Abstract
Antibodies against a conserved RNA-binding protein, the Ro 60-kDa autoantigen, occur in 24–60% of all patients with systemic lupus erythematosus. Anti-Ro antibodies are correlated with photosensitivity and cutaneous lesions in these patients and with neonatal lupus, a syndrome in which mothers with anti-Ro antibodies give birth to children with complete congenital heart block and photosensitive skin lesions. In higher eukaryotes, the Ro protein binds small RNAs of unknown function known as Y RNAs. Because the Ro protein also binds misfolded 5S rRNA precursors, it is proposed to function in a quality-control pathway for ribosome biogenesis. Consistent with a role in the recognition or repair of intracellular damage, an orthologue of Ro in the radiation-resistant eubacterium Deinococcus radiodurans contributes to survival of this bacterium after UV irradiation. Here, we show that mice lacking the Ro protein develop an autoimmune syndrome characterized by anti-ribosome antibodies, anti-chromatin antibodies, and glomerulonephritis. Moreover, in one strain background, Ro–/– mice display increased sensitivity to irradiation with UV light. Thus, one function of this major human autoantigen may be to protect against autoantibody development, possibly by sequestering defective ribonucleoproteins from immune surveillance. Furthermore, the finding that mice lacking the Ro protein are photosensitive suggests that loss of Ro function could contribute to the photosensitivity associated with anti-Ro antibodies in humans.
Patients suffering from systemic rheumatic diseases such as scleroderma, systemic lupus erythematosus, and polymyositis often produce antibodies against highly conserved RNA–protein complexes. A fascinating but poorly understood facet of these diseases is that specific autoantibodies are associated with distinct rheumatic disease syndromes. For example, antibodies against tRNAs and tRNA–synthetase complexes are commonly found in polymyositis, whereas antibodies against nucleolar components are associated with scleroderma (1). This association of particular autoantibodies with specific syndromes has been useful for establishing diagnoses and for predicting sequelae that may accompany the particular rheumatic disorder.
In two rheumatic diseases, systemic lupus erythematosus and Sjögren's syndrome, a major target of the immune response is an RNA-binding protein known as the Ro 60-kDa autoantigen. In lupus patients, anti-Ro antibodies are highly associated with photosensitivity and photosensitive skin lesions, particularly those of subacute cutaneous lupus erythematosus (1, 2). In addition, anti-Ro antibodies are associated with neonatal lupus, a syndrome in which maternal autoantibodies cross the placenta, resulting in infants with photosensitive skin lesions and a cardiac conduction defect, complete congenital heart block (3).
Studies of the Ro 60-kDa protein have revealed that it is present in both the nucleoplasm and cytoplasm of vertebrate cells (4–6). In the cytoplasm, the Ro protein is complexed with one of several small RNAs known as Y RNAs. These Y RNAs are ≈100 nt long, transcribed by RNA polymerase III, and bound almost entirely by the Ro protein (7). The Ro protein/Y RNA complex is conserved evolutionarily, because orthologues have been described in mammals, the frog Xenopus laevis, and the nematode Caenorhabditis elegans, as well as the radiation-resistant eubacterium Deinococcus radiodurans (8–12). Although the function of the Y RNAs remains unknown, genetic depletions of the Ro protein from C. elegans and D. radiodurans have revealed that Ro protein binding stabilizes Y RNAs from degradation (12, 13). Both mammalian cells and Xenopus oocytes contain a nuclear pool of Ro protein, which is not complexed with Y RNAs (4–6).
Experiments in X. laevis and C. elegans have led to the proposal that the Ro 60-kDa protein functions in a quality-control pathway for ribosome biogenesis (6, 13). In Xenopus oocyte nuclei, the Ro protein associates with a large class of variant 5S rRNA precursors. To synthesize the large numbers of ribosomes needed for early development, X. laevis contains ≈20,000 genes encoding the oocyte 5S rRNA, many of which diverge from the consensus 5S rRNA sequence (14). The 5S rRNA variants bound by the Ro protein contain additional gene-encoded nucleotides at their 3′ ends, suggesting that they are generated by read-through of the first termination signal for RNA polymerase III. The variants also contain one or more changes from the consensus 5S rRNA sequence that cause the RNAs to misfold into a structure recognized by the Ro protein (15). Because these variant pre-5S rRNAs are processed inefficiently to mature 5S rRNA and eventually degraded, the Ro protein is proposed to function in a quality-control pathway for 5S rRNA biogenesis (6, 15). Consistent with a role in ribosomal quality control, nematodes lacking the Ro protein have increased numbers of variant 5S rRNAs (13).
Genetic analyses in C. elegans and D. radiodurans have implicated the Ro 60-kDa protein in the resistance to environmental stress. C. elegans lacking the Ro protein are defective in the formation of dauer larvae, a stress-resistant stage formed when environmental conditions are unfavorable for growth (16). D. radiodurans lacking the Ro protein exhibit decreased survival after UV irradiation. Moreover, both the D. radiodurans Ro protein and a Y RNA orthologue are up-regulated after UV irradiation, suggesting a role for the Ro ribonucleoproteins (RNPs) in the recognition and/or repair of intracellular damage (12).
To examine the role of Ro RNPs in a mammalian organism, we created mice lacking the Ro 60-kDa protein. We report that mice lacking the Ro autoantigen develop an autoimmune syndrome that shares several features with the human disease systemic lupus erythematosus. Similar to lupus patients, Ro–/– mice exhibit anti-ribosome and anti-chromatin antibodies, glomerulonephritis, and sensitivity to sunlight. Thus, one normal function of this major lupus autoantigen may be to protect against the development of autoimmune disease.
