Abstract
Chemotherapy of malaria parasites is limited by established drug resistance and lack of novel targets. Intraerythrocytic stages of Plasmodium falciparum are wholly dependent on host glucose for energy. Glucose uptake is mediated by a parasite-encoded facilitative hexose transporter (PfHT). We report that O-3 hexose derivatives inhibit uptake of glucose and fructose by PfHT when expressed in Xenopus oocytes. Selectivity of these derivatives for PfHT is confirmed by lack of inhibition of hexose transport by the major mammalian glucose and fructose transporters (Gluts) 1 and 5. A long chain O-3 hexose derivative is the most effective inhibitor of PfHT and also kills P. falciparum when it is cultured in medium containing either glucose or fructose as a carbon source. To extend our observations to the second most important human malarial pathogen, we have cloned and expressed the Plasmodium vivax orthologue of PfHT, and demonstrate inhibition of glucose uptake by the long chain O-3 hexose derivative. Furthermore, multiplication of Plasmodium berghei in a mouse model is significantly reduced by the O-3 derivative. Our robust expression system conclusively validates PfHT as a novel drug target and is an important step in the development of novel antimalarials directed against membrane transport proteins.
Keywords: Xenopus oocyte, malaria, antimalarial, glucose analogues, transport
Infection with Plasmodium falciparum causes malaria, which kills 1 million children and afflicts a further 400 million individuals every year. New drugs are urgently needed to treat malaria because conventional cheap treatment options are compromised by drug resistance. Parasite membrane transport proteins have not yet been actively exploited as drug targets (1). Asexual stage parasites require a continuous supply of glucose to survive and multiply (2), suggesting that the hexose transporter (PfHT) of P. falciparum is a potential drug target (3). Large increases in glucose utilization by infected erythrocytes may also divert this essential substrate from host tissues to parasites sequestered in microvasculature, thereby exacerbating pathophysiological processes in cerebral malaria and providing another reason to search for inhibitors of PfHT (4).
There are other reasons for supposing that PfHT is a good novel drug target. It is a single-copy gene with no close paralogues in the fully sequenced falciparum genome (5), and there is no variation in derived amino acid sequence of PfHT in laboratory and field isolates that we have studied (6). However, identification of a specific inhibitor of PfHT will only validate this transporter as a drug target if it also kills parasites. With this investigative approach, redundant pathways for hexose uptake by intraerythrocytic parasites are effectively excluded.
We therefore functionally characterized PfHT by using the Xenopus laevis heterologous expression system and previously identified important differences in the interaction of substrates with PfHT and the major mammalian hexose transporter (Glut) 1. For example, PfHT transports d-fructose as well as d-glucose, whereas Glut1 is selective for d-glucose, and 3-O-methyl-d-glucose (3-OMG) is accommodated significantly better by PfHT (Km = 1.3 ± 0.3 mM) compared with Glut1 (Km > 30 mM) (7, 8). These differences provided the basis for deriving selective inhibitors of PfHT.
Experimental Procedures
Plasmodium vivax Hexose Transporter (PvHT) Cloning. Primers containing BglII restrictions sites, and for the 5′ end, a strong eukaryotic Kozak consensus sequence before the start codon (underlined; CACCATG) were designed based on the P. vivax hexose transporter nucleotide sequence (PvHT, Belem strain; GenBank accession no. AJ488939). These oligonucleotides were used in a PCR to amplify wild-type PvHT by using DNA extracted from a patient returning from India with vivax infection (GenBank accession no. AJ549815). PCR product was ligated into pSPGT1, which contains 5′ and 3′ untranslated Xenopus β-globin sequences as described (8).
