Abstract
Nanobacteria-like objects evidenced at the surface of the orthopyroxenes of the Tataouine meteorite in South Tunisia have been studied by scanning and transmission electron microscopies. A method of micromanipulation has been developed to ensure that exactly the same objects were studied by both methods. We have shown that the nanobacteria-like objects are spatially correlated with filaments of microorganisms that colonized the surface of the meteoritic pyroxene during its 70 years of residence in the aridic Tataouine soil. Depressions of a few micrometers in depth are observed in the pyroxene below the carbonates, indicating preferential dissolution of the pyroxene and calcite precipitation at these locations. The nanobacteria-like small rods that constitute calcium carbonate rosettes are well crystallized calcite single crystals surrounded by a thin amorphous layer of carbonate composition that smoothes the crystal edges and induces rounded shapes. Those morphologies are unusual for calcite single crystals observed in natural samples. A survey of recent literature suggests that the intervention of organic compounds derived from biological activity is likely in their formation.
Several recent studies have questioned the use of morphological criteria for the identification of fossil terrestrial or extraterrestrial life (e.g., refs. 1–5). Consecutive to the suggestion by McKay et al. (6) that ALH84001 contained evidence of Martian fossil life, several biogenicity criteria that can be checked with scanning electron microscopy (SEM) observations were discussed (e.g., refs. 7 and 8). For example, a packed distribution (colony-like), no preferred orientation with respect to the crystallographic directions of the substrate, rod-shaped forms displaying round edges, a narrow size distribution, or binary division images have been proposed as criteria for discriminating biological from abiotic objects.
The size criterion is still a matter of debate (9). Nanometer-scale spherical-, rod-, or ovoid-shaped objects in the 25- to 300-nm range have been considered by many authors as bacteria and named nanobacteria (10). They have been observed in a wide variety of samples: for example, terrestrial carbonates (10, 11), sulfides (12), mammalian blood (13), and Martian meteorites (6). They thus could be an important component on Earth (14) and could be related to life on Mars (6). The biological nature of these objects, however, has been challenged by both the geological and microbiological communities. The main objections are that these forms may have several possible abiotic origins, e.g., amorphous materials, etched crystallographic edges, and heavy-metal coating artifacts (4), and that they are too small to be living autonomous cells (15). Despite the interest associated with those problems, only a few studies have been carried out for characterizing the exact chemical composition and structure of such objects (e.g., refs. 16 and 17).
Nanometer-sized rod-shaped objects have been observed at the surface of the Tataouine meteorite (18). They developed in <63 years, which is the duration between the fall of the meteorite and the first observation of these features. The fall of the Tataouine meteorite was observed in 1931 in South Tunisia (19). The material broke up along mineral grain boundaries before the impact. Consequently, the sampling mainly consists of clasts ranging from a few hundred micrometers to several centimeters in size. Many samples were collected soon after the fall. Unaltered Tataouine specimens are composed mainly of a green orthopyroxene. The composition of the highly homogeneous crystals is uniform and clusters around Wo1.5En75Fs23.5 (20). The strewnfield was revisited in 1994, and several fragments of the meteorite were recovered by sifting the first centimeters of the sand. These samples contain secondary minerals that have formed during the terrestrial residence (18). Among these minerals, calcitic aggregates occur either as a partial filling with a rosette texture or they completely fill some fractures. It has been shown that carbonates associated with the orthopyroxene fragments have similar carbon and oxygen isotopic compositions as local sedimentary carbonates, but individual rosettes have not been analyzed (18). The surface of the meteorite, moreover, is invaded by small rod-shaped objects with rounded ends, sometimes curved, 70–80 nm wide and 100–600 nm long. They are found on all types of substrates at the surface of the meteorite (calcite, orthopyroxene, or chromite). Moreover, the same rodshaped forms are found on several other objects collected in the Tataouine sand (21).
In this work we studied those rod-shaped nanobacteria-like objects, collected from the surface of the pyroxenes of the meteorite, by transmission electron microscopy (TEM), using the same material characterized by SEM, and we determined their structure and chemistry.
Materials and Methods
Ten-millimeter-sized grains of the Tataouine meteorite were sorted under a binocular with sterile tweezers. They were mounted on aluminum stubs covered with carbon-conductive adhesive tape, and then they were gold-coated for 30 s (14). Operating conditions of the XL30 S FEG-SEM (Philips, Eindhoven, The Netherlands) were an accelerating voltage of 5 kV with a working distance of 5–15 mm. For energy-dispersive x-ray (EDX) chemical analyses, an acceleration voltage of 20 kV was used.
