Abstract
Biodiversity responses to ongoing climate and atmospheric changes will affect both ecosystem processes and the delivery of ecosystem goods and services. Combined effects of co-occurring global changes on diversity, however, are poorly understood. We examined plant diversity responses in a California annual grassland to manipulations of four global environmental changes, singly and in combination: elevated CO2, warming, precipitation, and nitrogen deposition. After 3 years, elevated CO2 and nitrogen deposition each reduced plant diversity, whereas elevated precipitation increased it and warming had no significant effect. Diversity responses to both single and combined global change treatments were driven overwhelmingly by gains and losses of forb species, which make up most of the native plant diversity in California grasslands. Diversity responses across treatments also showed no consistent relationship to net primary production responses, illustrating that the diversity effects of these environmental changes could not be explained simply by changes in productivity. In two- to four-way combinations, simulated global changes did not interact in any of their effects on diversity. Our results show that climate and atmospheric changes can rapidly alter biological diversity, with combined effects that, at least in some settings, are simple, additive combinations of single-factor effects.
Keywords: California grassland, plant diversity, functional groups, global change interactions
Human activity is altering the world's atmosphere and climate in multiple ways. Anthropogenic increases in global atmospheric CO2 concentrations are contributing both to rising global temperatures and to precipitation increases over some continents, including North America (1). Global anthropogenic N fixation now exceeds all natural sources of N fixation, and its products include greenhouse gases such as N2O that further contribute to climate change (2). These major changes in the world's atmosphere and climate are not just co-occurring but also are causally linked. In light of these trends, anticipating ecosystem responses to combined climate and atmospheric changes is critical in the guidance of management, policy, and conservation efforts to mitigate their effects.
At least three classes of interactions could characterize biodiversity and ecosystem responses to multiple, simultaneous global changes. Biodiversity effects of climate and atmospheric changes might not interact (additive response) and therefore might be predicted directly from single-factor experiments (3–5). Alternatively, effects of multiple global changes may be synergistic (amplifying) or antagonistic (canceling or damping) (6), producing larger or smaller biodiversity changes, respectively, than expected based on single-factor results. Changes in environmental conditions can alter the diversity of plant communities by changing resource availability and by affecting individual species' performances and, in turn, the strength and outcomes of inter-specific competition (7, 8). In systems of moderate-to-high productivity, environmental changes that alleviate limits to production, such as higher temperatures in cold-limited systems or direct increases in the supply of limiting nutrients, water, or carbon, generally reduce diversity (9–13). In systems limited by availability of more than one of these resources, co-occurring environmental changes could produce larger or smaller diversity responses than expected by alleviating multiple resource limitations or producing antagonistic changes in the availability of these resources. Net primary production (NPP) responses to some combinations of simulated global changes, such as N fertilization and elevated CO2, have been characterized by important interactions suggestive of alleviated resource colimitation in certain systems, including California grassland (14–16), but not in others (17). Because diversity seems sensitive to NPP changes, its responses to combined global changes could be coupled to NPP responses, producing similar interactions.
Modeling efforts illustrate that the nature of global change interactions will strongly influence both ecosystem responses and our ability to anticipate and understand them based on single-factor studies (6, 18). Yet actual ecological responses in natural systems to simultaneous elevated atmospheric CO2, warming and precipitation changes, and N deposition have not been investigated previously. To date, other global change factors, such as changes in land use and biological invasions, have been the major drivers of grassland biodiversity change. The effects of climate and atmospheric changes are likely to grow in importance, particularly because they influence communities and their component species over their entire ranges. We examined the responses of a California grassland community to 3 years of treatments simulating single and combined global changes to empirically evaluate the importance of climate and atmospheric change interactions in causing biodiversity change. In our study system, production was limited by cold during and drought at the end of the winter growing season, as well as by soil nitrogen (15). Environmental changes that alleviate these limitations thus could interact in their effects.