Materials and Methods
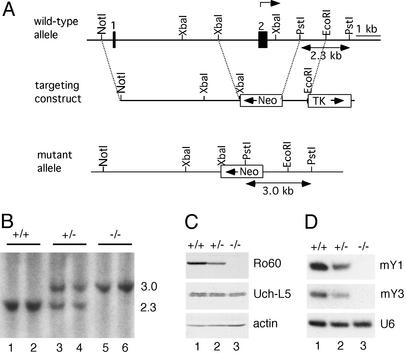
Generation and Characterization of Ro–/– Mice. DNAs corresponding to the first 10 kb of the mouse Ro gene (17) were isolated from a λFIXII mouse genomic library (129/Sv strain; Stratagene) by using human cDNA (18) as a hybridization probe. To construct the targeting vector shown in Fig. 1 A, we used plasmids (19) in which the PGKneo cassette (pBS-neo) and herpes simplex virus thymidine kinase genes (pBS-TK) were cloned into pBluescript SK (Stratagene). First, a 2.3-kb PstI fragment containing intronic sequences between the second and third coding exons was inserted into the pBS-neo vector at the EcoRI site. Next, a 3-kb NotI/EcoRI fragment containing the 1.6-kb PGKneo cassette and 1.4 kb of Ro sequences was excised from the plasmid and inserted into the corresponding sites of pBS-TK. Last, a 4.7-kb NotI/XbaI fragment derived from the 5′ end of the Ro genomic clone was ligated into the corresponding sites of the TK/PGKneo/Ro-containing plasmid. The resulting plasmid was linearized with NotI and electroporated into W9.5 embryonic stem cells. Genomic DNA from transformants resistant to G418 (200 μg/ml) and ganciclovir (2 μM) was digested with PstI and subjected to Southern blot analysis. Clones containing the targeted mutation were injected into C57BL/6 blastocysts, followed by implantation into pseudopregnant foster mothers. Male chimeric mice were mated with C57BL/6 female mice, and germ-line-transmitted progeny were obtained. Light and electron microscopic examination of kidneys was performed as described (20).
Fig. 1.
Disruption of the Ro gene. (A) Diagram showing the Ro gene locus, the targeting construct, and the recombined mutant allele. The first two exons are indicated by filled boxes, and the translation initiation site is indicated (arrow). (B) Southern analysis of PstI-digested DNA from wild-type (lanes 1 and 2), Ro+/– (lanes 3 and 4), and Ro–/–(lanes 5 and 6) mice. (C) Western blots of brain extracts with a rabbit anti-Ro serum. The blot was reprobed to detect Uch-L5, which runs as a doublet (Middle), and actin (Bottom). (D) Northern blot analyses of brain RNA reveal that Y RNAs are reduced in Ro+/– and Ro–/– mutant mice.
For Northern blot analyses to detect Y RNAs, total brain tissue was lysed in TRIzol (Invitrogen), and RNA was isolated as described by the supplier. After fractionation in 5% polyacrylamide/8 M urea gels, the RNA was transferred to Zetaprobe GT nylon membranes (Bio-Rad) in 0.5× TBE (1× TBE = 89 mM Tris/89 mM boric acid/2.0 mM EDTA, pH 8.3) at 150 mA for 16 h. Oligonucleotides used to detect mY1 and mY3 RNA were 5′-AAGGGGGGAAAGTGTAGAACAGGA-3′ and 5′-GAGCGGAGA AGGA ACA A AGA A ATCTG-3′, respectively.
For Western blot analyses, brain extracts were fractionated in SDS/polyacrylamide gels, transferred to nitrocellulose as described (21), and probed with a monoclonal anti-mouse Ro antibody. To prepare the antibody, oligonucleotides 5′-GGCGCGGGATCCGAAGGATCTGCAAACCAGTTGC-3′ and 5′-GCCGCGGAGCTCT TA A ATGACATCCA ATGTGAAATTCCG-3′ were used to amplify the mouse Ro protein-coding sequence. The DNA was digested with BamHI and SacI and inserted into pTrcHisA (Invitrogen). Because the recombinant protein was insoluble, it was solubilized in guanidine·HCl, purified from Escherichia coli lysates, and renatured as described (12). After renaturation, the protein was used to immunize Ro–/– mice. Spleen cells were fused to Ag8.653 cells by using standard methods. Antiserum against human Uch-L5 was a generous gift of R. Cohen (University of Iowa, Iowa City).
Analysis of T and B Cell Function. Thymus, spleen, lymph nodes, and bone marrow were isolated from Ro–/– and wild-type mice, stained with the appropriate antibodies [CD4, CD8, T cell receptor, CD69, CD44, CD25, CD45R, CD43, IgM, and IgD antibodies (Pharmingen)] and analyzed by using a FACSCalibur analysis instrument (Becton Dickinson). Adult Ro–/–, Ro+/–, and wild-type mice were immunized s.c. with keyhole limpet hemocyanin (Calbiochem) that was emulsified in complete Freund's adjuvant. After 10 days, mice were killed and lymph node cells were assayed for proliferation by [3H]thymidine incorporation (New England Nuclear) and cytokine production by ELISA (Pharmingen). Blood was collected at this time, and anti-keyhole limpet hemocyanin antibodies were measured by ELISA (Southern Biotechnology Associates). Ig levels were measured by ELISA in unimmunized mice and in age-matched Ro–/–, Ro+/–, and wild-type mice.
