Abstract
We report here the case of a metazoan parasite, a strepsipteran, that manipulates host epidermal tissue and wraps itself within it; which probably camouflages the endoparasite and is recognized as ``self'' by the host. This mechanism is one of immune avoidance among parasitoid insects. The host-derived epidermal ``bag'' might have enabled Strepsiptera to radiate to disparate hosts compared with the relatively few taxa (596 species) described so far. They have been recorded as parasitizing 34 families belonging to seven orders of Insecta. We also report a mechanism of insect ecdysis between the first- and second-instar larva, while enclosed in the bag.
The obligate endoparasites of the order Strepsiptera (Insecta) have a wide range of insect hosts, including Apterygota, Exopterygota, and Endopterygota (1), although it has been said that as a general rule koinobiont endoparasites have a narrower host range than ectoparasitic ones (2, 3). This view is held because endoparasitic insects have to deal directly with the host's immune system for which specialized adaptations are required (4). However, in the case of Strepsiptera, although only 596 species have been described so far, they have been recorded as parasitizing seven orders and 34 families of Insecta. Such diversity of hosts is probably greater than any group of parasitoid insects of comparable size and would require immune avoidance mechanisms effective for a spectrum of hosts.
Strepsiptera are one of the most complex groups of parasitoids: the major part of the life cycle of the adult free-living male is spent as a larval endoparasite, and the female (except in one family) spends its entire life in the host. The first-instar larva is the only free-living stage of both sexes as it is the host-seeking stage and is produced by the ovoviviparous, neotenic (larviform) female (1). The mechanism whereby Strepsiptera flourish and reproduce (in some species the female produces a million first-instar larvae) without any interference from the host has been a mystery. The stylopised host is the disperser of the strepsipteran (especially of the female). Furthermore, the life cycle of the host is extended, because the host only dies after the emergence of the free-living male, or after all of the first-instar larvae have emerged from the endoparasitic mother (1).
Perhaps even more mysterious is that males and females of the family Myrmecolacidae parasitize hosts belonging to different orders: the males parasitize Hymenoptera (ants) and females parasitize Orthoptera (grasshoppers, mantids, and crickets). Is there a common mechanism that permits Strepsiptera to overcome the immune response of such diverse hosts, or are there separate adaptations used by each species, and even by the male and the female of a single species in the family Myrmecolacidae?
The host-seeking stage of Strepsiptera is the first-instar larva, and entry into the host is through the body, egg, or tarsi of the host (5). The first-instar larva takes considerable time penetrating the outer cuticle, and also while just below the cuticle, before it migrates into the host body cavity. Stichotrema dallatorreanum Hofeneder was used as a model species to study its entry into its katydid (Tettigoniidae) hosts, Segestidae novaeguineae (Brancsik) and S. defoliaria defoliaria (Uvarov), and its molt to the second-apodous larval stage. The first-instar strepsipteran parasitizes the nymphal stage of S. novaeguineae and S. d. defoliaria. This study was conducted by observing the following: (i) in vivo video recordings of entry of the first-instar larva and its subsequent development; (ii) endoparasitic larvae in vitro in standard insect saline; (iii) timed samples of second-, third-, and fourth-instar larvae, including all associated tissues dissected from the hosts and fixed appropriately for light microscopy and histology; and (iv) molecular analysis of the above tissue.
This article is, to our knowledge, the first in vivo and in vitro study of the larvae of these unusual parasitoids. Understanding the interactions of Strepsiptera and their hosts in vivo is vital for successful rearing of this parasite for biological control programs, among other applications. It also reports of a unique mechanism of host avoidance among insects, where a parasitoid wraps itself with host tissue, which probably enables them to masquerade as ``self,'' and thereby allows Strepsiptera to exploit an exceptional diversity of hosts.
Methods
Insects Studied. Stichotrema dallatorreanum is a parasite of Segestidea novaeguineae and S. d. defoliaria (Orthoptera: Tettigoniidae), both of which are serious pests of oil palm in Papua New Guinea in the Oro and West New Britain Provinces (6). S. dallatorreanum is a parthenogenetic species (J.K., unpublished work), so the reference to endoparasitic larvae will be to females.
In Vivo Studies. Stylopised hosts of S. novaeguineae and S. d. defoliaria were dissected along the mid-dorsal line from the thorax to the tip of the abdomen. The host was pinned to expose the inside of the abdomen. Observations of the early stages of live first-instar larvae of S. dallatorreanum after penetration into the cuticle were recorded by a Leica MZ EPO microscope [Leica Microscopy Systems (Schweiz), Heerbrugg, Switzerland], which was attached to a video recorder.