Methods
Site and Design. Our study site was a California grassland at the Jasper Ridge Biological Preserve in the San Francisco Bay area (37°24′N, 122°13′W). The study area included annual grasses, annual and biennial forbs, and occasional perennial bunch-grasses, forbs, and shrubs. Annual grasses are the community dominants, generally undergoing more rapid above-ground growth than forbs and contributing the majority of peak plant biomass. The small stature and short lifespan of the annual plants at our site make it an excellent model system in which to observe multiple generations of community response over a period of years. Our study plots contained a total of 43 plant species, including annual and perennial grasses and forbs, with a mean of 10.1 species in each 0.78-m2 study plot. Whereas many global change experiments have occurred in extremely low-diversity systems, our study system provides insight into the dynamics of response in a moderately diverse system. The processes that seem to control interannual variation in diversity at our study site include small-scale disturbances and the availability of light and soil resources during several critical windows in the growing season. Our findings thus can provide insight into the roles of a class of diversity regulators important in a range of ecosystem types.
In 1997 we established 32 circular plots at our grassland site and divided each one into four 0.78-m2 quadrants with solid below-ground and mesh above-ground partitions. We exposed plots to elevated CO2 (ambient plus 300 ppm), warming (80 W·m–2 of thermal radiation, resulting in a soil-surface warming of 0.8–1°C), elevated precipitation (increased by 50%, including a growing-season extension of 20 days), and N deposition (increased by 7 g·m–2·yr–1) in one- to four-way treatment combinations, each replicated eight times. Warming was applied with infrared lamps suspended over plot centers. CO2 was elevated with a free-air system with emitters surrounding each plot and delivering pure CO2 at the canopy level (19). Extra precipitation was delivered with sprinklers and drip lines within 48 h of the end of each natural rain event to avoid changing the frequency or distribution of rainfall, which can affect grassland diversity. The growing season extension was delivered as two waterings 10 and 20 days after the last natural rain event. Within each plot, control, N deposition, precipitation, and N deposition plus precipitation treatments were assigned randomly to each of the four quadrants. N deposition was administered with liquid (in autumn) and slow-release (in winter) Ca(NO3)2 applications each year. Precipitation was enhanced with overhead sprinklers that supplemented natural rain events and extended the wet season by 20 days in the spring. Invasion of two of the eight replicate blocks by the large, exotic biennial Dipsacus sativus led us to exclude them from analysis of NPP responses (n = 6), but it did not affect diversity (n = 8). We began treatments in November 1998 and report responses observed at the end of 3 years of treatments.
Diversity and Production. In our region's Mediterranean-type climate, annual plants germinate in the fall after the first significant wet-season rains and set seed and senesce during the summer drought between April and August. Plant diversity was measured through exhaustive searches in May 1998–2001, supplemented by species lists generated from each year's biomass harvest. We chose to measure diversity as species richness because it is the most sensitive diversity metric (20). We computed annual values for the entire plant community in each study plot and for individual plant functional groups (21). To evaluate the contributions of species gains and losses from different functional groups to overall diversity changes, we analyzed treatment effects on the diversity of forbs (herbaceous dicots), annual grasses, and perennial grasses. Forbs are the most species-rich group overall at our site and include more native species than the grasses. Because many forb species are uncommon and/or restricted in their distributions at the site, however, grass diversity tended to exceed forb diversity at the plot scale. Forbs made up 9.9 ± 0.9% of total biomass across treatments in 2001.
Peak production and end-season litter biomass were measured by harvesting all above-ground material from 144-cm2 subplots in each quadrant on April 17–19 and May 16–18, 2001. Litter and live material were separated. April live samples were sorted by species; May live samples were sorted by functional group (annual grasses, perennial grasses, annual/biennial forbs, perennial forbs, and other). All samples were oven-dried and weighed. Peak annual grass and annual/biennial forb production, respectively, were each determined for every quadrant as the greater of the two harvest biomass values.