We ensured that the objects studied by TEM were exactly the same as those observed by SEM, which is not an easy task generally. We could precisely locate under an optical microscope the clusters of nanoforms of interest studied by SEM, which are several micrometers wide, and selectively sampled them using a micromanipulator. The clusters were placed on a copper grid of 200 mesh covered with a silicon film and were studied by TEM. Moreover, because the meteorite grains were gold-coated, we could check that the objects observed in TEM contained gold and were thus no contaminants brought during the micromanipulation procedure. TEM experiments were carried out on a JEOL 2010F microscope operating at 200 kV, equipped with a field emission gun, a high-resolution UHR pole piece, and a Gatan energy filter GIF 100. EDX analyses were performed by using a Kevex detector with an ultrathin window allowing detection of light elements. Finally, we observed again the same grains by SEM after micromanipulation to check that only the clusters of nanoforms had been removed from the pyroxene grains. We think that such a procedure provides an efficient and trustable way to couple SEM and TEM observations of such small-sized objects present at the surface of natural solids.
Results
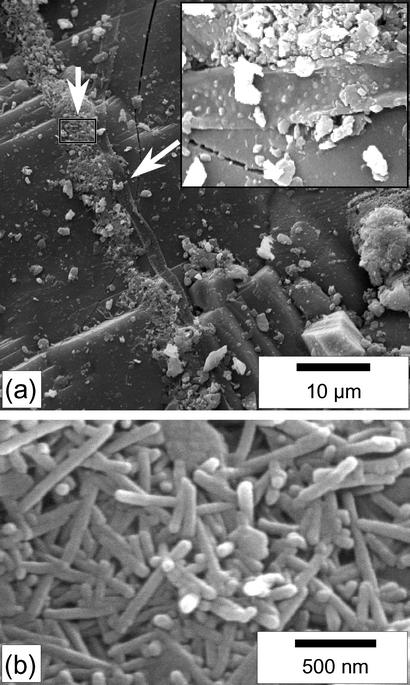
We first investigated by SEM the surface of pyroxene grains collected in 2000. Isolated rod-shaped nanobacteria-like objects were observed on many grains, although not with the same density on each grain. Their sizes have a slightly wider distribution than those mentioned by Barrat et al. (18), with lengths ranging between 200 and 800 nm and diameters ranging between 30 and 80 nm; most are 70–80 nm wide and 300–500 nm long. The nanoforms have been observed regularly in the course of a 3-year study, and no significant evolution could be seen. Several clusters of hundreds or thousands of rod-shaped objects were also observed (Fig. 1). The clusters were frequently near or directly associated with biological filaments that are 1 μm wide and tens of micrometers long (Fig. 1a). Those filaments are absent from reference orthopyroxenes of the meteorite collected immediately after the fall. As observed in previous studies (18), the EDX analyses on the clusters were indistinguishable from calcite analyses. No euhedral calcite crystal could be evidenced in the clusters.
Fig. 1.
SEM images (secondary electrons) of the surface of the Tataouine meteorite. (a) At the center of the image, a filament (the arrow and Inset show the filament at a higher magnification) is bordered all along its axis with clusters of nanobacteria-like rods. The euhedral crystal at the bottom right of the picture is a chromite. (b) Close-up view of the area denoted by a rectangle in a. The nanobacteria-like rods are 80 nm wide and a few hundreds of nanometers long. They display round edges.
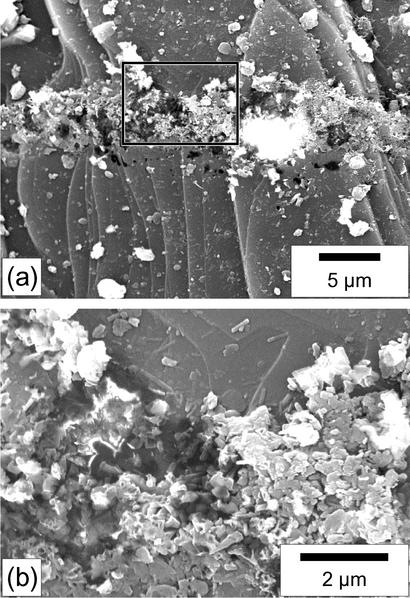
The pyroxene grains from which clusters were sampled for TEM observation (see below) were then observed again by SEM without any further gold coating. They systematically show etch pits at the places from which clusters have been removed (Fig. 2). We could verify on subsequent cross sections that the clusters of nanobacteria-like objects are not lying on the surface of the pyroxene but that they fill excavations (Fig. 2b). Such excavations are absent on neighboring cluster-free pyroxene surfaces.