In Vitro Studies. The endoparasitic larvae of S. dallatorreanum that had detached from the epidermis were carefully removed from the host and placed in standard insect saline. The development of live stages of the later instars of S. dallatorreanum in insect saline was recorded by using a Leica MZ EPO microscope attached to a video recorder.
Light Microscopy 5-μm Sections. S. dallatorreanum larvae were dissected from the hosts in standard insect saline. Specimens of: (i) larvae plus ``bag,'' and (ii) just ``bags,'' from which larvae were removed (Fig. 2D) and washed in saline. The specimens were placed in 70% alcohol, passed through a graded series of alcohols for ≈1 h each, placed in Histoclear (National Diagnostics) for 1 h with two changes, then in hot wax at 57°C for half an hour with two changes, and embedded in Polywax (Merck). Sections (5-μm each) were cut in an American Optical 820 rotary microtome, mounted on 1% gelatinized slides with 1% glycerin albumen, blotted with wet fiber-free paper, dried on a hot plate, stained with Masson's Trichrome, and observed under a Leica MPS 30 compound microscope. The above process was also carried out for the 5-μm sections of the cuticle of the host.
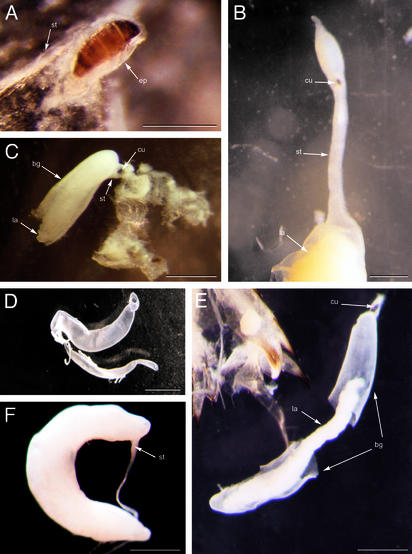
Fig. 2.
Stichotrema dallatorreanum Hofeneder wrapped with the host-derived bag. (A) First-instar larva having penetrated the exocuticles and endocuticles is wrapped with host-derived tissue (ep) and is suspended by the stalk (st). (Scale bar, 0.6 mm.) (B) Stalk soon after detachment from the epidermis containing exuviae of first-instar larva (cu) and second-instar larva (la). (Scale bar, 0.6 mm.) (C) Second-instar larva wrapped with bag (bg) suspended from the epidermis with exuviae of the first-instar larva within the stalk. (Scale bar, 1.7 mm.) (D) Host-derived bag dissected from the second-instar larva. (Scale bar, 1 mm.) (E) Second-instar larva with split bag and exuviae of the first-instar larva. (Scale bar, 1 mm.) (F) Late third-instar larva with stalk, which is still evident. (Scale bar, 1 mm.)
Whole specimens of both larvae and bag were preserved in 75% alcohol and photographed with a Zeiss SR stereomicroscope.
Numbers Studied in Vivo and in Vitro. Thirty specimens of live S. dallatorreanum were observed soon after the first-instars had penetrated S. novaeguineae and S. d. defoliaria, and the observation continued until the larvae suspended themselves in the host hemolymph. The larvae were then removed and placed in saline and were further observed until they died.
Histological 5-μm Sections. Ten specimens of S. dallatorreanum (with bags), and four cuticles of S. novaeguineae consisting of exocuticle, endocuticle, and epidermis, were used for histological sections.
Molecular Techniques. Microscope-assisted, dissected samples of bag corresponding to each larval stage of S. dallatorreanum, and the two known hosts of S. dallatorreanum, were washed thoroughly in sterile TE buffer (10 mM Tris/1 mM EDTA, pH 8.0) before DNA extractions to minimize the occurrence of contamination. DNA extractions were performed by using the Qiagen QiAmp DNA mini kit as specified by the manufacturer (7). Fragments of 28S rRNA (D2) and the mitochondria 12S gene were amplified for each extracted DNA sample. Amplified fragments were separated on a 1% agarose gel, gel-extracted by using a Bio 101 GENECLEAN III kit, and were sequenced by using an Applied Biosystems Big Dye kit and an Applied Biosystems 377 sequencer. Nucleotide sequences were aligned by using clustal x, version 1.5b.
Results
Description. J.K. observed the entry of the host-seeking first-instars of S. dallatorreanum into each of the two hosts and their subsequent development into apodous endoparasitic larvae.
