Abstract
Oxidative phosphorylation involves the coupling of ATP synthesis to the proton-motive force that is generated typically by a series of membrane-bound electron transfer complexes, which ultimately reduce an exogenous terminal electron acceptor. This is not the case with Pyrococcus furiosus, an archaeon that grows optimally near 100°C. It has an anaerobic respiratory system that consists of a single enzyme, a membrane-bound hydrogenase. Moreover, it does not require an added electron acceptor as the enzyme reduces protons, the simplest of acceptors, to hydrogen gas by using electrons from the cytoplasmic redox protein ferredoxin. It is demonstrated that the production of hydrogen gas by membrane vesicles of P. furiosus is directly coupled to the synthesis of ATP by means of a proton-motive force that has both electrochemical and pH components. Such a respiratory system enables rationalization in this organism of an unusual glycolytic pathway that was previously thought not to conserve energy. It is now clear that the use of ferredoxin in place of the expected NAD as the electron acceptor for glyceraldehyde 3-phosphate oxidation enables energy to be conserved by hydrogen production. In addition, this simple respiratory mechanism readily explains why the growth yields of P. furiosus are much higher than could be accounted for if ATP synthesis occurred only by substrate-level phosphorylation. The ability of microorganisms such as P. furiosus to couple hydrogen production to energy conservation has important ramifications not only in the evolution of respiratory systems but also in the origin of life itself.
Almost all life forms conserve energy for growth and development by the process of oxidative phosphorylation. This involves the spontaneous, thermodynamically favorable transfer of electrons from a suitable donor through a complex chain of membrane-bound redox proteins, enzymes, and electron carriers, to a suitable acceptor. The concomitant pumping of protons across the membrane enables energy to be conserved by chemiosmotic coupling in the form of a proton-motive force (pmf) (1). The enzyme ATP synthase uses the pmf to couple proton transfer back across the membrane to the synthesis of ATP from ADP and inorganic phosphate (Pi). Higher life forms typically use NADH generated from glucose oxidation as the electron donor, and molecular oxygen serves as the acceptor, a process accomplished by the multicomplex, aerobic respiratory chain of mitochondria (2, 3). On the other hand, microorganisms vary (and can be classified) by the electron donors and acceptors that they use for respiratory purposes. Some microbes contain a respiratory chain that uses the same NADH/oxygen donor/acceptor pair, but others may use a variety of compounds, such as methane, ammonia, hydrogen, or carbon monoxide as donors, and carbon dioxide, nitrate, sulfate, or ferric iron as acceptors (2, 3). In these cases, specialized oxidoreductase-type enzymes donate electrons directly or indirectly into complex respiratory chains that ultimately, by means of both organic (quinones) and protein redox carriers, reduce the added electron acceptor by the appropriate enzyme complex.
Of course, the simplest and most readily available electron acceptor for a respiratory system is the proton. Proton reduction has the advantage that the product, hydrogen gas (H2), does not accumulate. Typically, however, H2 is produced by fermentative microorganisms rather than by respiratory ones (3). Fermentative microbes synthesize ATP solely by the direct phosphorylation of ADP by using so-called high-energy phosphate compounds such as acetyl phosphate or phosphoenolpyruvate, a process known as substrate-level phosphorylation. These high-energy phosphate compounds are produced by the oxidative metabolism of sugars and amino acids. In the absence of a respiratory system with which to dispose of the electrons, these organisms have to remove excess reductant as reduced carbon compounds, such as ethanol, or as H2 gas.
Pyrococcus furiosus, a strictly anaerobic hyperthermophile (optimal growth temperature ≈100°C) that grows on sugars and peptides and produces organic acids, CO2, and H2 (4), was thought to have a purely fermentative metabolism (5, 6). In fact, it contains a modified Embden–Meyerhof pathway in which the oxidation of glyceraldehyde 3-phosphate (GAP) and conversion to 3-phosphoglycerate is catalyzed by a single enzyme, GAP:ferredoxin (Fd) oxidoreductase, and this conversion is not directly coupled to a phosphorylation step (7, 8). Consequently, the conversion of glucose to pyruvate appears to result in no net gain of ATP. Rather, energy is conserved during the conversion of pyruvate to acetate and CO2. This involves pyruvate:Fd oxidoreductase and the ATP-producing enzymes acetyl-CoA synthetase I and II (9). However, the nature of the metabolic pathways and mechanisms of energy conservation in P. furiosus was brought into question with the discovery of its H2-evolving, membrane-bound hydrogenase (MBH) (10). This enzyme complex uses the ubiquitous redox protein Fd as an electron donor, which is itself reduced during oxidative metabolism by a dozen or more different Fd-linked oxidoreductases, including GAP:Fd oxidoreductase and pyruvate:Fd oxidoreductase (11, 12). MBHs that appear to be involved in H2 production have been previously identified in several microorganisms, although none has been shown to couple H2 evolution with proton translocation and ATP synthesis.