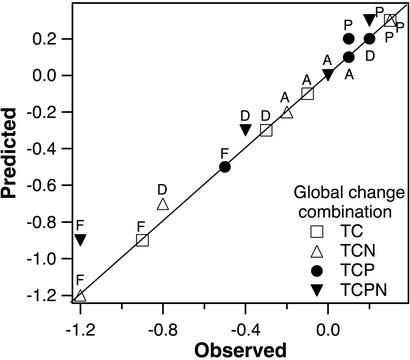
Analyses. We analyzed plant diversity responses to individual global change factors, using a split-plot analysis of covariance (ANCOVA) with two levels each of CO2, warming, precipitation, and temperature and with pretreatment (1997) species richness as a covariate to control for preexisting differences among study quadrants. We interpret main factor effects and all two-, three-, and four-factor interactions based on the ANCOVA results. We do not report measures of variance for the diversity data because analyses are on ANCOVA-adjusted means to which the variances of before (pretreatment) and after (year 3) data do not correspond. Responses to treatments are reported as the percent of species present at ambient level that were gained or lost at the elevated level: X = 100% of Delevated-ambient/Dambient. To further investigate interactions among global change factors in their effects on grassland biodiversity, we directly compared the sums of individual factor effects with the observed effects of treatment combinations (Fig. 2).
Fig. 2.
Predicted and observed effects of combined global change treatments on forbs (F), annual grasses (A), perennial grasses (P), and total diversity (D). Predicted values are the summed effects of individual global change factors that make up each selected treatment combination. Points on the 1:1 line indicate perfectly additive (no interaction) responses to combined global change treatments. T, elevated temperature; C, elevated CO2; P, elevated precipitation; N, nitrogen deposition.
To test whether variation in total plant production and surface litter biomass across treatments explained diversity responses, we compared results for the split-plot ANCOVA of diversity with and without each of these measures as additional covariates. To evaluate the contributions of individual species to single-factor diversity responses in 2001, we tallied gains and losses of each species in each plot, using 1998 as the reference year. We then compared gains and losses under ambient and elevated levels of each single-factor treatment and tested for significant treatment effects, using χ2 goodness of fit for each species and global change factor. Reported P values are based on χ2 values computed with the Yates continuity correction, with degree of freedom = 1 (22).
All regions of the world are expected to experience elevated CO2 and warming in the next century, but increases in N deposition and precipitation will vary geographically and affect only some areas. Four of the treatment combinations included in our experiment simulate the range of alternative scenarios likely to be experienced by different regions in the future: elevated CO2 plus warming, elevated CO2 plus warming plus N deposition, elevated CO2 plus warming plus precipitation, and elevated CO2 plus warming plus N deposition plus precipitation. To test the effects of these specific two-, three-, and four-way treatment combinations relative to controls, we performed t tests or their nonparametric equivalent, the Kruskal–Wallis test, on the eight replicates of the treatment combination of interest versus the eight replicates of the control (no simulated global changes) treatment.
Results and Discussion
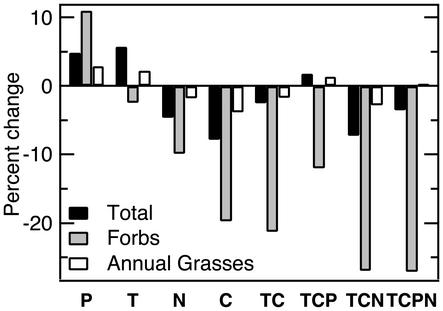
Individual Global Changes. At the end of 3 years, three of the four individual global change treatments had altered total plant diversity (Fig. 1 and Table 1). N deposition reduced total diversity by 5% (X = –0.5/10.3 species, F = 4.600, P = 0.041). Elevated CO2 also reduced total diversity, by 8% (X =–0.8/10.5 species, F = 3.499, P = 0.072). However, elevated precipitation increased total diversity by 5% (X = 0.5/9.8 species, F = 3.976, P = 0.057). Warming had no significant effect but tended to increase diversity (X = 0.6/9.8 species, P > 0.20). These changes in species richness, although numerically small, occurred despite large natural variation from plot to plot in initial species composition and the relatively small proportion of the total site species pool captured by each plot. Treatment-driven species losses at the level of the entire site were also consistent with plot-scale patterns. For example, after 3 years the total species pool across all plots that received N deposition experienced a small decline relative to the total species pool across all plots that did not receive extra N. These rapid responses to individual global change treatments support and build on the previous suggestion that grasslands consisting of fast-growing, short-lived species are sensitive to climate changes (23).