Fig. 2.
SEM images (secondary electrons) of the area shown in Fig. 1 after the removing of the clusters of nanobacteria-like objects by micromanipulation. (a) The surface of the pyroxene crystal is deeply pitted along the linear feature where clusters were present. Some remnant clusters have been left by the micromanipulator. (b) Close-up view of the area denoted by a rectangle in a. In this view it is shown that remnant clusters are not lying on the flat surface of the pyroxene but that they fill an underlying excavation. Some crystals with subhedral and euhedral shapes can be observed among the nanobacteria-like rod clusters. They result either from the adhesion of small crystals of the Tataouine sand at the surface of the meteorite (quartz, iron oxides, and clay minerals are homogeneously scattered at the surface of the meteorite) or from precipitation at the surface of the meteorite.
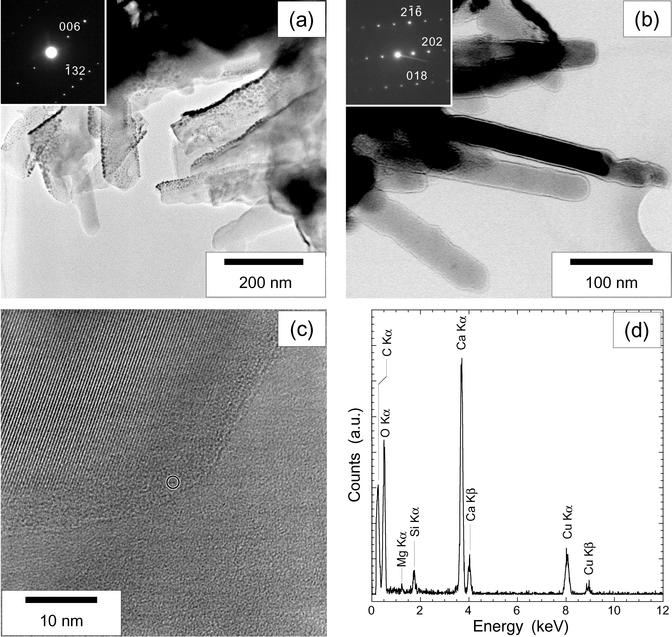
Several chips of clusters taken from the meteorite surface by micromanipulation were then observed by TEM (Fig. 3). Packed rod-shaped forms are not always easy to recognize, because thick samples are opaque to electrons. We studied several rod-shaped forms on the sides of thick aggregates of such objects. The sizes of the objects seen by TEM are consistent with those observed by SEM. The rods sometimes display convex or concave curvatures on their sides (Fig. 3a). EDX analyses were performed on each particle retrieved from the surface of the meteorite (Fig. 3d). All the rod-shaped forms and most of the other analyzed objects display calcium, carbon, and oxygen as the main elements and a more or less intense gold peak resulting from the coating of the sample (Fig. 3d). The high-resolution images and the associated electron diffraction patterns (Fig. 3 b and c) of the rods show that they are well crystallized calcite single crystals. All the numerous electron diffraction patterns collected were unambiguously indexed as calcite, and their inconsistency with the other polymorphs of CaCO3, especially vaterite, which is structurally close to calcite, was systematically checked. Most of the rods display round edges at low magnifications with TEM (Fig. 3a). However, high-resolution images show that they are actually facetted crystals at the nanometer scale (Fig. 3c). The smooth appearance is due to a 5- to 10-nm-thick amorphous layer (Fig. 3c) surrounding the crystals. This amorphous layer was analyzed with a probe size of 1 nm. Its analysis is indistinguishable from that of the crystallized part.
Fig. 3.
