sown on
-free MS supplemented with 25 mM Suc, Glc, mannitol (Man), or sorbitol (Sor). (C) Seeds from WT or antiAtGLR1.1 lines were sown on  -free MS medium supplemented with 87 mM (3%) Suc and 5 mM
-free MS medium supplemented with 87 mM (3%) Suc and 5 mM 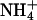 or
or  .
.
To test the specificity of Suc as a C source involved in the inhibition of germination of antiAtGLR1.1 seeds, antiAtGLR1.1 and WT seeds were germinated on MS plates minus inorganic N in the presence of different C (25 mM) sources (Fig. 2B). Previously we determined that 25 mM Suc was the lowest effective concentration to inhibit germination of the antiAtGLR1.1 seeds (data not shown). Germination of antiAtGLR1.1 seeds was inhibited by 25 mM Suc, whereas under the same conditions WT seeds germinated and seedlings developed normally. The antiAtGLR1.1 and WT seeds germinated and seedlings developed normally when germinated in MS minus inorganic nitrogen supplemented with 25 mM Glc, mannitol, or sorbitol. High levels of C (300 mM Suc or Glc) arrest a variety of plant developmental processes (1), through the elevation of ABA (20). Our observations suggest that (i) AtGLR1.1 may be associated with Suc-sensing or -mediated regulation; (ii) Suc has a negative or inhibitory effect on AtGLR1.1, because Suc in the absence of N, like the DNQX treatment, results in the inhibition of seed germination; (iii) AtGLR1.1 also effects Glc sensing or Glc-mediated regulation because the antiAtGLR1.1 lines are hypersensitive to Suc and not Glc; and (iv) AtGLR1.1 alters ABA biosynthesis because Suc-treatment antiAtGLR1.1 seeds did not germinate.
To test the specificity of different forms of inorganic N necessary for the reversal of the Suc-mediated inhibition of germination of antiAtGLR1.1 seeds, seeds from antiAtGLR1.1 and WT lines were maintained on MS plates plus 3% Suc in the presence of 5 mM  or
or  (Fig. 2C). The Suc-mediated inhibition of germination of the antiAtGLR1.1 seeds was reversed by
(Fig. 2C). The Suc-mediated inhibition of germination of the antiAtGLR1.1 seeds was reversed by 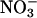 but not
but not 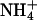 . Our observations suggest that (i) AtGLR1.1 may be associated with
. Our observations suggest that (i) AtGLR1.1 may be associated with 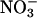 -sensing or -mediated regulation, and (ii)
-sensing or -mediated regulation, and (ii)  has a positive or stimulatory effect on AtGLR1.1, because
has a positive or stimulatory effect on AtGLR1.1, because 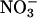 reverses Suc-mediated inhibition of seed germination. This reversal is analogous to the BMAA reversal of the DNQX treatment.
reverses Suc-mediated inhibition of seed germination. This reversal is analogous to the BMAA reversal of the DNQX treatment.
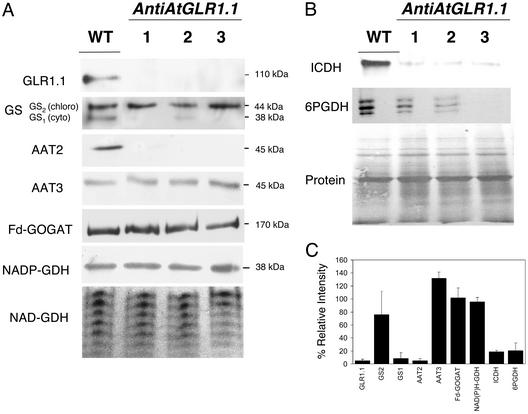
AtGLR1.1 Regulates the Accumulation of C- and N-Metabolic Enzymes. To elucidate the effects of antiAtGLR1.1 on C/N metabolism, we tested three independent transgenic lines by immunoblot analysis or specific enzyme activity stains for changes in the accumulation of isoenzymes involved in N or C metabolism (Fig. 3). In antiAtGLR1.1 plants, the levels of the cytosolic isoforms of GS (GS1) (21) and AAT (AAT2) (22) were 90% and 93% less, respectively, than those levels observed in WT plants (Fig. 3 A and C). The corresponding chloroplast isoforms, GS2 and AAT3, remained unchanged compared with WT plants. The accumulation of other C- and N-metabolic enzymes, including the chloroplastic isoenzymes (23, 24) Fd-GOGAT and NADP(H)-GDH and the mitochondrial (16) NAD-GDH, were unaltered compared with WT plants as determined by immunoblot analysis or enzyme-specific activity gel stain (Fig. 3C). Enzyme-specific activity stains for the cytosolic C-metabolic isoenzymes (25, 26) 6PGDH and NADP-dependent isocitrate dehydrogenase (ICDH) showed decreased levels (80% and 78%), respectively, of both isoforms in antiAtGLR1.1 plants (Fig. 3B) compared with WT. Collectively, these results suggest that the AtGLR1.1 gene product is involved in the maintenance of steady-state levels of specific N- and C-metabolic enzymes.