Entry and penetration of the first-instar larva. The first-instar infective larva of S. dallatorreanum sits on the host cuticle for ≈20 min (either on the body or the footpads; ref. 5; Fig. 1A). During the 20 min, the head of the first-instar larva constantly jabs the cuticle of the host. The larval entry into the exocuticle and endocuticle might occur both by digestion and piercing, but this was not studied in detail here. Once the whole body of the first-instar larva has penetrated the exocuticle and endocuticle of the host, it remains on the surface of the epidermis for 24–36 h and continues its constant motion. After ≈1 day the host epidermis separates from the endocuticle, as in apolysis during the beginning of a normal molting cycle (ref. 8; Fig. 1B). The area of the separation is restricted to about the length of the first-instar larva (≈0.15–2 mm). The first-instar larva continues its movements and eventually becomes enclosed by the host epidermis (Figs. 1C and 2A).
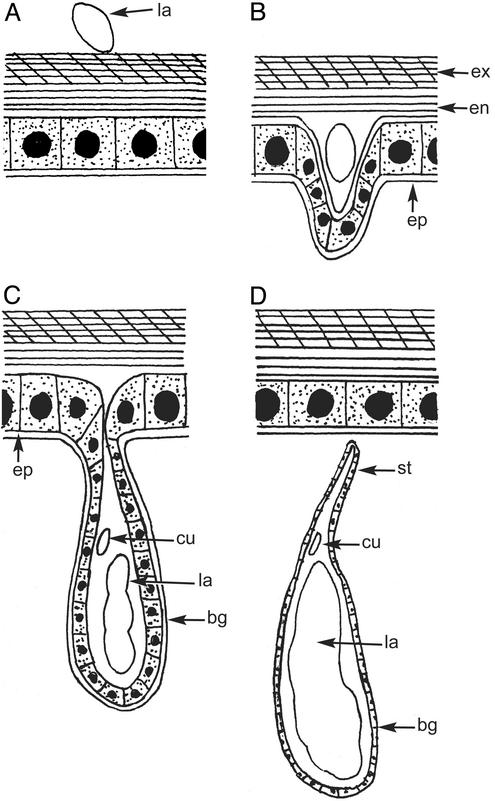
Fig. 1.
Diagram of the entry of first-instar larva and subsequent endoparasitic stages during formation of the bag. (A) First-instar larva (la) sitting on the host cuticle. (B) First-instar larva having entered the exocuticles (ex) and endocuticles (en) initially separates the epidermis (ep) from the endocuticle to form the bag. (C) Suspension of the bag (bg) from the epidermis containing the apodous second-instar larva (la) and exuviae of first-instar cuticle (cu). (D) Stalk (st) with the bag enclosing the second-instar larva with exuviae of the first-instar cuticle after detachment from the epidermis. Diagram is not to scale.
Suspension of the endoparasitic larva. The first-instar enclosed by the epidermis extends into the host hemolymph and the structure forms a bag, suspended by a thin stalk attached to the epidermal layer on the cuticle of the host (Fig. 2 A). Within this bag the first-instar molts to a second-instar apodous larva (Fig. 1C). This process takes 2–4 days. The stalk, which suspends the second-instar (Figs. 2C and 3A), eventually pinches off from the overlying epidermal layer (Figs. 1D and 2B), and the second-instar larva (enclosed by the bag; Fig. 2E) moves deeper into the host.
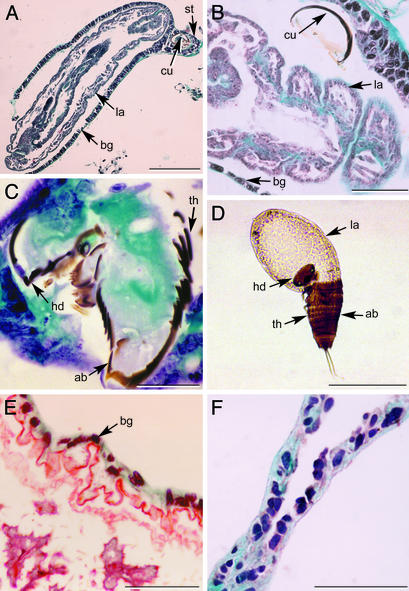
Fig. 3.