Fig. 1.
Changes in total and forb diversity under single and combined global change treatments. Values are percent differences between control and elevated levels of each treatment. T, elevated temperature; C, elevated CO2; P, elevated precipitation; N, nitrogen deposition. Table 1 provides means and significance levels.
Table 1. Plant diversity responses to single and combined global change treatments.
| Treatment | Level | n | Annual grasses | Forbs | Perennial grasses | Total |
|---|---|---|---|---|---|---|
| C | Low | 64 | 6.0 | 4.0 | 0.35 | 10.5 |
| High | 64 | 5.7 | 3.2* | 0.47 | 9.7** | |
| T | Low | 64 | 5.8 | 3.6 | 0.34 | 9.8 |
| High | 64 | 5.9 | 3.5 | 0.47 | 10.4 | |
| N | Low | 64 | 5.9 | 3.8 | 0.39 | 10.3 |
| High | 64 | 5.8 | 3.4*** | 0.43 | 9.8* | |
| P | Low | 64 | 5.7 | 3.4 | 0.42 | 9.8 |
| High | 64 | 5.9 | 3.8* | 0.39 | 10.3* | |
| C + T | Low | 32 | 5.9 | 4.1 | 0.36 | 10.3 |
| High | 32 | 5.8 | 3.2*** | 0.59** | 10.0 | |
| C + T + N | Low | 16 | 5.9 | 4.4 | 0.24 | 10.7 |
| High | 16 | 5.7 | 3.2 | 0.57 | 9.9 | |
| C + T + P | Low | 16 | 5.9 | 3.8 | 0.62 | 10.5 |
| High | 16 | 6.0 | 3.4 | 0.48 | 10.6 | |
| C + T + N + P | Low | 8 | 5.9 | 4.3 | 0.35 | 10.4 |
| High | 8 | 5.9 | 3.1* | 0.54** | 10.0 |
Diversity shown in mean number of species. Diversity means are ANCOVA-adjusted for preexisting differences among plots. C, elevated CO2; T, elevated temperature; N, N deposition; P, precipitation. *, P = 0.05. **, P = 0.10. ***, P = 0.01.
The effects of elevated CO2, N deposition, and precipitation on total diversity were driven mainly by significant gains and losses of forb species (Fig. 1). Annual grass diversity was relatively unresponsive to all individual global change treatments. Perennial grasses, which made up a small proportion of species in our study area, did not respond significantly to individual global change treatments. Entire plant functional groups within a community hence can vary widely in their sensitivity to climate and atmospheric change factors.
Previous studies have shown that grassland diversity responses to changes in ecosystem productivity also are driven mainly by gains and losses of forb species (11, 24–26). We hypothesized that the restriction of species gains and losses to forbs reflected their vulnerability to shading by the dominant grasses or by accumulated surface litter (27), both of which could result from increased plant production. Total plant production, however, explained almost none of the variation in plant diversity within or among treatments (F = 0.014, P = 0.907). In fact, some treatments with similar effects on grassland production (16) had contrasting effects on plant diversity. For example, N deposition and elevated precipitation treatments each significantly increased total above-ground NPP at our site (28), but N deposition reduced total and forb diversity, whereas elevated precipitation increased them. Litter biomass, which can respond to changes in both production and decomposition and can strongly influence grassland diversity by eliminating small-statured forbs (24), also failed to explain the observed variation in plant diversity after three years (F = 0.372, P = 0.547). The observed diversity responses to global change treatments thus cannot be explained directly through changes in productivity, despite evidence that productivity changes exert strong control over diversity in many grasslands and other ecosystems (9, 11–13, 24).