Characterization of Autoantibodies. Mouse NIH 3T3 cells were fixed in 3% formaldehyde/120 mM sodium phosphate, pH 7.4, and subjected to immunofluorescence analysis. Sera were used at dilutions between 1:125 and 1:1,000. Antibodies against RNA-containing antigens were detected by performing immunoprecipitations from mouse L1210 cells as described (8, 22). RNAs contained within immunoprecipitates were labeled with [32P]pCp and T4 RNA ligase (23) and fractionated in 5% polyacrylamide/8 M urea gels. Reference sera were anti-Sm and anti-ribosome mouse monoclonal antibodies (24) and human anti-P antibodies (a gift of K. Elkon, University of Washington, Seattle). Antibodies against histones were detected by Western blotting extracts of mouse L1210 cells as described (21) by using sera diluted to 1:500 and 1:1,000. Antibodies against double-stranded and single-stranded DNA (Euroimmun, Lübeck, Germany) and nucleosomes (Medizym, Medipan Diagnostics, Selchow, Germany) were detected by ELISA using precoated plates. For ELISAs, the mean reactivity for 18 wild-type antinuclear antibody (ANA)-negative mice was determined. Values higher than three standard deviations above the mean were considered positive.
To identify the ≈30-kDa doublet recognized by many sera, L1210 cells were sonicated in 40 mM Tris·HCl, pH 7.5/150 mM NaCl/2 mM MgCl2 by using a Branson sonifier (twice for 20 sec at setting 3). After sedimentation at 10,000 × g for 10 min, the 30-kDa doublet was found in the pellet. The pellet was subjected to sequential salt extraction using 300 and 600 mM NaCl. The doublet corresponded to two bands in the 600 mM NaCl supernatant, which were microsequenced at the Yale Keck Facility. For the ≈15-kDa band, embryonic stem cells were lysed in a Dounce homogenizer in 40 mM Tris·HCl, pH 7.5/150 mM NaCl/2 mM MgCl2/0.1% Nonidet P-40/10 mM DTT, and nuclei were sedimented at 10,000 × g for 10 min. After resuspension in the same buffer, nuclei were disrupted by sonication and the lysate was sedimented at 10,000 × g for 10 min. After washing the pellet with 300 mM NaCl in the above buffer, the pellet was incubated in 0.2 M HCl for 30 min on ice to extract histones, followed by sedimentation at 10,000 × g for 10 min. The supernatant was applied to a PD-10 column (Amersham Biosciences) equilibrated in 50 mM sodium phosphate, pH 8.5. The column eluate was applied to an SP Sepharose column equilibrated in the same buffer. Histones were eluted with 1 M NaCl.
UV Sensitivity. Mice (6–8 weeks old) were shaved on the back and irradiated 24 h later by using UVB lamps (FS20T12/UVB, National Biological, Twinsburg, OH; ref. 25). After 24 h, animals were killed and the exposed skin was excised, fixed, and examined for sunburn cells as described (25).
Results
Generation of Mice Lacking the Ro 60-kDa Autoantigen. Ro-deficient mice were generated through homologous recombination in embryonic stem cells. The targeting vector replaced the first coding exon of the Ro gene and parts of the adjacent introns with a neo gene cassette (Fig. 1 A and B). Targeted embryonic stem cells were injected into C57BL/6 blastocysts, and the resulting chimeric males were bred with C57BL/6 females to generate F1 hybrid (129/Sv × C57BL/6) heterozygotes. The F1 hybrid mice were inbred to generate F2 and F3 hybrid progeny. In addition, Ro–/– F3 hybrid progeny were backcrossed three generations to C57BL/6 mice and then inbred to generate F1 progeny. Ro–/– mice from both backgrounds were viable and fertile. Western blotting of brain extracts confirmed that Ro protein levels were reduced in Ro+/– mice and undetectable in Ro–/– mice (Fig. 1C). Reprobing of the blot revealed that expression of the neighboring Uch-L5 gene, whose transcription start site is 2.9 kb from the neo insertion, was unaffected (Fig. 1C). Northern analyses revealed that the levels of the two mouse Y RNAs, mY1 and mY3 (26, 27), were reduced in Ro+/– mice and undetectable in Ro–/– mice (Fig. 1D), consistent with previous reports that Ro protein binding is required for stable accumulation of these RNAs (12, 13).