Herein we demonstrate that protons are used as a terminal electron acceptor for respiration by P. furiosus, and that the production of H2 is coupled directly to ATP synthesis, according to the scheme depicted in Fig. 1. Moreover, this involves the simplest of respiratory systems, a single multiprotein complex (MBH), and a ubiquitous low-potential electron donor (Fd), that together couple electron transfer to both proton reduction and proton translocation.
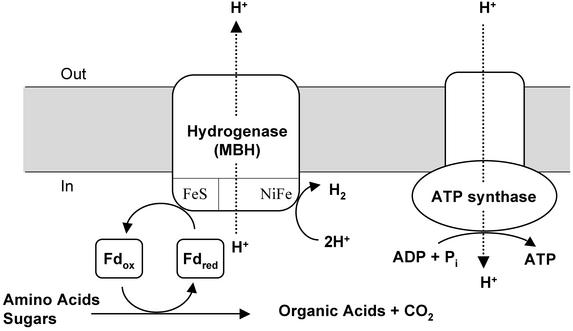
Fig. 1.
Model of chemiosmotic coupling between the MBH and the ATP synthase in intact cells of P. furiosus. Fdred and Fdox represent the reduced (by oxidative metabolism) and oxidized forms of the redox protein Fd, respectively. Fdred is reoxidized by the MBH, which reduces H+ to produce H2. As the primary proton-translocating complex, MBH also pumps H+ across the membranes, resulting in a transmembrane proton gradient. This gradient can then be used by the secondary proton translocating complex, ATPase, to produce ATP. See text for details, and note that experiments were conducted with inverted vesicles.
Materials and Methods
Growth of the Organism. P. furiosus (DSM 3638) was grown in a 600-liter fermenter at 90°C under pH-controlled conditions in the absence of elemental sulfur by using maltose, tryptone, and yeast extract as the carbon source, each at a concentration of 5 g per liter as described (13).
Membrane Isolation and Vesicle Preparation. All procedures for membrane isolation were carried out at 23°C under anaerobic conditions. All solutions were vacuum degassed and maintained under a positive pressure of argon. The buffer used throughout was 50 mM Tris (pH 8.0), containing 2 mM sodium dithionite (DT) (buffer A). Cell-free extracts of P. furiosus were prepared by suspending 15 g (wet weight) of frozen cells in 45 ml of buffer A and 10 μg of DNase I. The cell suspension was sonicated for 30 min under a constant flow of argon. Unbroken cells were removed by centrifugation at 5,000 × g for 15 min. The membranes were isolated by ultracentrifugation at 120,000 × g for 2.0 h, suspended in buffer A, and then subjected to two successive washes with buffer A. After each wash the suspension was centrifuged for 2 h at 120,000 × g, and the membrane fraction was resuspended in buffer A in an anaerobic chamber. The final preparation of membrane vesicles was suspended in buffer A to a protein concentration of 10 mg per ml. That vesicles were formed spontaneously (14) was confirmed by transmission electron microscopy analysis (Center for Ultrastructural Research, University of Georgia).