Fig. 3.
Accumulation of C and N metabolic isoenzymes in WT and antiAtGLR1.1 plants. (A) Crude total protein preparations from the leaves of 30-day-old Arabidopsis plants were subjected to SDS/PAGE, blotted onto nitrocellulose, stained for protein by using BLOT-FastStain (Geno Technology, St. Louis), destained, and used for immunoblot analysis with antiserum to specific N/C-metabolic enzymes, GS, Fd-GOGAT, AAT, and NADP(H)-dependent GDH. An enzyme activity stain was used to visualize the seven NAD-dependent GDH isoenzymes. (B) Accumulation of select C-metabolic isoenzymes was studied with enzyme stains to visualize the activities of 6PGDH and ICDH. (C) Results from densitometric analysis of immunoblots and enzyme stains. The band intensity for each of the three transgenic lines was determined and used to calculate the mean intensity for each band. The results were expressed as a percentage of the control (100%) ± SD.
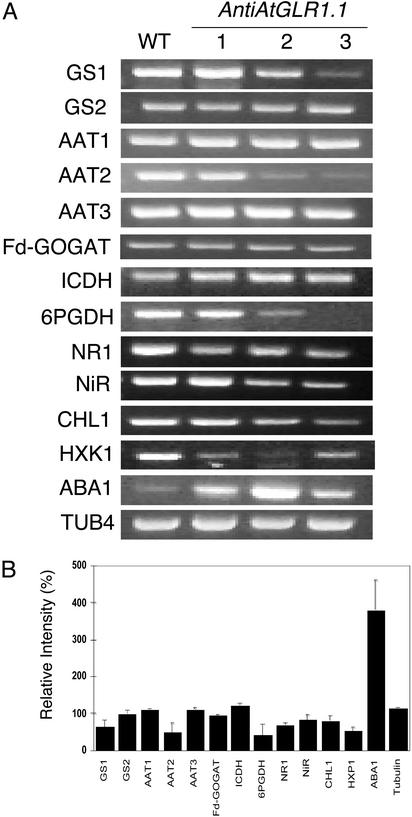
AtGLR1.1 Regulates the Accumulation of Transcripts Involved in C and N Metabolism, Glc Sensing, and ABA Biosynthesis. To determine whether the C- and N-metabolic isoenzymes were transcriptionally or translationally controlled, we used RT-PCR (Fig. 4). For all of the transcripts that we previously tested by immunoblotting or isoenzyme analysis, i.e., GS1, AAT2, and 6PDGH, except one ICDH, there was a parallel decrease in the peptide or isoenzyme (Fig. 3) and the corresponding transcript (Fig. 4A). In each instance there was an ≈50% decrease in the transcript in the antiAtGLR1.1 versus the WT plants (Fig. 4B). These findings suggest that AtGLR1.1 controls C- and N-metabolic pathways by decreased gene expression or RNA stability. In addition, the transcripts for other genes involved in N metabolism, namely NR, NiR, and CHL1, were less abundant (30% decrease) in antiAtGLR1.1 plants compared with WT. Because the antiAtGLR1.1 seedlings were more sensitive to Suc than to Glc (Fig. 2B), we hypothesized that the antiAtGLR1.1 plants would have less HXK1, the Glc sensor, than WT plants. Indeed, there was less accumulation (50% decrease) of the HXK1 transcript in antiAtGLR1.1 than in the WT plants, thus the decreased accumulation of the HXK1 transcript in antiAtGLR1.1 lines may explain why these lines are more sensitive to Suc as opposed to Glc (Fig. 2B). This notion is supported by earlier findings with antisense HXK1 plants, which were shown to be less sensitive to Glc than WT (3). Interestingly, the transcript for ABA1, a gene involved in the regulation of ABA biosynthesis, was more abundant (300% increase) in antiAtGLR1.1 plants compared with WT. This observation is consistent with the notion that a decrease in functional AtGLRs results in elevated ABA biosynthesis.