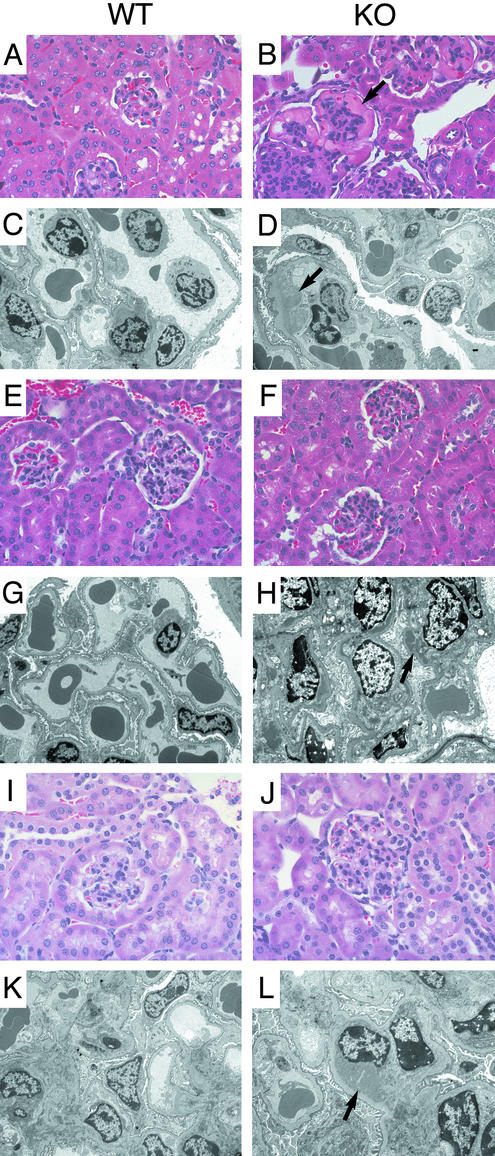
Mice Lacking the Ro Protein Develop Membranoproliferative Glomerulonephritis. Although histologic examination of 6-week-old Ro–/– mice revealed no significant abnormalities, ≈10% of the original F2 and F3 hybrid Ro–/– mice (8 of 74), but none of the wild-type mice (0 of 55), were dead within 6 months. By 12 months, this mortality had increased to 28% (21 of 74) of the Ro–/– mice, compared with 4% (2 of 55) of the wild-type mice. Mice showing signs of distress, such as fur ruffling and lethargy, were killed and subjected to histologic examination. All had evidence of membranoproliferative glomerulonephritis (Fig. 2). Examination of asymptomatic mice revealed that most (8 of 9) Ro–/–mice between 6 and 8 months of age showed changes characteristic of membranoproliferative glomerulonephritis. Inspection of other organs revealed no apparent differences compared with wild-type littermates. After three backcrosses to C57BL/6 mice, the Ro–/– mice no longer exhibited increased mortality compared with their wild-type counterparts. Nevertheless, examination of kidneys revealed that all Ro–/– mice >6 months of age exhibited histologic changes. The glomeruli were hypercellular with leukocytes in the capillary lumens (Fig. 2B), and immunofluorescence microscopy demonstrated the presence of IgG in a granular pattern (data not shown). Electron microscopy confirmed the presence of electron-dense deposits consistent with immune complexes (Fig. 2D). Deposits initially were seen predominantly in the glomerular mesangium (Fig. 2H) but, with increasing age, became larger and extended into the subendothelial region (Fig. 2L). These lesions were not as severe in the backcrossed mice as they were in the original, mixed background Ro–/– mice, suggesting the presence of modifier loci in the C57BL/6 background. Heterozygotes showed essentially normal histology by light microscopy, but those mice that developed antibodies (see below) demonstrated mild lesions characterized by the presence of small, electron-dense deposits by electron microscopy.
Fig. 2.
Glomerulonephritis in Ro–/– mice. Light (A, B, E, F, I, and J; ×200) and electron (C, D, G, H, K, and L; ×5,000) microscopy of kidneys from Ro–/– mice and age-matched controls. Ro–/– and wild-type mice from the original hybrid strain (A–D) were examined at 16.5 months, and backcrossed mice were examined at 7 (E–H) and 12.5 (I–L) months. Age-matched controls show essentially normal histology. The hybrid Ro–/– mice show glomerular hypercellularity with large eosinophilic capillary deposits (B, arrow), which correspond to subendothelial immune complexes (D, arrow). Lesions in the backcrossed mice are less severe, with mesangial immune complexes at 7 months (H, arrow) and larger subendothelial complexes at 12.5 months (L, arrow).
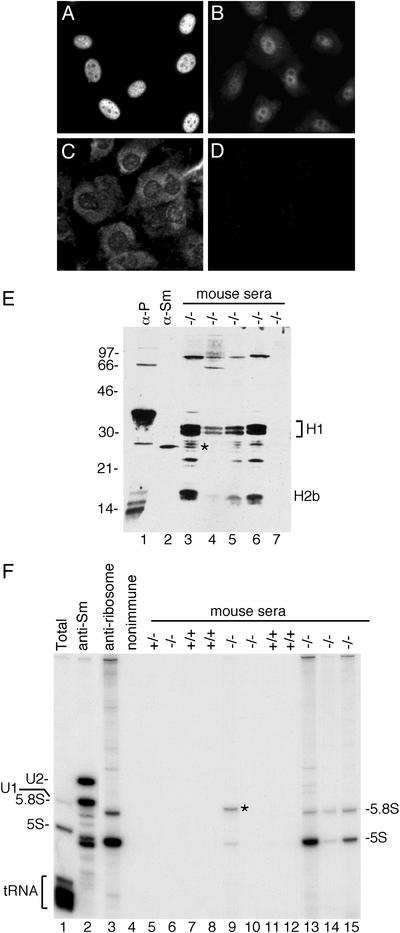
Anti-Chromatin and Anti-Ribosome Antibodies in Ro–/– Mice and Heterozygotes. To confirm the presence of autoantibodies, sera from the mice were analyzed by performing indirect immunofluorescence on NIH 3T3 cells. Autoantibodies (antinuclear and/or anticytoplasmic) with titers of at least 1:1,000 were detectable by 6 months of age in five of six (83%) Ro–/– mice from the original hybrid strain (Table 1; also see Fig. 3 A–D). After three backcrosses to C57/BL6 mice, antibodies remained detectable in the majority of Ro–/– mice (68%; 17 of 25). Autoantibodies also were detected in 10 of 26 (38%) Ro+/– heterozygotes but only 2 of 24 (8%) wild-type mice (Table 1). Although these antibodies tended to be of lower titer, between 1:125 and 1:500, high-titer antibodies (≥1:1,000) were detected in 7 of 25 (28%) backcrossed Ro–/– mice and 5 of 26 (19%) Ro+/– heterozygotes but were not detected in wild-type mice. Western blotting of mouse whole-cell extracts revealed that many sera detected a prominent doublet of 30 kDa and a second band of ≈16 kDa (Fig. 3E, lanes 3–6). Purification of these antigens, followed by protein microsequencing, revealed that they were histones H1 and H2b. Consistent with a prominent anti-chromatin response, characterization of the sera by ELISA revealed that many sera contained antibodies against nucleosomes, single-stranded DNA, and double-stranded DNA (Table 2).