Uptake of Hexoses in Xenopus Oocytes. X. laevis oocytes were assayed as described previously in detail (7). cRNA for each transporter was transcribed (MEGA-script T7 or SP6, Ambion, Austin, TX) from different linearized pSPGT1 plasmids containing cDNA encoding for Glut1 (GenBank accession no. NM_138827), Glut5 (GenBank accession no. AF161071), PfHT (GenBank accession no. AJ131457), Plasmodium knowlesi hexose transporter (GenBank accession no. AJ488937), Plasmodium yoelii hexose transporter (GenBank accession no. AJ488938), or Toxoplasma gondii glucose transporter 1 (GenBank accession no. AF518411). Oocytes were injected with cRNA (≈10 ng per oocyte) (9, 10) and RNase-free water-injected oocytes acted as controls. Competition assays on hexose uptake were performed 36–48 h after microinjection, at room temperature and for 20–30 min, on groups of eight oocytes in Barth's medium. Competition by hexose analogues on glucose uptake was studied in Barth's medium containing radiolabelled glucose (2.69 μM, 323 mCi·mmol–1 d-[U-14C]glucose; Amersham Pharmacia; 1 Ci = 37 GBq) and unlabelled glucose (35 μM) with varying amounts of competitor. Competition experiments by hexose analogues for fructose uptake were conducted in identical conditions using radiolabeled fructose (16.66 μM, 323 mCi·mmol–1 d-[U-14C]fructose; Amersham Pharmacia) and unlabelled fructose (495 μM) as permeant. All solutions were allowed to equilibrate for at least 10 h before competition experiments. A one-site competition model was fitted to results by using prism (version 3; GraphPad, San Diego).
Uptake assays on PvHT were performed at room temperature for 20–30 min, on groups of eight oocytes in Barth's medium containing permeant d-[U-14C]glucose (323 mCi·mmol–1) from Amersham Pharmacia with varying amounts of unlabelled glucose. Estimation of kinetic parameters by nonlinear regression analysis used a Michaelis–Menten model using prism.
Each result was confirmed by at least three independent experiments. Statistical comparisons between groups were carried out with Student's t test or multivariate ANOVA as appropriate.
Experiments on Cultured P. falciparum. Parasites (clones 3D7 and K1) were cultured as described (11) and routinely grown in 11 mM glucose. For experiments that varied glucose or fructose concentrations, glucose-free RPMI medium 1640 was supplemented with stated concentrations of d-glucose or d-fructose. IC50 assays were carried out in sextuplicate at 1% hematocrit and 0.5% initial parasitemia, by using asynchronous cultures. [3H]hypoxanthine (0.5 μCi per well) was added after 24 h. Cells were harvested at 48 h and [3H]hypoxanthine incorporation measured by scintillation counting (1450 MicroBeta Plus, Wallac, Turku, Finland).
Hexose Analogues. Methyl analogues (12) were a gift of P.-M. Léo and 3-O-ethyl- (13), 3-O-benzyl- (14) and 3-O-((undec-10-en)yl)-d-glucose were prepared as published (15). 3-O-(2-Hydroxyethyl)-d-glucose was obtained by acidic hydrolysis of the corresponding acetal (16).
Results
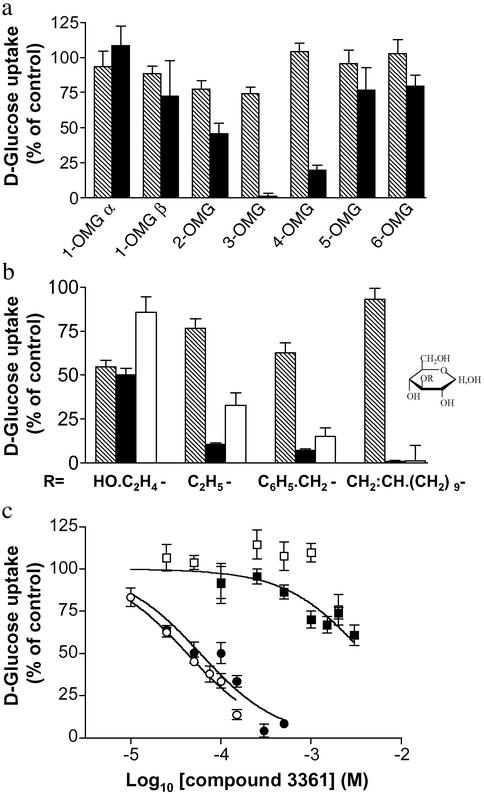
Identification of an Inhibitor of PfHT. Our previous work suggested that differences between PfHT and Glut1 in interacting at the -3 position in glucose could be usefully exploited. To confirm this, we initially examined other positions in d-glucose by using O-methyl derivatives as competitors in uptake assays of d-glucose mediated by PfHT and Glut1 (Fig. 1a). Highest selectivity for inhibition of d-glucose uptake for PfHT was confirmed for 3-OMG, with intermediate selectivity retained for O-2 and O-4 positions. The O-1, O-5, and O-6 derivatives did not compete for glucose uptake mediated by either the parasite or host transporter.