Because species composition varied among replicate plots, species gains and losses contributing to treatment effects on diversity also varied. Reduced diversity under N deposition was driven by losses of several forbs, including all three N-fixing species present at our site (Vicia sativa, Medicago polymorpha, and Lotus purshianus). No single species, however, made a significant individual contribution to this reduced diversity. Increased diversity under elevated precipitation was caused partly by gains of an annual forb, Anagallis arvensis (χ2 = 3.5, P < 0.10), whose indeterminate growth habit permits continued growth as long as resources remain available. Slightly higher diversity under elevated, rather than ambient, temperatures reflected significantly fewer losses of three species: an indeterminate forb (M. polymorpha, χ2 = 5.14, P < 0.05), a late-flowering forb (Torilis arvensis, χ2 = 3.06, P < 0.10), and a small-statured annual grass (Briza minor, χ 2 = 3.37, P < 0.10). These species' small stature or late phenology suggests responsiveness to the amount and timing of late-season light and soil resource availability. Diversity declines under elevated CO2 reflected increased losses of two such species: a late-flowering annual forb (Epilobium brachycarpum, χ2 = 5.34, P < 0.05) and a small-statured forb (Erodium botrys, χ2 = 3.23, P < 0.10). Losses of an annual grass, Bromus diandrus (χ2 = 5.06, P < 0.05), also contributed to diversity declines under elevated CO2. Different species dominated the diversity effects of each global change factor, suggesting that the four factors each influenced diversity through different mechanisms. This differs from previous suggestions that elevated CO2 and warming affect communities largely through their effects on water and nutrient availability (e.g., refs. 29–32). The individual responses of the 10 most common species at our site to the global change treatments are detailed in ref. 33.
Combined Global Changes. All four treatment-combination scenarios produced mean declines in forb diversity of >10% (Fig. 1). These forb declines were significant under elevated CO2 plus warming (X = –0.9/4.1 species, P < 0.01) and the combination of all four global change factors (X =–1.2/4.3 species, P < 0.05). Diversity of this functional group, which includes many of the remaining native and rare species in California grasslands (34), seems susceptible to decline regardless of whether N deposition and precipitation increase.
Effects of these four treatment combinations on total diversity (forbs, annual grasses, and perennial grasses) were not significant, largely because increases in perennial grass diversity partially offset losses of forb species (Table 1). Effects on total diversity did vary somewhat in magnitude among the four global change treatment combinations. Diversity did not respond to the combination of elevated CO2 plus warming plus precipitation (P > 0.20). The other three global change combinations each tended to reduce total diversity (P < 0.20), with the largest decline (–7%) under the combination of elevated CO2 plus warming plus N deposition. The magnitude of diversity responses to climate and atmospheric changes thus could depend strongly on regional variation in N deposition and precipitation changes, suggesting a need for improved regional models of these changes in the future.
Interaction terms indicated no significant two-, three-, or four-way interactions among treatment factors for total or forb diversity, the two components of diversity that exhibited responsiveness to simulated global changes. Moreover, the accuracy with which summed effects of individual global change factors predicted combined treatment effects on the diversity of every functional group was striking (Fig. 2). The least well predicted response was the mean decline in forb diversity of 1.2 species under the combination of all four global change factors, which we predicted would be a loss of only 0.9 species based on individual factor effects. The responses of our study system, at least to 3 years of the global change levels we simulated, resulted in essentially no interactions among co-occurring climate and atmospheric changes in their diversity effects.
Previous findings provide possible mechanisms for the rapid diversity responses to individual global change treatments that we observed. N deposition has been repeatedly observed to reduce grassland diversity by increasing the dominance of common species through increases in their production. Forb diversity declines followed fertilization-driven increases in annual grass production within 2 years in California serpentine grassland (26) and in other early successional, temperate grasslands (11, 13, 24, 35–37). The effects of N deposition that we observed are consistent with a mechanism of reduced diversity through increased production by the dominant annual grasses in our study system (16). It is also possible that reduced soil moisture availability in spring and summer under the N deposition treatment (28) contributed to losses of indeterminate and late-season forb species that rely on soil resources present at the end of the growing season.