Table 1. Autoantibodies in Ro–/–, Ro+/–, and wild-type mice.
| No. of mice with autoantibodies
|
|||
|---|---|---|---|
| Age, months | Ro-/- | Ro+/- | Wild type |
| 129/Sv × C57BL/6 hybrid mice (ANA ≥ 1:1,000) | |||
| 6-7 | 5/6 (83%) | ND | ND |
| 12-14 | 18/22 (82%) | ND | 1/17 (6%) |
| After three backcrosses to C57BL/6 (ANA ≥ 1:125) | |||
| 9 | 17/25 (68%) | 10/26 (38%) | 2/24 (8%) |
ANA, anti-nuclear antibody; ND, not done.
Fig. 3.
Autoantibodies in Ro mutant mice. NIH 3T3 cells were stained with sera (diluted 1:1,000) from 6- to 7-month-old Ro–/– mice (A–C) and a 12-month-old wild-type mouse (D). (E) Reference sera (lanes 1 and 2) and sera from Ro–/– mice were used to probe Western blots of L1210 cell extracts. *, Doublet detected by most sera containing anti-ribosomal antibodies. (F) Reference sera (lanes 2–4) and sera from 129/Sv × C57BL/6 hybrid mice were used to immunoprecipitate from L1210 cell extracts. RNAs in immunoprecipitates were labeled with [32P]pCp. *, Band identified as U1 RNA by cDNA sequencing.
Table 2. Subclassification of autoantibodies in Ro–/– and wild-type mice.
| No. of mice with autoantibodies
|
|||||
|---|---|---|---|---|---|
| (129/Sv × C57BL/6)
|
C57BL/6 backcross
|
||||
| Antigen | Wild type (n = 18) | Ro-/- (n = 18) | Wild type (n = 19) | Ro+/- (n = 23) | Ro-/- (n = 23) |
| Histones | 0 | 10 | 0 | 1 | 9 |
| Ribosomes | 1 | 9 | 0 | 3 | 10 |
| Sm | 0 | 0 | 0 | 0 | 0 |
| U1 snRNP | 0 | 1 | 0 | 0 | 0 |
| Ro | 0 | 0 | 0 | 0 | 0 |
| La | 0 | 0 | 0 | 0 | 0 |
| dsDNA | 2 | 9 | 0 | 3 | 6 |
| ssDNA | 2 | 6 | 1 | 3 | 5 |
| Nucleosomes | 2 | 10 | 1 | 8 | 6 |
snRNP, small nuclear RNP; dsDNA, double-stranded DNA; ssDNA, single-stranded DNA.
To characterize further the targeted antigens, sera were used to immunoprecipitate from mouse whole-cell extracts, and the RNAs within the immunoprecipitates were labeled at the 3′ end. This assay detects many RNA-containing lupus antigens, including Sm, Ro, La, tRNA/synthetase complexes, and ribosomes (22). Although none of the mice contained detectable anti-Sm, anti-Ro, anti-La, or anti-tRNA/synthetase antibodies, a large fraction of the Ro–/– mice contained anti-ribosome antibodies, because both 5S and 5.8S rRNAs were contained within the immunoprecipitates (Fig. 3F, lanes 13–15). In addition, one mouse had antibodies directed against the U1 small nuclear RNP (snRNP; Fig. 3F, lane 9, asterisk), a common lupus antigen (1). For the original hybrid strain, anti-ribosome antibodies were detected in 50% of Ro–/– mice older than 6 months (Table 2). This was also a major specificity in sera from the backcrossed mice, as 43% of Ro–/– mice possessed these antibodies (Table 2). The majority of sera positive for anti-ribosome antibodies detected a doublet migrating below the histone H1 band (Fig. 3E, lane 3, asterisk). Fractionation of cell extracts revealed that the doublet was found in the pellet after sedimentation at 100,000 × g for 1 h, consistent with a ribosomal protein (data not shown). None of the anti-ribosome antibodies recognized the ribosomal P proteins, which are common autoantigens in lupus patients (Fig. 3E, lane 1; ref. 1).