Fig. 1.
O-3 derivatives of d-glucose selectively inhibit PfHT. (a) The importance of the different d-glucose hydroxyl groups in interacting with Glut1 (hatched bars) and PfHT (black bars) transporters was assessed by testing different O-methyl-d-glucose analogues (10 mM) as competitors in uptake assays of d-glucose. (b) Uptake assays (mean ± SE of eight oocytes per condition) were carried out with d-[14C]glucose or d-[14C]fructose. Percentage of d-glucose or d-fructose uptake for each condition was compared with uptake in uncompeted oocytes (controls). All compounds were used at 10 mM except compound 3361 (1 mM). Hatched bars, Glut1; black bars, PfHT with d-glucose as substrate; white bars, PfHT with d-fructose as substrate. (c) Inhibition by compound 3361 of d-glucose uptake in PfHT (•) and Glut1 (▪) and d-fructose uptake in PfHT (○) and Glut5 (□).
We examined in greater detail O-3 derivatives of d-glucose as selective inhibitors of hexose uptake by PfHT (Fig. 1b). 3-O-hydroxyethyl-d-glucose was neither selective for PfHT nor did it significantly inhibit uptake of fructose. 3-O-ethyl-d-glucose and 3-O-benzyl-d-glucose inhibited d-glucose uptake but in relatively high concentrations (10 mM, Fig. 1b), whereas 3-O-((undec-10-en)-1-yl-d-glucose (1 mM, compound 3361) was a highly selective inhibitor of both d-glucose and d-fructose uptake mediated by PfHT (>98% inhibition; P < 0.0001 for both substrates). No inhibition of Glut1 mediated d-glucose uptake was observed at this concentration of compound 3361 (P > 0.1; Fig. 1c). KI values for PfHT were determined for these O-3 hexose derivatives and confirmed that compound 3361 is a specific inhibitor of PfHT (KI = 53 ± 19 μM) compared with Glut1 (KI = 3.3 ± 0.21 mM), giving a selectivity index (Glut1/PfHT) SIglucose = 62. Compound 3361 inhibits d-glucose uptake by PfHT at least 30 times more actively than the other derivatives. This selectivity is even greater for d-fructose uptake mediated by PfHT (Table 1), for two reasons; first because the KI value for compound 3361 is lower using d-fructose as substrate and secondly because highly selective Glut5-mediated d-fructose uptake cannot be inhibited by 3361 (2 mM) when it is expressed in Xenopus oocytes (Fig. 1c).