TEM characterization of the clusters of nanobacteria-like rod-shaped forms from the surface of the Tataouine meteorite. (a) Image of a cluster. (Inset) Electron diffraction pattern obtained on a single rod (zone axis [310]). Tiny dense spots in the image correspond to the gold-coating particles. (b) Image of another cluster. At this magnification, the edges of nanobacteria-like rods seem to be smooth. A strong contrast at the borders of the nanobacteria-like rods reinforced by overfocusing can be noticed. Zone axis [18-1] is shown. (c) High-resolution image of the border of a nanobacteria-like rod. The crystallized core showing lattice fringes is facetted and surrounded by an amorphous layer. The circle indicates the size of the EDX probe that was used. (d) EDX analysis obtained from the rod showing the background signal of the silicon-coated copper grid on which the samples have been deposited. Calcium, carbon, and oxygen are measured in both the core and the amorphous layer of the nanoforms. No other element has been evidenced. a.u., arbitrary units.
Discussion
Relationships Between Calcite Precipitates and Pyroxene Weathering. SEM observations consecutive to the micromanipulator sampling show that the pyroxene is etched under the clusters of nanoforms (Fig. 2). The possibility of these pits being artifacts due to the removal of the samples with the capillary of the micromanipulator can be excluded as shown by the observation of the sections created in the clusters by the micromanipulation (Fig. 2b). Remnant carbonates are observed in place at the bottom of such excavations. Thus, calcite precipitation is spatially associated with the dissolution of pyroxene at the surface of the Tataouine meteorite. A relationship between pyroxene weathering and carbonate precipitation is therefore likely. Jull et al. (22) observed carbonates (nesquehonite) resulting from the weathering of iron-magnesium-rich silicates in an Antarctic meteorite and proposed a similar relationship between carbonate precipitation and silicate alteration. The elucidation of the mechanisms allowing this correlation are beyond the scope of this article; several indications about the processes involved, however, can be noticed. First, the very localized occurrences of the calcite precipitates at the surface of the Tataouine meteorite are not related to areas enriched in calcium or of modified chemistry, because the orthopyroxene is chemically highly homogeneous (for a mineralogical study see ref. 23). Then, we point out that this localized intensive weathering of the pyroxene is frequently associated with the presence of biological filaments themselves observed near the clusters of nanobacteria-like objects (Fig. 1). The action of microorganisms in the alteration/precipitation process, either direct or indirect through local modifications of pH or ion activities, is thus highly probable. Indeed, several studies have shown that microorganisms can enhance the dissolution of ferromagnesian silicates (e.g., ref. 24). A molecular approach (sequencing of 16S rRNA genes) has just been carried out for determining the microorganisms present at the surface of the Tataouine pyroxene grains. Among other numerous bacteria, Actinomycetes and Cyanobacteria have been identified (K.B., V. Chapon, and T. Heulin, unpublished data). The filaments observed in association with the calcite clusters and the pyroxene weathering areas thus could be bacteria similar to those observed by Steele et al. (25) at the surface of the Martian meteorite ALH84001; they might also be fungi.
Nature and Origin of the Calcite Rods. Five criteria were used by Folk (10) to attribute a bacterial nature to similar forms of similar size observed in different carbonate deposits. More generally, these criteria are used to infer the biogenicity of fossils. (i) Bacteria often form colonies. Clustering of the rods at the surface of the Tataouine meteorite (e.g., Fig. 2) make them consistent with this criterion. (ii) The size variation of the objects can be normal, bimodal, but also less sorted if there are mixed stages of nutrition. The forms observed here are compatible with this criterion. (iii) A small upper size limit (<2 μm) and a narrow size range can also be associated with biogenic features. This is the case in the clusters observed at Tataouine. (iv) Folk (9, 10) noticed that he knew of no minerals that form sausage shapes with rounded ends (see, e.g., Fig. 3). (ν) Finally, chemical analyses should reveal silicon, calcium, phosphorus, or any elements commonly found in mineralization of microorganisms, which is the case in the present study (Fig. 3). All these criteria are satisfied by the rods observed at the surface of the Tataouine meteorite.