Evaluation of T and B Cell Populations and Function. T and B cell surface markers were evaluated in Ro–/– and wild-type mice by flow cytometric analysis. No obvious changes were found in mature T cell markers (CD4/CD8 ratios) or in the most immature (T cell antigen receptor-negative CD25/CD44 populations) cells in the thymus. Likewise in the bone marrow, B cell development appeared normal (using CD43/CD45R and IgM markers). Lymph node and spleen populations did not reveal differences in T and B cell activation status (using CD69 and CD25 markers). To determine whether Ro–/– mice respond abnormally to challenge with protein antigens, animals were immunized with the T cell-dependent antigen keyhole limpet hemocyanin. No significant differences in T cell proliferation, cytokine production, or anti-keyhole limpet hemocyanin antibody production were detected. However, total Ig levels in unimmunized 8-week-old Ro–/– mice were elevated slightly (1.4-fold). Among the Ig isotypes, increases were found in IgM (1.5-fold in the Ro–/– mice) and IgG2a (3-fold in Ro+/– mice and 3- to 29-fold in Ro–/– mice) (data not shown).
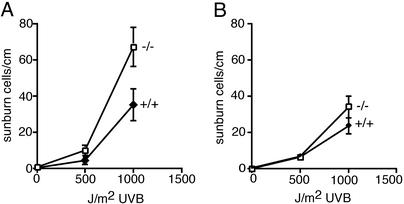
Sensitivity of Ro–/– Mice to UV Irradiation. Because a prokaryotic orthologue of the Ro protein is important for survival of the eubacterium D. radiodurans after UV irradiation (12), we examined the sensitivity of Ro–/– mice to UV light. The backs of wild-type and Ro–/– mice were shaved and the dorsal skin was exposed to UVB light at the physiologically relevant doses of 500 and 1,000 J/m2. Twenty-four hours later, histological sections were prepared. For mice that had been backcrossed six times to C57BL/6 mice, the number of apoptotic keratinocytes (sunburn cells) in the Ro–/– mice after 1,000 J/m2 UVB light was twice that of wild-type littermates (P = 0.02; Fig. 4A). Examination of mice from the original (129/Sv × C57BL/6) hybrid strain revealed that both wild-type and Ro–/– mice were less sensitive to irradiation than the backcrossed mice (Fig. 4B). However, although Ro–/– mice from the original hybrid strain also had higher numbers of apoptotic keratinocytes than did wild-type controls (Fig. 4B), the difference was not statistically significant (P = 0.08). Nonetheless, in at least one genetic background, the absence of the Ro protein results in significant photosensitivity, although modifier loci also may contribute.
Fig. 4.
Ro–/– mice are more sensitive to UVB irradiation. (A) After six backcrosses to C57BL/6 mice, the backs of wild-type (n = 8) and Ro–/– (n = 6) mice were shaved and exposed to the indicated doses of UVB. After 24 h, apoptotic keratinocytes (sunburn cells) were counted. (B) The backs of mice from the original hybrid 129/Sv × C57BL/6 strain were shaved and exposed to UVB, and apoptotic keratinocytes were counted. For the 1,000-J/m2 dose, 14 wild-type and 13 Ro–/– mice were examined.
Discussion
Although small RNA–protein complexes are major autoantigens in patients suffering from systemic lupus erythematosus, there have been few links between the RNP antigens and the disease pathology. We have shown that mice lacking the Ro 60-kDa autoantigen develop a lupus-like syndrome consisting of antiribosome and anti-chromatin antibodies, glomerulonephritis with subendothelial immune deposits, and photosensitivity. Thus, the Ro autoantigen may not be simply a passive target in the lupus immune response but, instead, may be important for the prevention of autoimmune disease.
Although a number of gene disruptions result in at least some degree of autoimmunity (28), our work is unique in several ways. First, we report that disruption of an RNA-binding protein can result in autoimmunity. Second, although photosensitivity occurs in a significant fraction of lupus patients, our backcrossed mice are unusual in displaying both autoimmune disease and sensitivity to sunlight. Although the observed difference is only 2-fold, similar changes in cell survival after UV are seen in cells from patients with the XP-E, XP-F, and XP-V forms of xeroderma pigmentosum, hereditary syndromes in which patients are predisposed to sunlight-induced skin cancers (29, 30). Last, our finding that mice lacking a major lupus autoantigen develop autoimmune disease raises the possibility that the anti-Ro antibodies seen in patients may not be an epiphenomenon but, instead, may contribute to the development or perpetuation of the autoimmune response and/or the observed photosensitivity.
How might the absence of an intracellular RNA-binding protein result in autoimmunity? Because the Ro protein binds misfolded, defective pre-5S rRNAs in X. laevis oocytes, it has been proposed to function in a quality-control pathway for ribosome biogenesis (6, 13, 15). Thus, one possibility is that the release of intracellular contents from Ro–/– cells that occurs normally during cell turnover triggers the autoimmune response. In this model, the presence of a small fraction of ribosomes containing subtle structural alterations resulting from the presence of misfolded 5S rRNAs causes a breach of tolerance by exposing normally cryptic determinants to the immune system. In support of this hypothesis, it often has been proposed that autoimmunity can be triggered by presentation of cryptic epitopes (31, 32). Such a mechanism would be reminiscent of the lupus-like syndromes that develop in mice lacking components of pathways involved in clearing extracellular debris, in that failure to remove cellular detritus triggers an autoimmune response (33–36). Alternatively, it remains possible that the absence of the Ro protein results in a subtle defect in immune system function that we have not yet uncovered.