Different mechanisms than production change, however, seem to drive the effects of the other three factors. Environmental effects on the temporal complementarity of resource use among plant functional groups are an intriguing possibility (38, 39) supported by the relatively large contributions to diversity change of late-season, indeterminate, and small-statured species that are likely responsive to changes in the timing and duration of resource availability late in the growing season. Increased precipitation could enhance California grassland diversity (40) by prolonging soil moisture availability at the end of the growing season, providing opportunities for perennial and late-season species to grow and reproduce after the dominant grass canopy has senesced (41, 42). At our site, warming accelerated senescence of the dominant, early-season grass canopy, increasing subsequent resource availability, including soil moisture availability, to late-season species (28). Warming also may enhance forb performance in the cold middle of the growing season through both suppressed herbivory (28) and accelerated growth at a time when the dominant grasses are small.
The contrasting effects of elevated CO2 and precipitation on diversity, despite their similar effects on soil moisture availability at our site (28), deserve more examination. Previous studies in lower-productivity grasslands than our site have found, in contrast to our results, that elevated CO2 increased species richness (43, 44) or evenness (45). These effects were attributed to the positive effect of elevated CO2 on soil moisture, an effect also observed at our site. Pot and mesocosm studies provide clues as to why our higher-productivity grassland might have responded differently. In one study, competition outcomes among grass species at elevated CO2 were best predicted from species' light-capturing abilities rather than from traits related to moisture needs or acquisition, but only under high levels of competition for light (46). A review of elevated CO2 experiments in planted mixtures concluded that, although soil moisture changes affect competitive outcomes in some low-fertility settings, elevated CO2 under high-fertility conditions favored good competitors for light rather than for soil resources (47). The relative importance of light- and water-mediated effects of elevated CO2 therefore likely depends on the productivity of the ecosystem in question. At our relatively productive site, elevated CO2 delayed senescence of the dominant plant canopy at the end of the growing season, narrowing the late-season window of light availability for late-emerging and small-statured species (E. E. Cleland, unpublished data). This might explain why elevated CO2 reduced diversity at our site despite soil moisture increases and NPP declines (16), whereas elevated precipitation and warming each tended to increase diversity despite NPP increases.
The absence of interactions among global change treatments in our study suggests that results from single-factor studies could be used to generate initial, additive estimates of the diversity effects of combined environmental changes in at least some systems. A recent analysis from the Jasper Ridge Global Change Experiment (JRGCE) showed that, across our two- to four-way treatment combinations, all factors except elevated CO2 also produced additive (noninteracting) NPP responses (16). This suggests that responses of some other important ecosystem characteristics also can be estimated from single-factor effect sums. However, at least one ecosystem variable, grassland invasion by woody species, responded with important interactions, producing highly synergistic responses to warming and elevated precipitation in the JRGCE (28). Important interactions between the global changes we simulated and other environmental changes, including change in land use and biological invasions, are also likely to occur and to increase in importance at larger spatial and longer temporal scales. There is no rule of thumb for understanding combined global change responses in natural ecosystems. Rather, the importance of interactions for particular ecosystem processes likely will depend on the mechanisms governing each global change effect and the degree to which these mechanisms interact and overlap, such as through alleviation of multiple resource colimitations on the response variable of interest (48). This study is one of many tests that will be required for a general picture of ecological response to multiple global changes. We expect the overall responses of this study, which we observed in a natural ecosystem operating under normal conditions, to extend to other systems as well. However, certain characteristics of Mediterranean grasslands, such as a winter growing season and a water-limited, rather than a cold-limited, growing-season length, are not shared by all other systems. Additional, multifactor experiments in other ecosystems and the development of a framework for identifying conditions where interactions are likely to matter are important next steps to improve prediction of the ecological consequences of global climate and atmospheric change.
Acknowledgments
We thank the dozens of volunteers and field assistants who made this project possible. The Jasper Ridge Global Change Experiment was supported by the National Science Foundation, the David and Lucile Packard Foundation, the Morgan Family Foundation, and the Jasper Ridge Biological Preserve. E.S.Z. received support from the U.S. Environmental Protection Agency STAR Fellowship Program, the Switzer Foundation, the Andrew W. Mellon Foundation, and a David H. Smith Conservation Research Fellowship from The Nature Conservancy. M.R.S. was supported by an Alexander Hollaender Postdoctoral Fellowship from the U.S. Department of Energy.