On the other hand, those rod-shaped nanobacteria-like objects observed at the surface of the Tataouine meteorite are indeed well crystallized calcite single crystals surrounded by an amorphous layer. Similar but longer and wider rod-shaped calcite crystals (width = 0.5 μm, length = 2 μm) have been observed in natural samples by Loisy et al. (26), who studied pedogenic micrite. Experimental studies have reproduced at least separately the different morphological features of these nanobacteria-like calcite crystals. (i) Calcite can grow as elongated crystals in the presence of some ions (Mg2+, e.g.; see ref. 27) as well as of some organic molecules (28). (ii) Calcite single crystals with such small sizes can be synthesized if growth is stopped briefly after the nucleation (e.g., refs. 29 and 30). (iii) Kile et al. (30) have shown that the crystal size distributions of abiotic calcites can have various shapes including log-normal (more or less skewed) and even bimodal shapes depending of the conditions of nucleation and growth. (iv) Calcite crystals can display rounded shapes when grown in the presence of various impurities in particular organic molecules (28, 31). It should be noticed that the calcite crystals actually observed at the surface of the Tataouine meteorite are facetted at the nanometer scale. (ν) Finally, the several-nanometers-thick amorphous layer that is surrounding the calcite crystals at the surface of the Tataouine meteorite is especially interesting. Atomic force microscopy studies have shown that a freshly cleaved calcite crystal surface examined under air restructures within a few hours (see, e.g., ref. 32). However, using low-energy electron diffraction Stipp and Hochella (33) demonstrated the persistence of an ordered surface 10 Å below the actual surface of the calcite crystal even after its rearrangement. An amorphous calcium carbonate phase is intrinsically unstable thermodynamically and kinetically at conditions similar to those prevailing at the surface of the Tataouine meteorite (34). The thin amorphous layer that is surrounding the crystals thus could be either the dissolution product of the calcite surface (29) or a precursor phase in the path of crystallization of calcite (see, e.g., ref. 35). The stabilization of such an amorphous phase can be achieved in the presence of specialized additives such as glycoproteins, for example, as shown by Aizenberg et al. (34). Hence the amorphous layer that surrounds the nanobacteria-like calcite single crystals observed at the surface of the Tataouine meteorite could suggest the role of organic molecules in their formation. Despite all the experimental results that give indications about the conditions needed for the formation of calcite crystals such as those observed at the surface of the Tataouine meteorite, such morphologies and structures have actually never been reported in natural samples. This does not preclude, however, the possibility that they might be common.
Two recent studies have been devoted specifically to the interpretation of such nanobacteria-like objects. Vecht and Ireland (36) propose that rod-shaped features in calcites observed in ALH84001 (6) and in the Tataouine diogenite (21) could result from the pseudomorphic replacement of vaterite by calcite. Indeed, they synthesized small spherical vaterite particles ranging between 50 and 100 nm in diameter, similar in shape and size to many of the nanobacteria-like objects reported in these studies. Moreover, many authors have suggested that metastable vaterite can act as a precursor phase spontaneously transforming into more stable calcite (e.g., refs. 37 and 38). Because the lattice structure of vaterite is similar to that of calcite, Vecht and Ireland (36) infer that calcite may result from the solid-state conversion of vaterite, thus conserving the same spherical morphology. Actually, this pseudomorphic replacement has only been experimentally reproduced by Maciejewski et al. (38) at 733 K, which is not relevant to the temperatures in the Tataouine sand. In solution, the transformation takes several hours (39) and consists in the recrystallization of calcite through the dissolution of vaterite with no conservation of the morphology (38, 40). Moreover, rod-shaped vaterite particles similar in shape to the Tataouine calcites have not been synthesized. For this reason and because we do not find any relict of vaterite in the observed calcites, the hypothesis of a vaterite precursor, although still possible, is not particularly supported by the observations in the Tataouine sand.
Kirkland et al. (29) propose two alternative origins for nanobacteria-like objects in calcite. They show that acid etching of surfaces of large euhedral calcite crystals can result in formation of rounded nanometer-scale objects. Clusters of carbonates observed at the surface of the Tataouine meteorite are not often associated with large euhedral calcite crystals and thus are not likely formed by this process. Kirkland et al. also performed several calcite precipitation experiments under purely inorganic, organic (addition of dextran or polyethylene glycol), and biotic (addition of full size bacteria or bacterial fragments and phages) conditions. They observed after 1 day small anhedral or rounded objects that have a strong resemblance to nanobacteria in all experiments. However, in experiments without organic molecules, most of the smallest component of the precipitate occurred as euhedral crystals, whereas in the experiments with a dissolved organic component nearly all the small precipitate occurred as masses of anhedral or rounded particles. They observed their experimental products with SEM only; thus, they could not discriminate the bacterial fragments from potential rounded calcite crystals in the experiments with bacteria. A TEM characterization thus would be interesting for actual comparison with the present study. After 3 days all the nanobacteria-like objects observed at the first stages by Kirkland et al. were absent in all the experiments, and calcite was present as large euhedral crystals. Mineralization conditions at Tataouine might have offered some equivalent frozen intermediate states of the experiments by Kirkland et al (29).