In lupus patients, photosensitivity occurs in up to 90% of patients with anti-Ro autoantibodies (2). Moreover, maternal anti-Ro antibodies that cross the placenta are highly associated with photosensitive skin lesions in infants. Because the disappearance of the skin lesions coincides with the loss of maternal antibodies from the circulation, it has been assumed that the antibodies play a direct role in the pathogenic process (37, 38). Our result that the absence of the Ro protein in one genetic background is associated with photosensitivity suggests that the photosensitivity long observed in patients could be related to a loss of Ro protein function in keratinocytes. However, although there are many reports that antibodies can enter cells (39–44), it is unlikely that they access the cytosol in quantities sufficient to ablate function of abundant target molecules (45). Nonetheless, inefficient entry of anti-Ro antibodies into a small fraction of patient keratinocytes potentially could enhance the sensitivity of these cells to sunlight, resulting in increased apoptotic cell death.
In summary, our genetic experiments have uncovered a new and surprising way in which the lack of an RNA-binding protein leads to an immune response to specific components of the subcellular machinery. Moreover, our experiments reveal that the Ro 60-kDa protein plays a critical role in the prevention of autoimmunity, possibly by removing defective RNPs from cells, allowing them to escape immune surveillance. In this regard, it is interesting that the human Ro protein maps to chromosome 1q31 (46), which has been linked to lupus (47–49).
Acknowledgments
We thank C. Delvecchio for technical assistance; R. Cohen, K. Elkon, and J. Steitz for providing antibodies; and J. Craft, R. Lifton, and M. Shlomchik for comments on the manuscript. Parts of this work were supported by grants from the National Institutes of Health (to M.K. and D.E.B.). R.A.F. is an Investigator and S.L.W. is an Associate Investigator of the Howard Hughes Medical Institute.
Abbreviation: RNP, ribonucleoprotein.
References
- 1.von Muhlen, C. A. & Tan, E. M. (1995) Semin. Arthritis Rheum. 24, 323–358. [DOI] [PubMed] [Google Scholar]
- 2.Provost, T. T., Watson, R. & Simmons-O'Brien, E. (1996) J. Am. Acad. Dermatol. 35, 147–169. [DOI] [PubMed] [Google Scholar]
- 3.Silverman, E. D. & Laxer, R. M. (1997) Rheum. Dis. Clin. North Am. 23, 599–618. [DOI] [PubMed] [Google Scholar]
- 4.Peek, R., Pruijn, G. J. M., van der Kemp, A. & van Venrooij, W. J. (1993) J. Cell Sci. 106, 929–935. [DOI] [PubMed] [Google Scholar]
- 5.Simons, F. H. M., Pruijn, G. J. M. & van Venrooij, W. J. (1994) J. Cell Biol. 125, 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien, C. A. & Wolin, S. L. (1994) Genes Dev. 8, 2891–2903. [DOI] [PubMed] [Google Scholar]
- 7.Wolin, S. L. & Steitz, J. A. (1983) Cell 32, 735–744. [DOI] [PubMed] [Google Scholar]
- 8.Wolin, S. L. & Steitz, J. A. (1984) Proc. Natl. Acad. Sci. USA 81, 1996–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien, C. A., Margelot, K. & Wolin, S. L. (1993) Proc. Natl. Acad. Sci. USA 90, 7250–7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farris, A. D., O'Brien, C. A. & Harley, J. B. (1995) Gene 154, 193–198. [DOI] [PubMed] [Google Scholar]
- 11.Van Horn, D. J., Eisenberg, D., O'Brien, C. A. & Wolin, S. L. (1995) RNA 1, 293–303. [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, X., Quinn, A. M. & Wolin, S. L. (2000) Genes Dev. 14, 777–782. [PMC free article] [PubMed] [Google Scholar]
- 13.Labbe, J. C., Hekimi, S. & Rokeach, L. A. (1999) Genetics 151, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson, R. C., Doering, J. L. & Brown, D. D. (1980) Cell 20, 131–141. [DOI] [PubMed] [Google Scholar]
- 15.Shi, H., O'Brien, C. A., Van Horn, D. J. & Wolin, S. L. (1996) RNA 2, 769–784. [PMC free article] [PubMed] [Google Scholar]
- 16.Labbe, J. C., Burgess, J., Rokeach, L. A. & Hekimi, S. (2000) Proc. Natl. Acad. Sci. USA 97, 13233–13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman, K. M., Farris, A. D., Gross, J. K., Kirby, M. Y. & Harley, J. B. (2000) Genes Immunol. 1, 265–270. [DOI] [PubMed] [Google Scholar]
- 18.Deutscher, S. L., Harley, J. B. & Keene, J. D. (1988) Proc. Natl. Acad. Sci. USA 85, 9479–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang, D., Tournier, C., Wysk, M., Lu, H. T., Xu, J., Davis, R. J. & Flavell, R. A. (1997) Proc. Natl. Acad. Sci. USA 94, 3004–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang, B., Gee, R. J., Kashgarian, M. J., Sharpe, A. H. & Mamula, M. J. (1999) J. Immunol. 163, 2322–2329. [PubMed] [Google Scholar]