Enzyme Assays. All enzyme assays were carried out at 80°C in anaerobic gas-tight serum-stoppered assay vials or cuvettes containing vacuum-degassed buffers. ATP hydrolysis was measured by Pi release from ATP in 50 mM N-2-hydroxyethylpiperazine-N′-3-propanesulfonic acid (EPPS; pH 8.0) buffer containing KCl (200 mM), MgCl2 (10 mM), and ATP (0.3 mM). ATP synthesis was measured by Pi consumption in 50 mM EPPS (pH 8.0) buffer containing KCl (200 mM), MgSO4 (20 mM), ADP (0.3 mM), and NaPi (0.3 mM). Pi was measured as described (15). Results are expressed as μmol of Pi formed or consumed per min per mg of protein. Hydrogenase activity was determined by H2 evolution by using methyl viologen (MV; 2 mM) (16) or Fd (15 μM) (12) as the electron carrier and sodium DT (10 mM) as the electron donor. The H2 produced was measured by gas chromatography. One unit of hydrogenase activity is defined as the production of 1 μmol of H2 per min. The H2 oxidation activity of the hydrogenase was determined spectrophotometrically by measuring the reduction of benzyl viologen (2 mM) (17) or MV (2 mM) at 578 nm. The assay buffer was saturated with H2, and the assays were performed at 80°C in serum-stoppered cuvettes. One unit of H2 oxidation activity corresponded to 1 μmol of H2 consumed per min. Hydrogenase activity was measured under the conditions that were used to measure ATP synthesis and ATP hydrolysis. All activities were calculated from constant rates of H2 production and ATP synthesis and hydrolysis measured over 20 min. For reactions with inhibitors, both assays were carried out after washed membrane vesicles were incubated at 80°C for 20 min with NaN3 (1.0 mM), NaNO3 (5 mM), N,N′-dicyclohexylcarbodiimide (DCCD; 0.5 mM), carbonyl-cyanide m-chlorophenylhydrazone (CCCP; 10 μM), or CuCl2 (0.1 mM).
pmf Measurements. Acridine orange (4 μM) was used to measure ΔpH (18) in membranes vesicles (100 μg of protein per ml) in 20 mM Hepes (pH 7.8) buffer containing KCl (60 mM), 2 mM MgSO4 (2 mM) (buffer B), and valinomycin (3 μM). Fd (15 μM), CCCP (4 μM), CuCl2 (0.1 mM), and ATP (0.3 mM) were added where indicated. Change in absorbance (A493–A530) was measured at 80°C by a dual-wavelength spectrophotometer (Shimadzu, UV-2501PC). For Δψ, additions were made as indicated to membranes (100 μg of protein per ml) in buffer B incubated with oxonol VI (2 μM; Molecular Probes) (19). The absorbance (A625–A587) was measured by using a dual-wavelength spectrophotometer.
Analytical Methods. Protein concentrations were determined with the Bradford dye-binding assay kit by using BSA as the standard.
Results
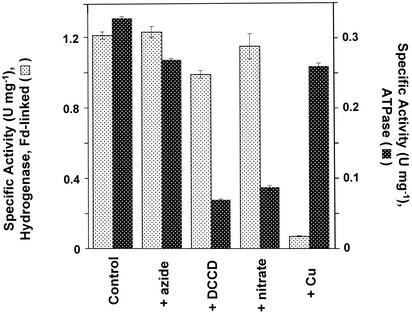
To investigate the bioenergetics of H2 production by P. furiosus, inverted vesicles were prepared from washed membranes (14). Circular structures of ≈100-μm diameter were visible by transmission electron microscopy (data not shown) and were <1/10th the size of intact P. furiosus cells. Freshly prepared vesicles catalyzed H2 production at 80°C with Fd (1.2 ± 0.1 units/mg of protein) or the redox dye MV (2.5 ± 0.1 units/mg) as the electron carrier by using DT as the common artificial electron source (Fig. 2, complete system). Although the P. furiosus genome indicates the presence of a membrane-bound ATP synthase, the activity of the enzyme had not been previously measured, and for this measurement its ATPase activity was used. Thus, in a separate experiment, the same vesicles catalyzed the hydrolysis of ATP at 80°C (1.3 ± 0.04 units·mg–1). This activity was inhibited by the ATP-synthase inhibitors, DCCD and nitrate, as expected for an archaeal-type ATP synthase. Although the H2 evolution activity of the MBH is unaffected by these ATP synthase inhibitors (Fig. 2), copper ions (CuCl2) are a very specific and powerful inhibitor of the enzyme (Fig. 2, +Cu). The MBH loses 95% of its H2 evolution activity after only 5-min incubation with 0.1 mM CuCl2, although the ATP hydrolysis activity of the ATP synthase is unaffected (Fig. 2, +Cu). Thus, copper ions and nitrate (and DCCD) represent two very specific inhibitors for the MBH and ATP synthase, respectively.