Fig. 4.
Comparison of transcript accumulation of genes involved in N or C metabolism, Glc sensing, or ABA biosynthesis in WT and antiAtGLR1.1 plants. (A) RT-PCR analysis was performed on 1 μg of total RNA isolated from the leaves of 30-day-old Arabidopsis, using gene-specific primers to cytosolic GS (GS1), chloroplastic GS (GS2), mitochondrial AAT (AAT1), cytosolic AAT (AAT2), chloroplastic AAT (AAT3), Fd-GOGAT, ICDH, 6PGDH, nitrate reductase 1 (NR1), nitrite reductase (NiR), nitrate transporter (CHL1), hexokinase 1 (HXK1), zeaxanthin epoxidase (ABA1), or tubulin 4 (TUB4). (B) Results from densitometric analysis of the RT-PCR data, conducted as described in the legend of Fig. 3. The results were expressed as a percentage of the control (100%) ± SD.
Implications for the Role and Function of AtGLR1.1. C and N metabolism are eminently integrated because C skeletons are required for N assimilation. Numerous studies have demonstrated that changes in the availability of different C compounds significantly affects the transcriptional or translational control of certain N-metabolic enzymes and vice versa (1, 2). Therefore, perturbations in the activity or accumulation of any C-metabolic isoenzyme might be expected to alter the activity of a particular N-metabolic isoenzyme. Likewise, similar reciprocating effects might be expected of a C-metabolic isoenzyme attributable to a change in a N-metabolic isoenzyme. But the fact that a reduction of AtGLR1.1 results in a global decrease of select C- and N-metabolic isoenzymes suggests that AtGLR1.1 is a regulator that coordinates and controls specific facets of C and N metabolism. Interestingly, only decreases in specific isoenzymes involved in C, N, or C/N metabolism were observed in antiAtGLR1.1 plants. These isoenzymes varied in their cellular locations, i.e., cytosol or chloroplast, whereas the mitochondrial isoenzymes tested in this study were unaffected, suggesting that there are distinct C/N sensor(s) or regulator(s) throughout the plant. This hypothesis is supported by reports of a PII-like element that functions as a N (4) and/or C (27) sensor in chloroplasts.
Our results are consistent with the hypothesis that AtGLR1.1 is a regulator of C and N metabolism, but the possibility that AtGLR1.1 may function as a sensor cannot be dismissed. Our results with the iGLR agonist and antagonist (Fig. 1) are consistent with the hypothesis that AtGLR1.1 also functions as a sensor or receptor. If AtGLR1.1 is a sensor, the question still remains: what is the true ligand? The most likely candidate is Glu. This hypothesis is supported by our results that show the reversal of the inhibitory effects of DNQX on seed germination by Glu, and by published reports of a Glu-induced voltage potential in Arabidopsis roots (10). Glu was not as effective as BMAA in reversing the inhibitory DNQX effect on germination. Probable explanations for this finding include the possibilities that (i) Glu uptake and/or distribution is limited in the antiAtGLR1.1 plants, or (ii) Glu is not the natural ligand and perhaps a BMAA-like molecule is the endogenous AtGLR1.1 ligand. The latter explanation is credible because BMAA is a plant-derived compound, found mainly in the cicads (28). Results from another laboratory demonstrated that the effects of an iGLR agonist are reversed only with high concentrations (10 mM) of Glu (8).
Collectively our data indicate that AtGLR1.1 functions as a C/N regulator, and/or sensor, that regulates C/N metabolism and distinct physiological processes such as germination through the control of ABA biosynthesis. We have demonstrated that the reduction of functional AtGLRs, either through antisense or by treatment with an iGLR antagonist, DNQX, results in elevated ABA contents (Table 1) or the accumulation of the ABA1 transcript (Fig. 4). Both findings are consistent with the notion that the inhibition of germination that we observed in DNQX and Suc treatments was a result of elevated ABA biosynthesis. ABA biosynthesis has been shown to alter physiological events such as seed germination (19) and lateral root formation (29).