Table 1. Inhibition by O-3 derivatives of d-glucose on hexose transport mediated by PfHT in oocytes and on cultured P. falciparum.
| C3 analogue | KI for D-glucose uptake | KI for D-fructose uptake | IC50 (P. falciparum growth) |
|---|---|---|---|
| 3-O-ethyl-D-glucose | 1.62 ± 0.72 | 2.67 ± 0.66 | 3.70 ± 1.041 |
| 3-O-benzyl-D-glucose | 2.19 ± 0.85 | 1.49 ± 0.93 | 5.23 ± 1.063 |
| 3-O-hydroxyethyl-D-glucose | 14.8 ± 1.4 | > 10 | > 10 |
| 3-O-((undec-10-en)-1-yl)-D-glucose (compound 3361) | 0.053 ± 0.019 | 0.027 ± 0.003 | 0.0157 ± 0.0017 |
Experiments were performed on strain 3D7 with 4 mM D-glucose. Values (mean ± SE of three independent replicates) are expressed in mM.
Site of Action of Compound 3361. In previous work, we showed that a single amino acid mutation (Q169N) in helix 5 of PfHT determines fructose selectivity (8). Fructose transport is abolished, but affinity for d-glucose is unaltered by this change. Strikingly, this helix 5 mutant is 5-fold less susceptible to inhibition of d-glucose uptake by compound 3361 (Table 2), suggesting that the molecular site of interaction with compound 3361 involves the ``fructose filter'' of helix 5. Interestingly, helix 7 mutants have a similar affinity for d-glucose compared with native sequence (17). These mutants are susceptible to inhibition by compound 3361 with similar inhibitory constants compared with wild-type sequence (Table 2).
Table 2. Inhibition (KI in mM) by compound 3361 of d-glucose uptake mediated by a variety of hexose transporters.
| Transporter | KI for compound 3361 |
|---|---|
| PfHT Q169N | 0.271 ± 0.054 |
| PfHT 302AGT → SGL | 0.044 ± 0.018 |
| PfHT S302A | 0.064 ± 0.022 |
| PyHT | 0.080 ± 0.016 |
| PvHT | 0.112 ± 0.014 |
| PkHT | 0.108 ± 0.051 |
| TgGT1 | 2.073 ± 0.59 |
PyHT, P. yoelii hexose transporter; PvHT, P. vivax hexose transporter; PkHT, P. knowlesi hexose transporter; TgGT1, T. gondii glucose transporter. Mutants of PfHT are indicated individually. The KI value for the Q169N (helix 5) mutant was significantly (P < 0.001; multiple ANOVA with Bonferroni's correction) higher than for native PfHT (Table 1) and helix 7 mutants.
Killing of P. falciparum in Cultures. IC50 values were determined for the O-3 glucose derivatives in Table 1, by using cultured parasites. The KI estimates for inhibition of d-glucose uptake for these derivatives are in a similar rank order when determined in oocytes as the IC50 values obtained in P. falciparum cultured with d-glucose. Furthermore, compound 3361 is at least 230 times more effective in inhibiting parasite growth than other O-3 derivatives shown in Table 1.
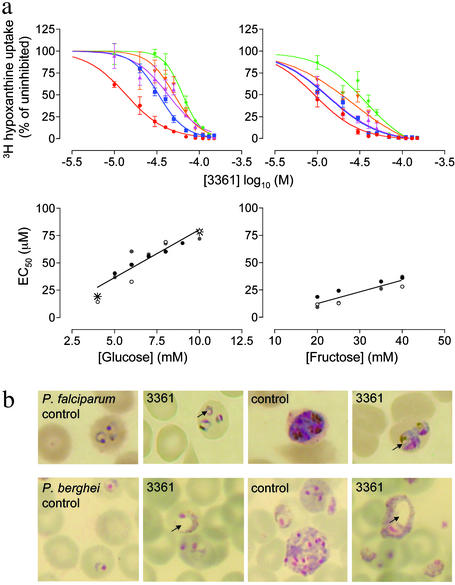
To confirm that PfHT is the target for compound 3361 in cultured parasites (as it is in oocytes expressing PfHT), we determined the relationship between extracellular hexose concentrations and IC50 values by inhibition of hypoxanthine incorporation. Results in Fig. 2a show that increasing d-glucose or d-fructose concentrations decreases the inhibitory activity of compound 3361. There is a linear relationship between extracellular hexose concentrations and increases in IC50 values (Fig. 2a). The magnitude of increase in extracellular d-glucose (e.g., 5 mM to 10 mM; 2-fold) is closely related to corresponding increases in IC50 values (from 36.7 ± 2.2 to 76.6 ± 2.2 μM; also 2-fold).