Fig. 2.
ATPase and hydrogen evolution activities of washed membrane vesicles of P. furiosus. Enzyme activities were measured as described in Materials and Methods. Fd was used as the electron carrier for H2 production. ATPase activity is specifically inhibited by DCCD and nitrate but not by azide. The membrane-bound hydrogenase activity is specifically inhibited by copper ions (CuCl2).
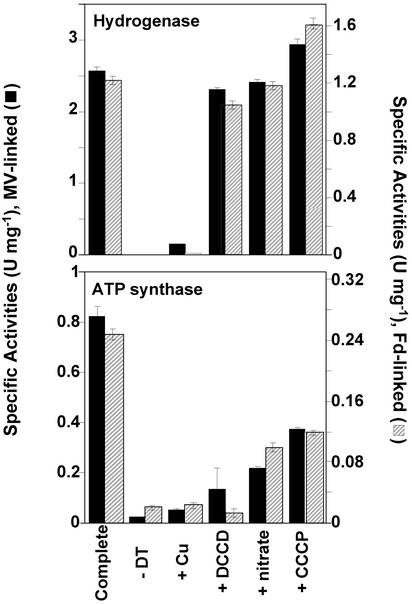
Vesicles exhibited negligible (<0.02 unit·mg–1) ATP synthesis activity with ADP and Pi as substrates when assayed under the same assay conditions used to measure ATPase activity. However, ATP synthesis activity increased dramatically (>40-fold, to 0.8 ± 0.4 unit·mg–1) when an electron donor (Fd) was added to the vesicles. Of course, these are the same conditions as those used to determine hydrogenase activity, and H2 was also evolved as ATP was synthesized (Fig. 3, complete system). When the electron donor was omitted from the assay, H2 was not produced and ATP was not synthesized (Fig. 3,–DT), suggesting an indirect coupling between the hydrogenase and the ATP synthase. Such a coupling could be demonstrated most effectively by the addition of copper ions. These are a potent inhibitor of the hydrogenase but they also abolish ATP synthesis (Fig. 3, +Cu) even though, at the same concentration, copper ions have no effect on the ATPase activity of the vesicles (Fig. 2). ATP synthesis was also inhibited, as expected, by DCCD and nitrate, although these had little effect on hydrogenase activity (Fig. 3).
Fig. 3.
Hydrogen production and ATP synthesis catalyzed simultaneously by membrane vesicles. Enzyme activities were measured as described in Materials and Methods. Fd or MV was used as the electron carrier for H2 production. Membrane vesicles synthesize ATP and produce H2 in the presence of either Fd or MV as electron carriers (complete). MBH-coupled ATP synthesis is not observed either in the absence of the electron donor (–DT) for H2 production or if the MBH is inhibited (+CuCl2). ATP is not produced in the presence of ATP-synthase inhibitors (+DCCD, +nitrate) or in the absence of the pmf (+CCCP).
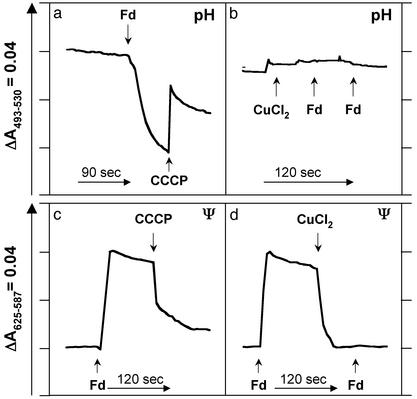
These results strongly suggest that the hydrogenase uses protons both as a substrate for H2 production and as a means of conserving energy by pumping them across the membrane. Direct evidence for such a chemiosmotic mechanism is shown in Fig. 4. Both pH (ΔpH, acidic inside) and electrochemical (Δψ, inside positive) gradients are generated when reduced Fd is added to intact vesicles with concomitant H2 production and ATP synthesis. Both types of gradient are dissipated by CCCP (Fig. 4 a and c). CCCP also inhibits ATP synthesis and actually stimulates H2 production, as would be expected in a coupled system (Fig. 3, +CCCP). If H2 production is prevented by the addition of CuCl2, a pH gradient is not formed when reduced Fd is added (Fig. 4b). Similarly, the Fd-induced electrochemical gradient is rapidly collapsed by inactivating the hydrogenase with CuCl2, and the further addition of reduced Fd does not generate Δψ (Fig. 4d). That such a proton gradient is used to drive ATP synthesis can be demonstrated by the generation of a similar gradient by ATPase activity independent of the Cu-inhibited hydrogenase (data not shown). The ATP-induced gradient does not form if DCCD is present, although DCCD has little effect on the Fd-induced gradient (data not shown).