(A) A 5-μm section of second-instar larva (la) (specimen in Fig. 2C) wrapped with bag (bg) containing exuviae of first-instar larva (cu) within the stalk (st). (Scale bar, 200 μm.) (B) A 5-μm section of second-instar larva covered with bag, containing exuviae of first-instar larva. (Scale bar, 50 μm.) (C) A 5-μm section of exuviae of first-instar cuticle showing the head (hd) broken along a circular ecdysial line separating it from the thorax (th) and abdomen (ab). (Scale bar, 30 μm.) (D) Second-instar larva emerging from the first-instar cuticle showing the head capsule broken from the thorax and abdomen along the circular ecdysial line. No bag is evident as emergence was in the mother. (Scale bar, 200 μm.) (E) A 5-μm section of neotenic female in region of the ``apron'' surrounded by host-derived bag. (Scale bar, 50 μm.) (F) A 5-μm section of bag that was dissected from the second-instar larva. (Scale bar, 50 μm.)
This same process of host entry by the first-instar larva applies to first-instars that enter the footpads (5). The apodous second-instar larvae move passively up the tibia and femur with the bag, enter the thorax, and eventually settle in the abdomen.
The molting process. The second-instar larva emerges from the exuvium of the first-instar larva anteriorly from the head. The ecdysial line of the first-instar larva splits along the base of the head capsule in a circle, which is unique to Strepsiptera. The head capsule does not split all of the way around and remains hinged to the thorax (Fig. 3 C and D). In all other insects the ecdysial line splits along the mid-dorsal line of the head and/or along one or more ventral lines (9). The exuvium of the first-instar remains within the bag (Figs. 1D, 2 B, C, and E, and 3 A and B) throughout the life of the larva, and is evident even in neotenic female adults.
The apodous second-instar larva goes through two more molts to reach the fourth-instar stage. The molting process during these stages is apolysis, but is not followed by ecdysis (10). This feature too is unique to Strepsiptera. At the late fourth-instar stage, the female S. dallatorreanum extrudes the anterior region (cephalothorax) through the host cuticle, and becomes sclerotized. The posterior region remains within the host. At this stage the female becomes a neotenic adult without an intervening pupal instar (11).
Development of the Bag. The bag consists of large columnar epidermal cells, each with a nucleus that is many times that of the strepsipteran nuclei (Fig. 3E). The cells initially surround a tiny first-instar larva (≈0.16 mm; Fig. 2A), then the apodous larval instars (Fig. 2 C, E, and F), and eventually the neotenic female (≈1.5–3.9 cm; Fig. 3E). The bag is not evident in the region of the extruded cephalothorax of the neotenic female, but is present in the posterior region, which is within the host and remains so throughout the strepsipteran adulthood.
Molecular Data. DNA of the larva, bag, and host were extracted to study whether the bag is host- or parasite-derived. Sequences of the larva, bag, and host revealed that the bag and host were identical. The hosts and bags showed no differences for either 28S rRNA (D2) or mitochondrial 12S gene fragments (Fig. 4A and B). In addition, no differences were observed within larval sequences for either fragments. In contrast, when the larvae and bag were compared, they differed significantly. For the 12S fragment, 47 differences, including three insertion-deletions (5, 3, and 1 nt, respectively), were observed (Fig. 4A). For the D2 fragment, 67 differences, including two single-nucleotide insertion-deletions, were observed (Fig. 4B).
Fig. 4.
clustal x, version 1.5b, multiple sequence alignment of mitochondrial 12S gene fragment (A) and nuclear D2 gene fragment (B). Shown is clustal x alignment of mitochondrial 12S and 28S (D2) DNA sequences of S. dallatorreanum hosts S. novaeguineae (Snov_host) and S. defoliaria (Sdef_host), and the bag (50c_bag and 50d_bag) and larval (50c_larvae and 50d_larvae) sequences of S. dallatorreanum samples 50c and 50d.
Discussion
This article is, to our knowledge, the first report of a parasitoid surrounding itself with host-derived epidermal tissue while still in the host hemolymph, which is a unique mechanism among parasitoid insects for avoiding immune responses of their hosts, and also of the unusual ecdysis that occurs between the first- and second-instar larva within the host-derived epidermal bag.
The Ontogeny of the Bag. It is known that columnar cells of arthropod epidermis are capable of spreading to form a single layer and repair wounds. Each cell is able to break the desmosomal contacts with its neighbors and move in an amoeboid fashion. It is also known that the epidermal cells, when detached from the cuticle, i.e., when the epidermis is naked, can divide by mitosis (12). The autonomous capacity of the epidermal cells may alone explain the ability of the bag, when detached from the endocuticle, to lengthen, grow, and initially surround the first-instar larva (≈1 mm in length) and subsequently the neotenic adult female (≈1.8–3.9 cm in length). What may also shed light on epidermal development is the role of the strepsipteran in this process. Is the epidermal bag also a consequence of the release of ecdysone during the entry of the first-instar larva?