- 21.Yoo, C. J. & Wolin, S. L. (1994) Mol. Cell. Biol. 14, 5412–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardin, J. A., Rahn, D. R., Shen, C., Lerner, M. R., Wolin, S. L., Rosa, M. D. & Steitz, J. A. (1982) J. Clin. Invest. 70, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.England, T. E., Bruce, A. G. & Uhlenbeck, O. C. (1980) Methods Enzymol. 65, 65–74. [DOI] [PubMed] [Google Scholar]
- 24.Lerner, E. A., Lerner, M. R., Janeway, C. A. & Steitz, J. A. (1981) Proc. Natl. Acad. Sci. USA 78, 2737–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziegler, A., Jonason, A. S., Leffell, D. J., Simon, J. A., Sharma, H. W., Kimmelman, J., Remington, L., Jacks, T. & Brash, D. E. (1994) Nature 372, 773–776. [DOI] [PubMed] [Google Scholar]
- 26.Hendrick, J. P., Wolin, S. L., Rinke, J., Lerner, M. R. & Steitz, J. A. (1981) Mol. Cell. Biol. 1, 1138–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farris, A. D., Gross, J. K., Hanas, J. S. & Harley, J. B. (1996) Gene 174, 35–42. [DOI] [PubMed] [Google Scholar]
- 28.Wakeland, E. K., Liu, K., Graham, R. R. & Behrens, T. W. (2001) Immunity 15, 397–408. [DOI] [PubMed] [Google Scholar]
- 29.Andrews, A. D., Barrett, S. F. & Robbins, J. H. (1978) Proc. Natl. Acad. Sci. USA 75, 1984–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bootsma, D., Kraemer, K. H., Cleaver, J. E. & Hoeijmakers, J. H. J. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D. (McGraw–Hill, New York), Vol. 1, pp. 677–703. [Google Scholar]
- 31.Lanzavecchia, A. (1995) J. Exp. Med. 181, 1945–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrade, F., Casciola-Rosen, L. & Rosen, A. (2000) Rheum. Dis. Clin. North Am. 26, 215–227. [DOI] [PubMed] [Google Scholar]
- 33.Botto, M., Dell'Agnola, C., Bygrave, A. E., Thompson, E. M., Cook, H. T., Petry, F., Loos, M., Pandolfi, P. P. & Walport, M. J. (1998) Nat. Genet. 19, 56–59. [DOI] [PubMed] [Google Scholar]
- 34.Bickerstaff, M. C., Botto, M., Hutchinson, W. L., Herbert, J., Tennent, G. A., Bybee, A., Mitchell, D. A., Cook, H. T., Butler, P. J., Walport, M. J., et al. (1999) Nat. Med. 5, 694–697. [DOI] [PubMed] [Google Scholar]
- 35.Napirei, M., Karsunky, H., Zevnik, B., Stephan, H., Mannherz, H. G. & Moroy, T. (2000) Nat. Genet. 25, 177–181. [DOI] [PubMed] [Google Scholar]
- 36.Scott, R. S., McMahon, E. J., Pop, S. M., Reap, E. A., Caricchio, R., Cohen, P. L., Earp, H. S. & Matsushima, G. K. (2001) Nature 411, 207–211. [DOI] [PubMed] [Google Scholar]
- 37.Lee, L. A. (2001) Curr. Rheumatol. Rep. 3, 391–395. [DOI] [PubMed] [Google Scholar]
- 38.Patel, P. & Werth, V. (2002) Dermatol. Clin. 20, 373–385. [DOI] [PubMed] [Google Scholar]
- 39.Alarcon-Segovia, D., Ruiz-Arguelles, A. & Fishbein, E. (1978) Nature 271, 67–69. [DOI] [PubMed] [Google Scholar]
- 40.Ma, J., Chapman, G. V., Chen, S. L., Melick, G., Penny, R. & Breit, S. N. (1991) Clin. Exp. Immunol. 84, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanase, K., Smith, R. M., Cizman, B., Foster, M. H., Peachey, L. D., Jarett, L. & Madaio, M. P. (1994) Lab. Invest. 71, 52–60. [PubMed] [Google Scholar]
- 42.Zack, D. J., Stempniak, M., Wong, A. L., Taylor, C. & Weisbart, R. H. (1996) J. Immunol. 157, 2082–2088. [PubMed] [Google Scholar]
- 43.Koscec, M., Koren, E., Wolfson-Reichlin, M., Fugate, R. D., Trieu, E., Targoff, I. N. & Reichlin, M. (1997) J. Immunol. 159, 2033–2041. [PubMed] [Google Scholar]
- 44.Deng, S. X., Hanson, E. & Sanz, I. (2000) Int. Immunol. 12, 415–423. [DOI] [PubMed] [Google Scholar]
- 45.Musunuru, K. & Darnell, R. B. (2001) Annu. Rev. Neurosci. 24, 239–262. [DOI] [PubMed] [Google Scholar]
- 46.Chan, E. K., Tan, E. M., Ward, D. C. & Matera, A. G. (1994) Genomics 23, 298–300. [DOI] [PubMed] [Google Scholar]
- 47.Moser, K. L., Neas, B. R., Salmon, J. E., Yu, H., Gray-McGuire, C., Asundi, N., Bruner, G. R., Fox, J., Kelly, J., Henshall, S., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 14869–14874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindqvist, A. K., Steinsson, K., Johanneson, B., Kristjansdottir, H., Arnasson, A., Grondal, G., Jonasson, I., Magnusson, V., Sturfelt, G., Truedsson, L., et al. (2000) J. Autoimmun. 14, 169–178. [DOI] [PubMed] [Google Scholar]
- 49.Johanneson, B., Lima, G., Von Salome, J., Alarcon-Segovia, D. & Alarcon-Riquelme, M. E. (2002) Am. J. Hum. Genet. 71, 1060–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]