Fig. 2.
Dependence on hexose concentrations of inhibition by compound 3361. (a Upper) Effect of compound 3361 on [3H]hypoxanthine incorporation in cultured parasites at different d-glucose (Left) and d-fructose (Right) concentrations. Red, blue, pink, orange, and green lines correspond respectively to 4, 6, 8, 10, and 12 mM glucose and 20, 25, 30, 35, and 40 mM fructose in culture medium. (Lower) Change in IC50 for compound 3361 depending on the d-glucose (Left) and d-fructose (Right) concentrations. Data from three independent assays are shown (for glucose r2 = 0.84 and P < 0.0001; for fructose r2 = 0.80 and P = 0.0002). Values obtained with the multidrug-resistant strain K1 at extremes of d-glucose concentrations are represented by asterisks. (b) Effect of compound 3361 on the morphology of P. falciparum in culture (30μM compound 3361; 4 mM extracellular glucose) and of P. berghei in mice. Arrows show vacuole formation.
To exclude the possibility that varying glucose concentrations changes IC50 values for conventional antimalarials, we measured the IC50 for chloroquine for parasites grown in 4 mM and 10 mM d-glucose and found it to be 13.5 nM and 15 nM, respectively. Furthermore, the IC50 values of compound 3361 for a multidrug resistant strain of P. falciparum (K1) (18) are comparable to those for a chloroquine-sensitive strain at glucose concentrations that encompass the range tested (Fig. 2, asterisks). Similar observations hold when extracellular d-fructose concentrations are varied (e.g., increasing fructose concentrations from 20 to 40 mM is associated with an increase in IC50 values from 13.4 ± 2.8 to 33.9 ± 2.9 μM; 2-fold change). Morphological changes in parasites exposed to compound 3361 are shown in Fig. 2b. These reveal abnormal vacuole formation on exposure to this compound at different stages of parasite development including ring stages and trophozoites. Because neither Glut1 nor Glut5 are inhibited by compound 3361 at concentrations that kill parasites (Fig. 1c and Table 1), we conclude that PfHT is the target for compound 3361 in vivo.
Inhibition of Plasmodium berghei Growth. We extended our in vitro observations to assess the inhibition of an orthologue of PfHT encoded by the rodent malaria parasite P. yoelii (PyHT) and expressed in Xenopus oocytes (19). For d-glucose uptake mediated by PyHT, the KI for compound 3361 (80 μM) is similar to that for PfHT (Tables 1 and 2). The closely related P. berghei/mouse model was used in a standardized 4-day suppression test with compound 3361 (25 mg/kg; administered i.p. twice daily). There was significant suppression of parasitaemia from 13.32 ± 1.46% in untreated infected mice to 8 ± 0.38% in treated mice (a reduction of 40%; P = 0.005), confirming the efficacy of 3361 in vivo. Comparable morphological changes were induced by compound 3361 in vivo in P. berghei (Fig. 2b) and P. falciparum cultures, and consist of extensive vacuole formation in parasites. More detailed pharmacokinetic studies will be needed to optimize in vivo efficacy.
Inhibition of Other PfHT Orthologues. P. knowlesi and P. vivax are phylogenetically closely related parasites. Their hexose transporters have ≈84% sequence identity (19). The Km for glucose of the vivax transporter, PvHT, is a mean ± SEM of 0.85 ± 0.22 mM, compared with a previously published value for P. knowlesi hexose transporter of 0.67 ± 0.16 mM (19). Both these hexose transporters are also inhibited with very similar KI values (≈0.11 mM) by compound 3361 when expressed in oocytes (Table 2).