Fig. 4.
Direct measurements of ΔpH and Δψ during hydrogen production. The change in pH (a and b) was measured by using acridine orange, and the change in electrochemical potential (c and d) was measured by using oxonol VI as described in Materials and Methods. ΔpH (a) and Δψ (c) are formed when the electron donor Fd is added to the vesicles. Δψ and ΔpH are collapsed by the addition of CCCP (a and c) and when the MBH is inhibited by copper ions (CuCl2, d), and these also prevent formation of ΔpH.
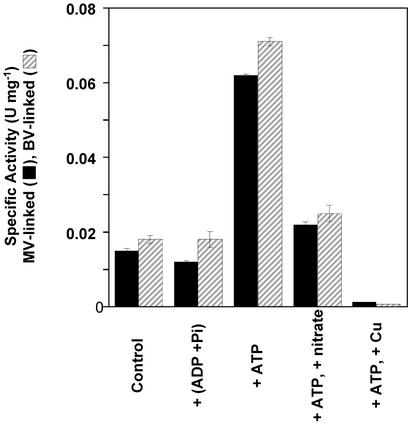
These results suggest a simple chemiosmotic model wherein the production of H2 by the membrane-bound hydrogenase, in the presence of reduced Fd (or MV), leads to proton translocation across the membrane and the formation of a pmf (Fig. 1). The ATP synthase then uses the energy to drive the energetically favorable transport of protons into the cell to synthesize ATP from ADP and Pi. In the absence of the pmf, the ATP synthase is unable to catalyze ATP synthesis, and without a substrate (electrons) for the hydrogenase, no H2 is evolved and no protons are translocated, thus ATP synthesis is negligible. The catalytic properties of P. furiosus MBH are consistent with this model because the enzyme has a dramatic preference for catalyzing H2 production in the standard hydrogenase assays (10). For example, when artificial electron carriers in vitro are used, the ratio of the H2 evolution to H2 oxidation activity catalyzed by the enzyme is >2,000, which compares with values typically near unity or less for the cytoplasmic hydrogenases from various microorganisms when assayed under comparable conditions (10, 20). The molecular mechanism responsible for this catalytic predilection of the P. furiosus enzyme is not known, but a consequence of the model (Fig. 1) is that the hydrolysis of ATP should stimulate H2 oxidation by proton-driven reverse electron transport. Accordingly, as shown in Fig. 5, the low H2 uptake activity of inverted vesicles is unaffected by ADP and Pi but increases 4-fold in the presence of ATP. The ATP effect is minimized in the presence of the ATPase inhibitor nitrate (Fig. 5) and the uncoupler CCCP (data not shown), and H2 oxidation is abolished by copper ions, which inactivate the hydrogenase (Fig. 5).
Fig. 5.
Hydrogen oxidation driven by reverse proton pumping. H2 uptake activity was measured by using benzyl viologen (BV) or MV as the electron acceptor as described in Materials and Methods. The activity is stimulated by the presence of ATP (+ATP) but is unaffected by ADP and Pi [+(ADP + Pi)] or when the ATPase is inhibited by nitrate (+nitrate).
Discussion
Our investigations into the bioenergetics of H2 production by P. furiosus were prompted by the discovery of its MBH (10). This enzyme was solubilized and purified and contained two subunits that were encoded by genes that appeared to be part of a large operon (Mbh) containing 14 ORFs. Coordinate regulation of the operon was subsequently demonstrated by DNA microarray analysis (10, 21). Several of the ORFs show sequence similarity to the products of genes found in operons that encode MBHs in other organisms, including the methanogen Methanosarcina barkeri (22, 23), the CO-oxidizing photosynthetic bacterium Rhodospirillum rubrum (24, 25), and in Escherichia coli (26). The H2-evolving enzymes of E. coli (encoded by the Hyc operon) and R. rubrum (the Coo system) are part of multienzyme respiratory complexes that oxidize formate and carbon monoxide, respectively (24, 25, 27), whereas that of M. barkeri (Ech) evolves H2 during CO oxidation and the conversion of acetate to methane (28).