Conclusion
We have shown that the nanobacteria-like rods observed at the surface of orthopyroxenes of the Tataouine meteorite are well crystallized calcite single crystals with nanometric sizes, surrounded by a thin amorphous layer of similar composition. We have also shown a spatial correlation of the clusters of those calcite single crystals with biological filaments that colonized the surface of the meteoritic pyroxene during its 70-year residence time in the aridic Tataouine soil. The morphologies and structures of the calcite single crystals forming the clusters had never been reported in natural samples and, although experimental studies provide several hints, their mechanism of formation remains to be explained, in particular as far as organic molecules and microorganisms present at the surface of the meteorite are concerned. Because nanobacteria have often been observed in carbonate-containing samples (29), we suggest that those could be, in several cases, calcite single crystals. Experimental studies with accurate structural tools are needed to understand the potential links between such single crystals and microorganisms.
Acknowledgments
We thank two anonymous reviewers for helping to improve significantly the science contained in this article, in particular by pointing out key connections with previous studies. This work was made possible by support by the French national program Géomicrobiologie des Environnements Extrêmes.
Abbreviations: SEM, scanning electron microscopy; TEM, transmission electron microscopy; EDX, energy-dispersive x-ray.
References
- 1.Schopf, J. W., Kudryavtsev, A. B., Agresti, D. G., Wdowiak, T. J. & Czaja, A. D. (2002) Nature 416, 73–76. [DOI] [PubMed] [Google Scholar]
- 2.Brasier, M. D., Green, O. R., Jephcoat, A. P., Kleppe, A. K., Van Kranendonk, M. J., Lindsay, J. F., Steele, A. & Grassineau, N. V. (2002) Nature 416, 76–81. [DOI] [PubMed] [Google Scholar]
- 3.Southam, G. & Donald, R. (1999) Earth Sci. Rev. 48, 251–264. [Google Scholar]
- 4.Bradley, J. P., Harvey, R. P. & McSween, H. Y., Jr. (1997) Nature 390, 454–456. [DOI] [PubMed] [Google Scholar]
- 5.Toporski, J. K. W., Steele, A., Westall, F., Thomas-Keprta, K. L. & McKay, D. S. (2002) Astrobiology 2, 1–26. [DOI] [PubMed] [Google Scholar]
- 6.McKay, D. S., Gibson, E. K., Thomas-Keprta, K. L., Vali, H., Romanek, C. S., Clemett, S. J., Chillier, X. D. F., Maechling, C. R. & Zare, R. N. (1996) Science 273, 924–930. [DOI] [PubMed] [Google Scholar]
- 7.McKay, D. S., Gibson, E. K., Thomas-Keprta, K. L., Vali, H., Romanek, C. S. & Vali, H. (1997) Nature 390, 455–456. [Google Scholar]
- 8.Westall, F. (1999) J. Geophys. Res. Planets 104, 16437–16451. [Google Scholar]
- 9.Folk, R. L. & Taylor, L. A. (2002) Meteorit. Planet. Sci. 37, 1057–1069. [Google Scholar]
- 10.Folk, R. L. (1993) J. Sediment. Petrol. 63, 990–999. [Google Scholar]
- 11.Vasconcelos, C. & McKenzie, J. A. (1997) J. Sediment. Petrol. 67, 378–390. [Google Scholar]
- 12.Sillitoe, R. H., Folk, R. L. & Sarie, N. (1996) Science 272, 1153–1155. [DOI] [PubMed] [Google Scholar]
- 13.Kajander, E. O. & Ciftçioglu, N. (1998) Proc. Natl. Acad. Sci. USA 95, 8274–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folk, R. L. & Lynch, F. L. (1997) J. Sediment. Res. 67, 597–603. [Google Scholar]