These KI values contrast with the KI determined for the glucose transporter of T. gondii, another apicomplexan parasite, which is relatively insusceptible to inhibition by 3361 (KI ≈ 2 mM). They confirm the relatively high specificity of compound 3361 for hexose transporters of Plasmodium spp., particularly for the two most important human pathogens: P. falciparum and P. vivax.
Discussion
We have previously suggested a simple model for hexose permeation in P. falciparum-infected erythrocytes in which glucose and fructose enter erythrocytes through Glut1 and Glut5, respectively, but both hexoses are taken up by PfHT into parasites (3). Our experimental findings using a specific inhibitor of PfHT are entirely consistent with this model for hexose permeation pathways in infected erythrocytes. Despite the amphipathic nature of compound 3361, nonspecific effects on membrane structure and function are likely to be minimal because the activity of Gluts is unimpaired in Xenopus oocytes at concentrations of compound 3361 that kill parasites. In addition, this inability of compound 3361 to inhibit hexose transport by mammalian Gluts in submillimolar concentrations excludes Gluts as being important targets that mediate parasite killing. The specificity of compound 3361 is also emphasised by the lack of inhibition of glucose uptake mediated by T. gondii glucose transporter 1, an apicomplexan hexose transporter with which Plasmodium hexose transporters share 35–42% amino acid identity, compared with <30% identity with mammalian Gluts (19).
It is possible that compound 3361 acts on posttransport metabolism of parasites such as phosphorylation by hexokinase, but several experimental results argue against this. First, the observation that both fructose and glucose compete with compound 3361 in killing parasites (Fig. 2a) supports the idea that PfHT is the molecular target in parasites, as it is in oocytes, because it is unlikely that similar competitive effects would be observed if compound 3361 were to act on different enzymes dealing with glucose and fructose in early glycolysis. Second, the rank order for inhibition of PfHT by O-3 derivatives in oocytes is preserved in the rank order for inhibition of growth of P. falciparum-infected erythrocytes (Table 1). Finally, the molecular site of interaction of PfHT with compound 3361 has been narrowed to the region of the ``fructose filter'' in helix 5 by analysis of previously characterized mutants of PfHT (Table 2). The sugar moiety in compound 3361 probably interacts with amino acid residues in Helix 5 of PfHT that are involved in substrate discrimination (17). This glucose portion of compound 3361 may be envisaged to be the ``ball'' of a lollipop, where the stick is the long aliphatic side chain that presumably associates with lipid leaflets of the plasma membrane. Compound 3361 appears to act as a competitive inhibitor of PfHT, and is effective in micromolar concentrations, compared with millimolar concentrations of substrate that are presented to the infected erythrocyte.
We have developed a novel specific inhibitor of the hexose transporter of P. falciparum and shown that it kills parasites (including a multidrug-resistant line) in vitro, as well as suppressing P. berghei development in vivo. These findings confirm our hypothesis that PfHT is the critical mediator of hexose delivery to P. falciparum in erythrocytes, and is a worthy novel drug target.
Rational antimalarial drug development directed against PfHT can now proceed, either with compound 3361 as a lead, or by using alternative combinatorial approaches. Concurrent comparisons with mammalian orthologues will continue to be invaluable in identifying molecular determinants of antimalarial specificity.
Acknowledgments
We dedicate this paper to the memory of P. Crabbé, founder of the International Organisation for the Chemical Sciences in Development. We thank Dr. Simon Croft and Wendy Davies (London School of Hygiene and Tropical Medicine) for in vivo assays, Dr. C. F. Burant for the gift of a clone encoding Glut5, and Dr. A.-C. Uhlemann for discussions. This work was supported by Medical Research Council (U.K.) Grant G9800300.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Glut, human facilitative hexose transporter; -OMG, -O-methyl-d-glucose; PfHT, Plasmodium falciparum hexose transporter; PvHT, Plasmodium vivax hexose transporter.