The gene in the MBH operons that encodes the catalytic subunit that is responsible for H2 production is the one whose product is homologous to the catalytic subunit of the well characterized cytoplasmic NiFe-hydrogenase (29). A phylogenetic relationship between NiFe-hydrogenases and proton-translocating enzymes such as NADH:ubiquinone oxidoreductase (complex I) of the aerobic respiratory chain is well documented (26, 30–33), and this supports the notion that the MBHs might be capable of coupling H2 production to proton translocation and thus ultimately to ATP synthesis. Results from inhibitor studies in R. rubrum and genetic studies in M. barkeri have also strongly suggested a link between proton reduction and ATP synthesis (25, 28), but there has been no direct biochemical demonstration of proton reduction coupled to proton translocation and to ATP synthesis. Moreover, understanding the bioenergetics of the H2-evolving respiratory systems in these other organisms is complicated by the fact that the oxidoreductases that are associated with, and provide electrons for, the hydrogenase, such as CO dehydrogenase and formate dehydrogenase, could be responsible for proton translocation during substrate oxidation. However, herein we show that in P. furiosus it is the hydrogenase system itself that is responsible for energy conservation. Furthermore, an accessory membrane-bound multiprotein complex that oxidizes an electron donor is not necessary in P. furiosus, because its respiratory system uses a small (7-kDa) cytoplasmic redox protein (Fd) that is reduced during primary metabolism (Fig. 1). This simplicity suggests that such a system could be the precursor of complex respiratory chains of the type now seen in higher organisms, an idea consistent with the proposed evolutionary position of hyperthermophilic organisms (34) such as P. furiosus.
The precise mechanism by which electron transfer and H2 production are coupled to proton pumping in the membranes of P. furiosus is unclear at present. Nevertheless, from the data presented herein (Figs. 3 and 4), the calculated ratio of moles of ATP produced per two electrons transferred (or mole of H2 evolved) is 0.3–0.4 for both the Fd-linked and the MV-linked assays. Interestingly, these values are comparable to those reported in respiratory systems where H2 is oxidized and used as a source of energy, e.g., 0.25 ATP per two electrons (35). Assuming three protons are translocated per molecule of ATP synthesized (36), approximately one proton is translocated per molecule of H2 evolved in the P. furiosus system.
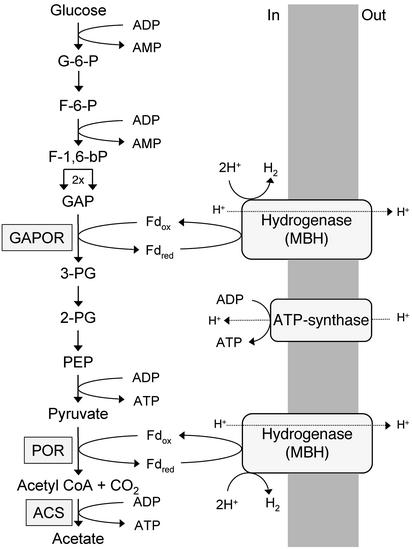
What impact does this respiratory mechanism have on the energetics of P. furiosus, an organism that was thought to obtain energy only by substrate-level phosphorylation (6, 11)? First, it readily explains why the organism has an unusual glycolytic pathway that uses Fd in place of the expected NAD as the electron acceptor for GAP oxidation. This reaction is catalyzed by the tungsten-containing enzyme GAP:Fd oxidoreductase (8) and generates reduced Fd. As shown in Fig. 6, this reduced Fd can be used directly for ATP synthesis by means of H2 production, whereas NADH could not because it has a much higher redox potential (Em = –320 mV, pH 7.0) than the H2/H+ redox couple (–420 mV, pH 7.0).
Fig. 6.
Pathways of electron flow and energy conservation during sugar catabolism by P. furiosus. G-6-P, glucose 6-phosphate; F-6-P, fructose 6-phosphate; F-1,6-bP, fructose 1,6-bisphosphate; GAPOR, GAP:Fd oxidoreductase; 3-PG, 3-phosphoglycerate; 2-PG, 2-phosphoglycerate; PEP, phosphoenolpyruvate; POR, pyruvate:Fd oxidoreductase; ACS, acetyl-CoA synthase. See text for details.