- 15.Nealson, K. H. (1997) J. Geophys. Res. Planets 102, 23675–23686. [PubMed] [Google Scholar]
- 16.Vali, H., McKee, M. D., Ciftcioglu, N., Sears, S. K., Plows, F. L., Chevet, E., Ghiabi, P., Plavsic, M., Kajander, E. O. & Zare, R. N. (2001) Geochim. Cosmochim. Acta 65, 63–74. [Google Scholar]
- 17.Cisar, J., Xu, D. Q., Thompson, J., Swaim, W., Hu, L. & Kopecko, D. J. (2000) Proc. Natl. Acad. Sci. USA 97, 11511–11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrat, J. A., Gillet, P., Lécuyer, C., Sheppard, S. M. F. & Lesourd, M. (1998) Science 280, 412–414. [DOI] [PubMed] [Google Scholar]
- 19.Lacroix, A. (1931) Comptes Rendus de l'Académie des Sciences 193, 305–309. [Google Scholar]
- 20.Gooley, R. & Moore, C. B. (1976) Am. Mineral. 61, 373–378. [Google Scholar]
- 21.Barrat, J. A., Gillet, P., Lesourd, M., Blichert-Toft, J. & Poupeau, G. R. (1999) Meteorit. Planet. Sci. 34, 91–97. [Google Scholar]
- 22.Jull, A. J. T., Cheng, S., Gooding, J. L. & Velbel, M. A. (1988) Science 242, 417–419. [DOI] [PubMed] [Google Scholar]
- 23.Benzerara, K., Guyot, F., Barrat, J. A., Gillet, P. & Lesourd, M. (2002) Am. Mineral. 87, 1250–1256. [Google Scholar]
- 24.Liermann, L. J., Kalinowsli, B. E., Brantley, S. & Ferry, J. F. (2000) Geochim. Cosmochim. Acta 64, 587–602. [Google Scholar]
- 25.Steele, A., Goddard, D. T., Stapleton, D., Toporsli, J. K., Peters, V., Bassinger, V., Sharpless, G., Wynn-Williams, D. D. & McKay, D. S. (2000) Meteorit. Planet. Sci. 35, 237–241. [DOI] [PubMed] [Google Scholar]
- 26.Loisy, C., Verrechia, E. P. & Dufour, P. (1999) Sediment. Geol. 126, 193–204. [Google Scholar]
- 27.Folk, R. L. (1974) J. Sediment. Petrol. 44, 40–53. [Google Scholar]
- 28.Weiner, S. & Addadi, L. (1997) J. Mater. Chem. 7, 689–702. [Google Scholar]
- 29.Kirkland, B. L., Lynch, F. L., Rahnis, M. A., Folk, R. L., Molineux, I. J. & McLean, R. J. C. (1999) Geology 27, 347–350. [Google Scholar]
- 30.Kile, D. E., Eberl, D. D., Hoch, A. R. & Reedy, M. M. (2000) Geochim. Cosmochim. Acta 64, 2937–2950. [Google Scholar]
- 31.Orme, C. A., Noy, A., Wierzbicki, A., McBride, M. T., Grantham, M., Teng, H. H., Dove, P. M. & DeYoreo, J. J. (2001) Nature 411, 775–779. [DOI] [PubMed] [Google Scholar]
- 32.Stipp, S. L. S., Gutmannsbauer, W. & Lehmann, T. (1996) Am. Mineral. 81, 1–8. [Google Scholar]
- 33.Stipp, S. L. S. & Hochella, F. J. (1991) Geochim. Cosmochim. Acta 55, 1723–1736. [Google Scholar]
- 34.Aizenberg, J., Lambert, G., Weiner, S. & Addadi, L. (2002) J. Am. Chem. Soc. 124, 32–39. [DOI] [PubMed] [Google Scholar]
- 35.Brecevic, L. & Nielsen, A. E. (1989)) J. Cryst. Growth 98, 504–510. [Google Scholar]
- 36.Vecht, A. & Ireland, T. G. (2000) Geochim. Cosmochim. Acta 64, 2719–2725. [DOI] [PubMed] [Google Scholar]
- 37.Ogino, T., Suzuki, T. & Sawada, K. (1987) Geochim. Cosmochim. Acta 51, 2757–2767. [Google Scholar]
- 38.Maciejewski, M., Oswald, H. R. & Reller, A. (1994) Thermochim. Acta 234, 315–328. [Google Scholar]
- 39.Yamaguchi, T. & Murakawa, K. (1981) Zairyo 30, 856–860. [Google Scholar]
- 40.Friedman, G. M. & Schultz, D. J. (1994) Mineral. Mag. 58, 401–408. [Google Scholar]