Abbreviations: NPP, net primary production; ANCOVA, analysis of covariance.
References
- 1.Intergovernmental Panel on Climate Change (2001) Working Group 1, Third Assessment Report (Cambridge Univ. Press, Cambridge, U.K.).
- 2.Vitousek, P. M., Mooney, H. A., Lubchenco, J. & Melillo, J. M. (1997) Science 277, 494–499. [Google Scholar]
- 3.Chapin, F. S., Shaver, G. R., Giblin, A. E., Nadelhoffer, K. J. & Laundre, J. A. (1995) Ecology 76, 694–711. [Google Scholar]
- 4.Nie, D., Kirkham, M. B., Ballou, L. K., Lawlor, D. J. & Kanemasu, E. T. (1992) J. Veg. Sci. 3, 673–678. [Google Scholar]
- 5.Reich, P. B., Knops, J., Tilman, D., Craine, J., Ellsworth, D., Tjoekler, M., Lee, T., Wedin, D., Naeem, S., Bahauddin, D., et al. (2001) Nature 410, 809–812. [DOI] [PubMed] [Google Scholar]
- 6.Sala, O. E., Chapin, F. S., Armesto, J. J., Berlow, E., Bloomfield, J., Dirzo, R., Huber-Sanwald, E., Huenneke, L. F., Jackson, R. B., Kinzig, A., et al. (2000) Science 287, 1770–1774. [DOI] [PubMed] [Google Scholar]
- 7.Tilman, D. (1988) Dynamics and Structure of Plant Communities (Princeton Univ. Press, Princeton).
- 8.Jeffree, E. P. & Jeffree, C. E. (1994) Funct. Ecol. 8, 640–650. [Google Scholar]
- 9.Mittelbach, G. G., Steiner, C. F., Schneider, S. M., Gross, K. L., Reynolds, H. L., Waide, R. B., Willig, M. R., Dodson, S. I. & Gough, L. (2001) Ecology 82, 2381–2396. [Google Scholar]
- 10.Grime, J. P. (1973) Nature 242, 344–347. [Google Scholar]
- 11.Goldberg, D. E. & Miller, T. E. (1990) Ecology 71, 213–225. [Google Scholar]
- 12.Tilman, D. (1987) Ecol. Monogr. 57, 189–214. [Google Scholar]
- 13.Tilman, D. (1993) Ecology 74, 2179–2191. [Google Scholar]
- 14.Owensby, C. E., Auen, L. M. & Coyne, P. I. (1994) Plant Soil 165, 105–113. [Google Scholar]
- 15.Chiariello, N. R. & Field, C. B. (1996) in Carbon Dioxide, Populations, and Communities, eds. Korner, C. & Bazzaz, F. A. (Academic, San Diego), pp. 139–175.
- 16.Shaw, M. R., Zavaleta, E. S., Chiariello, N. R., Cleland, E. E., Mooney, H. A. & Field, C. B. (2002) Science 298, 1987–1990. [DOI] [PubMed] [Google Scholar]
- 17.Reich, P. B., Tilman, D., Craine, J., Ellsworth, D., Tjoekler, M., Knops, J., Wedin, D., Naeem, S., Bahauddin, D., Goth, J., Bengston, W. & Lee, T. (2001) New Phytol. 150, 435–448. [DOI] [PubMed] [Google Scholar]
- 18.McGuire, A. D., Sitch, S., Dargaville, R., Esser, G., Foley, J., Heimann, M., Joos, F., Kaplan, J., Kicklighter, D. W., Meier, R. A., et al. (2001) Global Biogeochemical Cycles 15, 183–206. [Google Scholar]
- 19.Miglietta, F., Giuntoli, A. & Bindi, M. (1996) Photosynth. Res. 47, 281–290. [DOI] [PubMed] [Google Scholar]
- 20.Magurran, A. E. (1988) Ecological Diversity and Its Measurement (Princeton Univ. Press, Princeton).