Several models that describe the relationship between C or N sensing, ABA biosynthesis, and the control of physiological events have been recently published (29, 30). We present a model that incorporates portions of the above-mentioned models to explain how AtGLR1.1 regulates the relationship between Suc, N, and ABA biosynthesis to control seed germination (Fig. 5). In their explanation of C-mediated control of ABA, Cheng et al. (30) mention a HXK-independent ABA biosynthetic pathway. Our data suggest that AtGLR1.1 is a key component of the HXK1-independent ABA pathway. Furthermore, we have shown that AtGLR1.1 is differentially affected by Suc and/or N availability in a manner similarly described by Zhang and Forde (29) for an unnamed C/N regulator/sensor in one branch of their dual pathway model regulation of lateral root formation by 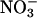 . Activation or stimulation of AtGLR1.1, through either increased
. Activation or stimulation of AtGLR1.1, through either increased 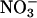 availability or reduced nitrogen in the form of amino acids, namely Glu or Gln, (i) maintains C- and N-metabolic pathways and (ii) decreases ABA biosynthesis. Inhibition of AtGLR1.1 by Suc results in decreased expression of distinct genes in C- and N-metabolic pathways, decreased HXK1, and elevated ABA biosynthesis, which results in physiological changes such as inhibition of germination. Because ABA has been shown to play an important role in drought tolerance, seed maturation, lateral root formation, and the regulation of vegetative growth (30), investigations into the link between AtGLR1.1 and these processes will prove to be an interesting and exciting area of research in the near future.
availability or reduced nitrogen in the form of amino acids, namely Glu or Gln, (i) maintains C- and N-metabolic pathways and (ii) decreases ABA biosynthesis. Inhibition of AtGLR1.1 by Suc results in decreased expression of distinct genes in C- and N-metabolic pathways, decreased HXK1, and elevated ABA biosynthesis, which results in physiological changes such as inhibition of germination. Because ABA has been shown to play an important role in drought tolerance, seed maturation, lateral root formation, and the regulation of vegetative growth (30), investigations into the link between AtGLR1.1 and these processes will prove to be an interesting and exciting area of research in the near future.
Fig. 5.
AtGLR1.1-mediated regulation of HXK-independent ABA biosynthesis in germinating seeds. AtGLR1.1 is negatively affected (Inhibitory) by Suc and positively affected (Stimulatory) by N. Negative signals (-) by antisense, Suc, or DNQX mediated result in decreased accumulation of the HXK1 transcript, elevated ABA levels or accumulation of the ABA1 transcript, and inhibited seed germination. N, in the form of amino acids, specifically Glu or Gln, positively (+) affects AtGLR1.1 (oval) and restores seed germination even in the presence of Suc or DNQX. Straight-dashed lines represent multiple-step or undefined processes. Curved-dashed lines represent positive or negative signals mediated by AtGLR1.1.
Acknowledgments
A portion of this work was funded by U.S. Department of Agriculture National Research Initiative Grant 2001-35100-09930 3125 (to F.J.T.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Suc, sucrose; Glc, glucose; Gln, glutamine; Glu, glutamate; iGLR, ionotropic Glu receptor; AtGLR, Arabidopsis thaliana Glu receptor; DNQX, 6,7-dinitroquinoxaline-2,3-(1H,4H)-dione; BMAA, S(+)-β-methyl-α,β-diaminopropionic acid; ABA, abscisic acid; GS, Gln synthetase; Fd-GOGAT, ferredoxin-dependent Glu synthase; AAT, asparate aminotransferase; GDH, Glu dehydrogenase; 6PGDH, 6-phosphogluconate dehydrogenase; ICDH, NADP-dependent isocitrate dehydrogenase; HXK1, hexokinase 1.