Second, the respiratory mechanism explains the discrepancies between the measured and calculated growth yields of P. furiosus (37). For example, the cell yield per mol of ATP during growth on a glucose-based sugar (cellobiose) was 17.8 ± 1.7 g/mol of ATP, which is much higher than expected (36). This calculation assumed that the only energy conservation step in glucose to acetate conversion is between pyruvate and acetate. However, the results presented herein show that 1.2 mol of ATP can be formed during the oxidation of 1 mol of glucose to 2 mol of acetate in addition to the 2 mol of ATP generated by substrate-level phosphorylation. The cell yield per mole of ATP therefore decreases to 11.1 ± 1.0 g/mol of ATP, a value that is much more consistent with the values reported for other organisms (36). Growth yields for P. furiosus with pyruvate also have to be reconsidered, as previous calculations (36) assumed a pyruvate/acetate/ATP ratio of 1:1:1. However, H2 is produced during the oxidation of pyruvate to acetyl-CoA, resulting in an additional 0.3 mol of ATP. Thus, based on a ratio of 1:1:1.3, the yield on pyruvate decreases from 9.5 ± 0.3 to 7.3 ± 0.2 g/mol of ATP.
Clearly, the ability of microorganisms such as P. furiosus to generate ATP as the result of H2 production has important ramifications not only in the evolution of respiratory systems, but also in the origin of life itself. If one accepts that life first evolved under anaerobic conditions, perhaps at elevated temperature, and in a highly reducing environment, the ability to use protons as a terminal electron acceptor and to couple this to proton translocation to conserve energy would be extremely advantageous. However, the precise mechanism by which this occurs in present-day anaerobic hyperthermophiles such as P. furiosus remains to be elucidated.
Acknowledgments
We thank Dr. Francis E. Jenney, Jr., for critical review of the manuscript and for many helpful discussions. This research was supported by funds from the Department of Energy (FG05-95ER20175), the National Science Foundation (MCB 9904624), and the Human Science Frontier Program (ST00021/2001-M).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GAP, glyceraldehyde 3-phosphate; pmf, proton-motive force; Fd, ferredoxin; MV, methyl viologen; MBH, membrane-bound hydrogenase; DCCD, N,N′-dicyclohexylcarbodiimide; CCCP, carbonyl cyanide m-chlorophenylhydrazone; DT, sodium dithionite; Pi, inorganic phosphate.
References
- 1.Mitchell, P. (1961) Nature 191, 144–148. [DOI] [PubMed] [Google Scholar]
- 2.Gottschalk, G. (1986) Bacterial Metabolism (Springer, New York).
- 3.Thauer, R. K., Jungermann, K. & Decker, K. (1977) Bacteriol. Rev. 41, 100–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiala, G. & Stetter, K. O. (1986) Arch. Microbiol. 145, 56–61. [Google Scholar]
- 5.Adams, M. W. & Kletzin, A. (1996) Adv. Protein Chem. 48, 101–180. [DOI] [PubMed] [Google Scholar]
- 6.de Vos, W. M., Kengen, S. W., Voorhorst, W. G. & van der Oost, J. (1998) Extremophiles 2, 201–205. [DOI] [PubMed] [Google Scholar]
- 7.Kengen, S. W., de Bok, F. A., van Loo, N. D., Dijkema, C., Stams, A. J. & de Vos, W. M. (1994) J. Biol. Chem. 269, 17537–17541. [PubMed] [Google Scholar]
- 8.Mukund, S. & Adams, M. W. (1995) J. Biol. Chem. 270, 8389–8392. [DOI] [PubMed] [Google Scholar]