- 21.Hooper, D. & Vitousek, P. (1997) Science 277, 1302–1305. [Google Scholar]
- 22.Zar, J. H. (1996) Biostatistical Analysis (Prentice–Hall, Upper Saddle River, NJ).
- 23.Grime, J. P., Brown, V. K., Thompson, K., Masters, G. J., Hillier, S. H., Clarke, I. P., Askew, A. P., Corker, D. & Kielty, J. P. (2000) Science 289, 762–765. [DOI] [PubMed] [Google Scholar]
- 24.Foster, B. L. & Gross, K. L. (1998) Ecology 79, 2593–2603. [Google Scholar]
- 25.Abrams, P. A. (1995) Ecology 76, 2019–2027. [Google Scholar]
- 26.Huenneke, L. F., Hamburg, S. P., Koide, R., Mooney, H. A. & Vitousek, P. M. (1990) Ecology 71, 478–491. [Google Scholar]
- 27.Newman, E. I. (1973) Nature 244, 310. [Google Scholar]
- 28.Zavaleta, E. S. (2001) Ph.D. dissertation (Stanford Univ., Stanford, CA).
- 29.Chapin, F. S. & Shaver, G. R. (1996) Ecology 77, 822–840. [Google Scholar]
- 30.Harte, J. & Shaw, R. (1995) Science 267, 876–880. [DOI] [PubMed] [Google Scholar]
- 31.Owensby, C. E., Ham, J. M., Knapp, A. K. & Auen, L. M. (1999) Global Change Biol. 5, 497–506. [Google Scholar]
- 32.Volk, M., Niklaus, P. A. & Korner, C. (2000) Oecologia 125, 380–388. [DOI] [PubMed] [Google Scholar]
- 33.Zavaleta, E. S., Shaw, M. R., Chiariello, N. R., Thomas, B. D., Cleland, E. E., Field, C. B. & Mooney, H. A. (2003) Ecol. Monogr., in press.
- 34.Harrison, S. (1999) Oecologia 121, 99–106. [DOI] [PubMed] [Google Scholar]
- 35.Bakelaar, R. G. & Odum, E. P. (1978) Ecology 59, 660–665. [Google Scholar]
- 36.Carson, W. P. & Barrett, G. W. (1988) Ecology 69, 984–994. [Google Scholar]
- 37.Wilson, S. D. & Tilman, D. (1991) Ecology 72, 1050–1065. [Google Scholar]
- 38.Chiariello, N. R. (1989) in Grassland Structure and Function: California Annual Grassland, eds. Huenneke, L. F. & Mooney, H. A. (Kluwer, Dordrecht, The Netherlands), pp. 47–58.
- 39.Hooper, D. U. (1998) Ecology 79, 704–719. [Google Scholar]
- 40.Hobbs, R. A. & Mooney, H. A. (1991) Ecology 72, 59–68. [Google Scholar]
- 41.Hamilton, J. G., Holzapfel, C. & Mahall, B. E. (1999) Oecologia 121, 518–526. [DOI] [PubMed] [Google Scholar]
- 42.Jackson, L. E. & Roy, J. (1986) Acta Oecol. 7, 191–212. [Google Scholar]
- 43.Potvin, C. & Vasseur, L. (1997) Ecology 78, 666–677. [Google Scholar]
- 44.Niklaus, P. A., Leadley, P. W., Schmid, B. & Korner, C. (2001) Ecol. Monogr. 73, 341–356. [Google Scholar]
- 45.Leadley, P. W., Niklaus, P. A., Stocker, R. & Korner, C. (1999) Oecologia 118, 39–49. [DOI] [PubMed] [Google Scholar]
- 46.Teyssonneyre, F., Picon-Cochard, C. & Soussana, J. F. (2002) New Phytol. 154, 53–64. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds, H. L. (1996) in Carbon Dioxide, Populations, and Communities, eds. Korner, C. & Bazzaz, F. A. (Academic, San Diego), pp. 273–286.
- 48.Chapin, F. S., Bloom, A. J., Field, C. B. & Waring, R. H. (1987) Bioscience 37, 49–57. [Google Scholar]