References
- 1.Ridley, R. G. (2002) Nature 415, 686–693. [DOI] [PubMed] [Google Scholar]
- 2.Kirk, K. (2001) Physiol. Rev. 81, 495–537. [DOI] [PubMed] [Google Scholar]
- 3.Krishna, S., Woodrow, C. J., Burchmore, R. J., Saliba, K. J. & Kirk, K. (2000) Parasitol. Today 16, 516–521. [DOI] [PubMed] [Google Scholar]
- 4.Krishna, S. & Woodrow, C. J. (1999) in Transport and Trafficking in the Malaria-Infected Erythrocyte, ed. Cardew, G. (Wiley, London), Vol. 226, pp. 126–144. [Google Scholar]
- 5.Gardner, M. J., Hall, N., Fung, E., White, O., Berriman, M., Hyman, R. W., Carlton, J. M., Pain, A., Nelson, K. E., Bowman, S., et al. (2002) Nature 419, 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishna, S., Eckstein-Ludwig, U., Joët, T., Uhlemann, A.-C., Morin, C., Webb, R., Woodrow, C. J., Kün, J. & Kremsner, P. (2002) Int. J. Parasitol. 32, 1567–1573. [DOI] [PubMed] [Google Scholar]
- 7.Woodrow, C. J., Penny, J. I. & Krishna, S. (1999) J. Biol. Chem. 274, 7272–7277. [DOI] [PubMed] [Google Scholar]
- 8.Woodrow, C. J., Burchmore, R. J. S. & Krishna, S. (2000) Proc. Natl. Acad. Sci. USA 97, 9931–9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould, G. W. (1997) in Molecular Biology Intelligence Unit (R. G. Landes Company and Chapman & Hall, Austin, TX), Vol. 1, pp. 235. [Google Scholar]
- 10.Corpe, C. P., Bovelander, F. J., Munoz, C. M., Hoekstra, J. H., Simpson, I. A., Kwon, O., Levine, M. & Burant, C. F. (2002) Biochim. Biophys. Acta 1576, 191–197. [DOI] [PubMed] [Google Scholar]
- 11.ter Kuile, F., White, N. J., Holloway, P. H., Pasvol, G. & Krishna, S. (1993) Exp. Parasitol. 76, 85–95. [DOI] [PubMed] [Google Scholar]
- 12.Bourne, E. J. & Peat, S. (1950) Adv. Carbohydr. Chem. 5, 145–190. [DOI] [PubMed] [Google Scholar]
- 13.Zeller, S. G., D'Ambra, A. J., Rice, M. J. & Gray, G. R. (1988) Carbohydr. Res. 182, 53–62. [DOI] [PubMed] [Google Scholar]
- 14.Charon, D. & Szabo, L. J. (1980) J. Chem. Soc. Perkin Trans. 1, 1971–1977. [Google Scholar]
- 15.Ikekawa, T., Irinoda, K., Saze, K., Katori, T., Matsuda, H., Ohkawa, M. & Kosik, M. (1987) Chem. Pharm. Bull. 35, 2894–2899. [DOI] [PubMed] [Google Scholar]
- 16.Bignan, G., Morin, C. & Vidal, M. (1993) Carbohydr. Res. 248, 371–375. [DOI] [PubMed] [Google Scholar]
- 17.Manning, S. K., Woodrow, C., Zuniga, F. A., Iserovich, P., Fischbarg, J., Louw, A. I. & Krishna, S. (2002) J. Biol. Chem. 277, 30942–30949. [DOI] [PubMed] [Google Scholar]
- 18.Thaithong, S. & Beale, G. H. (1981) Trans. R. Soc. Trop. Med. Hyg. 75, 271–273. [DOI] [PubMed] [Google Scholar]
- 19.Joët, T., Holterman, L., Stedman, T. T., Kocken, C. H., Van Der Wel, A., Thomas, A. W. & Krishna, S. (2002) Biochem. J. 368, 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]