References
- 1.Coruzzi, G. M. & Zhou, L. (2001) Curr. Opin. Plant Biol. 4, 247-253. [DOI] [PubMed] [Google Scholar]
- 2.Coruzzi, G. & Bush, D. R. (2001) Plant Physiol. 125, 61-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang, J. C., Leon, P., Zhou, L. & Sheen, J. (1997) Plant Cell 9, 5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh, M.-H., Lam, H.-M., van de Loo, F. J. & Coruzzi, G. (1998) Proc. Natl. Acad. Sci. USA 95, 13965-13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam, H.-M., Chiu, J., Hsieh, M.-H., Meisel, L., Oliveira, I. C., Shin, M. & Coruzzi, G. M. (1998) Nature 396, 125-126. [DOI] [PubMed] [Google Scholar]
- 6.Lacombe, B., Becker, D., Hedrich, R., DeSalle, R., Hollmann, M., Kwak, J. M., Schroeder, J. I., Le Novere, N., Nam, H.-G., Spalding, E. P., et al. (2001) Science 292, 1486-1487. [DOI] [PubMed] [Google Scholar]
- 7.Dingledine, R., Borges, K., Bowie, D. & Traynelis, S. F. (1999) Pharmacol. Rev. 51, 7-61. [PubMed] [Google Scholar]
- 8.Brenner, E. D., Martinez-Barboza, N., Clark, A. P., Liang, Q. S., Stevenson, D. W. & Coruzzi, G. M. (2000) Plant Physiol. 124, 1615-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, S. A., Kwak, J. M., Jae, S. K., Wang, M. H. & Nam, H. G. (2001) Plant Cell Physiol. 42, 74-84. [DOI] [PubMed] [Google Scholar]
- 10.Dennison, K. L. & Spalding, E. P. (2000) Plant Physiol. 124, 1511-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bechtold, N. & Pelletier, G. (1998) Methods Mol. Biol. 82, 259-266. [DOI] [PubMed] [Google Scholar]
- 12.Turano, F. J., Muhitch. M. J., Felker, F. C. & MacMahon, M. B. (2002) Plant Sci. 163, 43-51. [Google Scholar]
- 13.Turano, F. J. & Muhitch, M. (1999) Physiol. Plant. 107, 407-418. [Google Scholar]
- 14.Gebhardt, J. S., Wadsworth, G. J. & Matthews, B. F. (1998) Plant Mol. Biol. 37, 99-108. [DOI] [PubMed] [Google Scholar]
- 15.Wadsworth, G. J., Marmaras, S. M. & Matthews, B. F. (1993) Plant Mol. Biol. 21, 993-1009. [DOI] [PubMed] [Google Scholar]
- 16.Turano, F. J., Thakkar, S. S., Fang, T. K. & Weisemann, J. M. (1997) Plant Physiol. 113, 1329-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallejos, E. (1983) in Isozymes in Plant Genetics and Breeding, Part A, eds. Tanksley, S. D. & Orton, T. J. (Elsevier, Amsterdam), pp. 469-516.
- 18.Chiu, J., DeSalle, R., Lam, H.-M., Meisel, L. & Coruzzi, G. M. (1999) Mol. Biol. Evol. 16, 826-838. [DOI] [PubMed] [Google Scholar]
- 19.Garciarrubio, A., Legaria, J. L. & Covarrubias, A. A. (1997) Planta 203, 182-187. [DOI] [PubMed] [Google Scholar]
- 20.Arenas-Huertero, F., Arroyo, A., Zhou, L., Sheen, J. & Leon, P. (2000) Genes Dev. 14, 2085-2096. [PMC free article] [PubMed] [Google Scholar]
- 21.Tingey, S. V., Tsai, F. Y., Edwards, J. W., Walker, E. L. & Coruzzi, G. M. (1988) J. Biol. Chem. 263, 9651-9657. [PubMed] [Google Scholar]
- 22.Schultz, C. J. & Coruzzi, G. M. (1995) Plant J. 7, 61-75. [DOI] [PubMed] [Google Scholar]
- 23.Wallsgrove, R. M., Turner, J. C., Hall, N. P., Kendall, A. C. & Bright, S. W. J. (1987) Plant Physiol. 83, 155-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart, G. R., Mann, A. F. & Fentem, P. A. (1980) in The Biochemistry of Plants, ed. Miflin, B. J. (Academic, New York), Vol. 5, pp. 271-327. [Google Scholar]
- 25.Palomo, J., Gallardo, F., Suarez, M. F. & Canovas, F. M. (1998) Plant Physiol. 118, 617-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey-Serres, J., Tom, J. & Freeling, M. (1992) Biochem. Genet. 30, 233-246. [DOI] [PubMed] [Google Scholar]
- 27.Lancien, M., Gadal, P. & Hodges, M. (2000) Plant Physiol. 123, 817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh, C. H., Brownson, D. M. & Mabry, T. J. (1995) Planta Med. 61, 66-70. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, H. & Forde, B. F. (2000) J. Exp. Bot. 51, 51-59. [PubMed] [Google Scholar]
- 30.Cheng, W. H., Endo, A., Zhou, L., Penney, J., Chen, H. C., Arroyo, A., Leon, P., Nambara, E., Asami, T., Seo, M., et al. (2002) Plant Cell 14, 2723-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]