- 9.Mai, X. & Adams, M. W. (1996) J. Bacteriol. 178, 5897–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sapra, R., Verhagen, M. F. & Adams, M. W. (2000) J. Bacteriol. 182, 3423–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams, M. W. (1999) J. Appl. Microbiol. 85, 108S–117S. [DOI] [PubMed] [Google Scholar]
- 12.Silva, P. J., van den Ban, E. C., Wassink, H., Haaker, H., de Castro, B., Robb, F. T. & Hagen, W. R. (2000) Eur. J. Biochem. 267, 6541–6551. [DOI] [PubMed] [Google Scholar]
- 13.Verhagen, M. F., Menon, A. L., Schut, G. J. & Adams, M. W. (2001) Methods Enzymol. 330, 25–30. [DOI] [PubMed] [Google Scholar]
- 14.Dirmeier, R., Hauska, G. & Stetter, K. O. (2000) FEBS Lett. 467, 101–104. [DOI] [PubMed] [Google Scholar]
- 15.Hutchins, A. M., Holden, J. F. & Adams, M. W. (2001) J. Bacteriol. 183, 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryant, F. O. & Adams, M. W. (1989) J. Biol. Chem. 264, 5070–5079. [PubMed] [Google Scholar]
- 17.Ma, K. & Adams, M. W. (2001) Methods Enzymol. 331, 208–216. [DOI] [PubMed] [Google Scholar]
- 18.Teuber, M., Rogner, M. & Berry, S. (2001) Biochim. Biophys. Acta 1506, 31–46. [DOI] [PubMed] [Google Scholar]
- 19.Smith, J. C. (1990) Biochim. Biophys. Acta 1016, 1–28. [DOI] [PubMed] [Google Scholar]
- 20.Albracht, S. P. (1994) Biochim. Biophys. Acta 1188, 167–204. [DOI] [PubMed] [Google Scholar]
- 21.Schut, G. J., Zhou, J. & Adams, M. W. (2001) J. Bacteriol. 183, 7027–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunkel, A., Vorholt, J. A., Thauer, R. K. & Hedderich, R. (1998) Eur. J. Biochem. 252, 467–476. [DOI] [PubMed] [Google Scholar]
- 23.Meuer, J., Bartoschek, S., Koch, J., Kunkel, A. & Hedderich, R. (1999) Eur. J. Biochem. 265, 325–335. [DOI] [PubMed] [Google Scholar]
- 24.Fox, J. D., He, Y., Shelver, D., Roberts, G. P. & Ludden, P. W. (1996) J. Bacteriol. 178, 6200–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox, J. D., Kerby, R. L., Roberts, G. P. & Ludden, P. W. (1996) J. Bacteriol. 178, 1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauter, M., Bohm, R. & Bock, A. (1992) Mol. Microbiol. 6, 1523–1532. [DOI] [PubMed] [Google Scholar]
- 27.Bagramyan, K., Mnatsakanyan, N., Poladian, A., Vassilian, A. & Trchounian, A. (2002) FEBS Lett. 516, 172–178. [DOI] [PubMed] [Google Scholar]
- 28.Meuer, J., Kuettner, H. C., Zhang, J. K., Hedderich, R. & Metcalf, W. W. (2002) Proc. Natl. Acad. Sci. USA 99, 5632–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volbeda, A., Charon, M. H., Piras, C., Hatchikian, E. C., Frey, M. & Fontecilla-Camps, J. C. (1995) Nature 373, 580–587. [DOI] [PubMed] [Google Scholar]
- 30.Albracht, S. P. & Hedderich, R. (2000) FEBS Lett. 485, 1–6. [DOI] [PubMed] [Google Scholar]
- 31.Friedrich, T. (2001) J. Bioenerg. Biomembr. 33, 169–177. [DOI] [PubMed] [Google Scholar]
- 32.Albracht, S. P. (1993) Biochim. Biophys. Acta 1144, 221–224. [DOI] [PubMed] [Google Scholar]
- 33.Kashani-Poor, N., Zwicker, K., Kerscher, S. & Brandt, U. (2001) J. Biol. Chem. 276, 24082–24087. [DOI] [PubMed] [Google Scholar]
- 34.Wiegel, J. & Adams, M. W. (1998) Thermophiles: The Keys to Molecular Evolution and Origin of Life? (Taylor & Francis, Philadelphia).
- 35.Ide, T., Baumer, S. & Deppenmeier, U. (1999) J. Bacteriol. 181, 4076–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brune, A., Spillecke, J. & Kroger, A. (1987) Biochim. Biophys. Acta 893, 499–507. [DOI] [PubMed] [Google Scholar]
- 37.Kengen, S. W. & Stams, A. J. (1994) FEMS Microbiol. Lett. 117, 305–310. [Google Scholar]