Atalk by Richard Feynman, ``There is plenty of room at the bottom,'' was given >40 years ago at the California Institute of Technology during the annual meeting of the American Physical Society. Today, in the context of nanotechnology, this lecture is frequently cited because of its remarkable visionary power. Very early, Feynman envisaged nanotechnology as a whole new field and argued that a wealth of revolutionary technological advances and applications, like ultra-high density data storage media or ultra-small mechanical devices, would be feasible, with much room for improvements until finally fundamental physical limits at the atomic scale become relevant. To illustrate how much room there actually is at the bottom, Feynman asks us to imagine a miniaturization contest in which one participant manages to write on a pinhead ``how's this?'' Her competitor returns it, and in the dot of the ``i'' it says, ``not so hot,'' and so on.
The field has seen remarkable achievements since then, indeed, most notably the reduction of the area of transistors in microelectronic circuits by more than a factor of 107, or of the space required to store 1 bit of information on a magnetic surface by ≈108. Nanomechanical devices have seen tremendous progress, too, through impressive advances in scanning probe microscopy (1, 2).
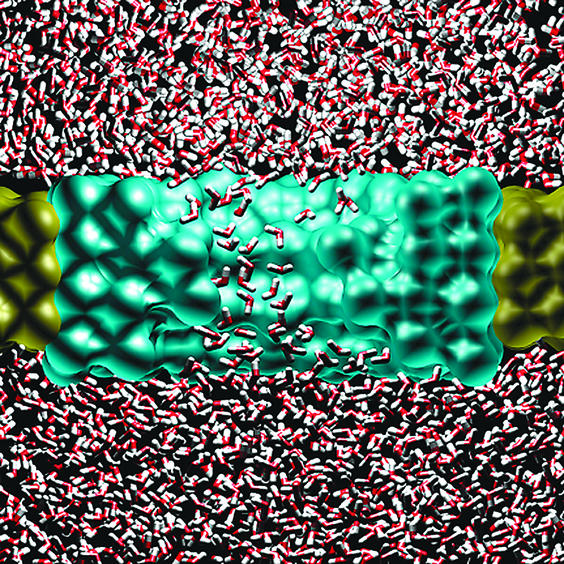
But what if the room at the bottom actually runs out? What if the physics like that appears at the limit of miniaturization? In their molecular dynamics study in a recent issue of PNAS, Beckstein and Sansom (3) address this question for the water flow through a short hydrophobic nanopore, where the fluid is essentially confined to one dimension (Fig. 1). In their simulations, they varied the radius of the pore from 1.0 nm down to 0.35 nm, which is a bit larger than the size of a water molecule. At first sight, one might expect a gradual, possibly stepwise, reduction of water flow with decreasing pore radius. What the authors and others (4) observed, however, is the appearance of oscillations between a fluid-like and a more vapor-like water phase within the pore, with a fluctuation frequency in the nanosecond range. With decreasing pore radius, the probability of finding the water in the pore in the vapor state increases. Eventually, below 0.4 nm, the water density within the pore drops to very small values.
Fig. 1.
Liquid/vapor oscillations are seen in molecular dynamics simulations of a hydrophobic model nanopore built from methane-like pseudo atoms (transparent). Water molecules (red/white) placed at both sides of the pore diffuse through the channel. The water molecules appear somewhat smaller than they actually are, because they are drawn as stick models, whereas the atoms forming the channel are drawn as Van der Waals spheres. The figure was kindly provided by Oliver Beckstein.
At this point, and typical for many other simulation studies, these simulations provide much of a full atomistic picture in a sense that bears some resemblance to Maxwell's daemon. However, and also quite typical, one finds that this wealth of simulation data does not immediately force on oneself any deeper understanding. Although already a lot of effort is involved in the set-up and execution of such molecular dynamics simulations, their physical interpretation is often much more demanding. Moreover, because of the structural and functional diversity of biological macromolecules and models thereof, it is even more challenging to find and isolate new phenomena that hold the promise to apply to a wide range of systems and processes.
In this respect, Beckstein and Sansom (3) have made an important contribution to the field. From their data, they developed a comprehensive picture of the underlying physics in terms of kinetics, dynamics, and thermodynamics. In particular, the Helmholtz free energy as a function of water density within the pore is found to exhibit two metastable minima for radii <0.55 nm, which explains the observed two-state behavior. Eventually, below 0.45 nm, the vapor state becomes the global minimum, whereas it vanishes for radii >0.7 nm, in agreement with the observations. Furthermore, their analysis of the kinetics of the vapor/fluid and fluid/vapor transitions points toward two different mechanisms involved for nano condensation and evaporation, respectively, which clearly deserve further investigation.
Studies like the one discussed here expand our basic knowledge of physics at the atomic scale. Moreover, they are not only interesting on their own, as a closer look into biology quickly reveals. Almost impossible to imagine 30 years ago, and still not fully acknowledged today, is the very fact that a much more advanced nanotechnology appeared on Earth >1 billion years ago, and we are only beginning to understand how this machinery works. In contrast to most technological approaches, during biomolecular evolution there was no need for miniaturization toward the (molecular) bottom, because it started out right at the bottom, generating biomolecules of increasing size and complexity. Thus, it is the rule rather than the exception for any biomolecular machinery to work within the realm of few-atoms physics, which we are only beginning to explore today.
In DNA, for example, one bit of genetic information occupies ≈0.5 nm3 (involving <100 atoms), a volume that is ≈108 times smaller than the one a bit requires on a 1-gigabit memory chip today. Proteins, just a few nanometers in size, have evolved to perform astonishingly complex tasks, such as pumping single protons against electrostatic fields and osmotic gradients in bacteriorhodopsin (which is achieved mainly by a lever arm, retinal, comprised of merely some 50 atoms) (5). Many different enzymes accelerate chemical reactions by factors of 1010 or more (6). The mitochondrial F-ATPase, for example, drives the synthesis of the energy currency of all living cells, ATP (7, 8), via a mechanical nanomotor (9, 10) that is ≈104 times smaller than the smallest manmade electromotor.
Also biological channels have evolved to near perfection. There is a huge diversity of channels, including ion channels, which currently receive pronounced attention today but lie outside the scope of this commentary. Rather, I would like to focus on water pores, which are essential for most living organisms (11), too, and are particularly addressed by Beckstein and Sansom. Up to now, ≈300 different channels have been discovered in bacteria, plants, and animals, forming the aquaglyceroporin membrane protein family (12). In the human body, 10 different aquaporins are known, expressed in tissues as diverse as the brain, eye lens, and kidney, and in red blood cells. A wide range of diseases are caused by malfunction of these water channels, such as nephrogenic diabetes insipidus, congenital cataract, and impaired hearing. The atomic structures of two of these channels, AQP1 and GlpF, have recently been solved by cryo-electron microscopy (13) and x-ray crystallography (14). Aquaporins are remarkably efficient water channels, yet are strikingly selective against ions and even protons. Recent molecular dynamics simulations of water permeation through the channels (15, 16) have revealed how these somewhat contradictory properties have been simultaneously optimized.
Aquaporins are efficient water channels, yet are strikingly selective against ions.
Also, Beckstein and Sansom have observed increased water mobility along their channel. They attribute it to the 1D confinement of the water molecules inside the pore rather than specific interactions with the channel. Admittedly, their simplified model differs in many respects from the pores formed by real channels. For example, biological channels typically show a quite heterogeneous channel interior, with less regular but highly optimized structure, a fine-tuned arrangement of hydrophobic and hydrophilic surface patches, and an equally fine-tuned set-up of electric fields. Nevertheless, one should not be too surprised if the observed liquid/vapor oscillations were also observed in complex biological channels, possibly with functional implications. Thus, in future studies on any transport process across membranes, passive or active, of water, protons, ions, or other solutes, we all might want to keep our eyes peeled. Along these lines, much work remains to be done to characterize this effect for increasingly complex systems, e.g., as a function of pore geometry, temperature, solvent composition, electrostatics, or hydrophobicity of the channel interior, or with respect to possible interactions with the conformational dynamics of the pore.
When the room at the bottom runs out, there is plenty of room for advancements.
See companion article on page 7063 in issue 12 of volume 100.
References
- 1.Binnig, G. & Rohrer, H. (1982) Helv. Phys. Acta 55, 726–735. [Google Scholar]
- 2.Hugel, T., Holland, N. B., Cattani, A., Moroder, L. & Gaub, H. E. (2002) Science 296, 1103–1106. [DOI] [PubMed] [Google Scholar]
- 3.Beckstein, O. & Sansom, M. S. P. (2003) Proc. Natl. Acad. Sci. USA 100, 7063–7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hummer, G., Rasaiah, J. C. & Noworyta, J. P. (2001) Nature 414, 188–190. [DOI] [PubMed] [Google Scholar]
- 5.Oesterhelt, D. & Stoeckenius, W. (1973) Proc. Natl. Acad. Sci. USA 70, 2853–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stryer, L. (1988) Biochemistry (Freeman, San Francisco).
- 7.Mitchell, P. (1961) Nature 191, 144–148. [DOI] [PubMed] [Google Scholar]
- 8.Boyer, P. D. (1993) Biochim. Biophys. Acta 1140, 215–250. [DOI] [PubMed] [Google Scholar]
- 9.Noji, H., Yasuda, R., Yoshida, M. & Kinosita, K. (1997) Nature 386, 299–302. [DOI] [PubMed] [Google Scholar]
- 10.Abrahams, J. P., Andrew, G. W. L., Lutter, R. & Walker, J. E. (1994) Nature 370, 621–628. [DOI] [PubMed] [Google Scholar]
- 11.Preston, G. M., Carrol, T. P., Guggino, W. B. & Agre, P. (1992) Science 256, 385–387. [DOI] [PubMed] [Google Scholar]
- 12.Heymann, J. B. & Engel, A. (2000) J. Mol. Biol. 295, 1039–1053. [DOI] [PubMed] [Google Scholar]
- 13.Murata, K., Mitsuoka, K., Hirai, T., Walz, T., Agre, P., Heymann, B., Engel, A. & Fujiyoshi, Y. (2000) Nature 407, 599–605. [DOI] [PubMed] [Google Scholar]
- 14.Fu, D., Libson, A., Miercke, L. J. W., Weitzman, C., Nollert, P., Krucinski, J. & Stroud, R. M. (2000) Science 290, 481–486. [DOI] [PubMed] [Google Scholar]
- 15.de Groot, B. L. & Grubmüller, H. (2001) Science 294, 2352–2357. [DOI] [PubMed] [Google Scholar]
- 16.Tajkhorshid, E., Nollert, P., Jensen, M. Ø., Miercke, L. J. W., O'Connell, J., Stroud, R. M. & Schulten, K. (2002) Science 296, 525–530. [DOI] [PubMed] [Google Scholar]