The Role of the Bag. The basal layer of the host epidermis becomes the outer layer of the bag, which is in direct contact with the hemolymph of the host. This situation might enable it to secrete and withdraw materials from the hemolymph of the host required for development and reproduction of the endoparasitic strepsipteran. The endoparasitic larva has a mouth and gut, which are lost when the female becomes a neotenic adult (11). Nutrients for the larva and later for the neotenic female (which produces ≈800,000 larvae) must pass from the hemolymph of the host to the developing endoparasitic strepsipteran through the epidermal bag.
While surrounded by the bag, the first-instar larva molts and a second-instar larva emerges from the exuvium of the first-instar through the head region. The exuvium of the first-instar remains in the bag throughout the life of the strepsipteran. The subsequent apodous larval instars, also surrounded by the bag, go through apolysis without ecdysis (10), which is highly unusual among insects. ``Ecdysless'' molting may have evolved because the growing endoparasitic strepsipteran larva becomes fairly closely fitted in its bag. Hence, the second to fourth strepsipteran larva would not be able to undergo ecdysis. However, the tiny first-instar is able to go through both apolysis and ecdysis because initially the bag fits loosely.
As Mitchenson (13) says, parasites and hosts have been playing chess with each other for millions of years, and have been in a ``continuous evolutionary arms race'' (sensu Dawkins and Krebs, ref. 14). The main defense against attacks by parasitoids in insects is the immune system. To avoid the immune system of their hosts and possible elimination through encapsulation, insect parasitoids have evolved a variety of strategies for overcoming the host immune response.
While living in the hemolymph of hosts, entomophagous larval parasitoids have active and passive mechanisms to evade encapsulation (3). Some of the active mechanisms occur as follows: (i) when the parasitoid eggs hatch in the host, the serosa disintegrates into single, large cells or tetrocytes (15, 16), or persists as an intact organ (17), which is embryonic in origin, as seen in Braconidae and Scelionidae (18–22); or, (ii) the mother injects virus-like particles into the host when laying the egg, as in Ichneumonidae, Braconidae, and Cynipidae, (23–27).
The strategy used by S. dallatorreanum that is described here is a good example of coevolution between host and parasite. It remains to be seen why this strategy, which is successfully used by S. dallatorreanum, has not been recorded in any other entomophagous parasite.
Is the strategy due to the strepsipteran's long association with its host, in the evolutionary sense? Further studies are needed to investigate: (i) whether this bag is universally present in all other strepsipteran species, and (ii) as apolysis is an event triggered by ecdysone (28), whether the first-instar larvae also secrete this hormone to trigger the detachment of the host epidermis from the endocuticle.
Acknowledgments
J.K. thanks the Papua New Guinea Oil Palm Research Association for assistance in the collection of specimens; Jenny Corrigan for the histology; Malcolm Ryder for the preparation of the plates; and Simon Hunt and the anonymous reviewers for their helpful comments. This work was supported by the Oxford University Challenge Fund, the Abraham Trust, and a Royal Society of London research grant for the purchase of the Leica MZ APO microscope and video recorder.
References
- 1.Kathirithamby, J. (1989) Syst. Entomol. 14, 41–92. [Google Scholar]
- 2.Askew, R. R. & Shaw, M. R. (1986) in Insect Parasitoids, eds. Wagge, J. & Greathead, D. (Academic, London), pp. 225–264.
- 3.Strand M. R. & Peach, L. L. (1995) Annu. Rev. Entomol. 40, 31–56. [DOI] [PubMed] [Google Scholar]
- 4.Strand, M. R. (1986) in Insect Parasitoids, eds. Wagge, J. & Greathead, D. (Academic, London), pp. 97–136.
- 5.Kathirithamby, J. (2001) Proc. R. Soc. London Ser. B 268, 2287–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kathirithamby, J., Simpson, S., Solulu, T. & Caudwell, R. (1998) Int. J. Pest Manage. 44, 127–133. [Google Scholar]
- 7.Halbert, N. R., Ross, L. D., Kathirithamby, J., Woolley, J. B., Saff, R. R. & Johnston, J. S. (2001) Tijdschr. Entomol. 144, 179–186. [Google Scholar]
- 8.Jenkin, P. & Hinton, H. E. (1966) Nature 211, 871. [DOI] [PubMed] [Google Scholar]
- 9.Hinton, H. E. (1963) Trans. Entomol. Soc. London 115, 39–61. [Google Scholar]
- 10.Kathirithamby, J., Smith, D. S., Lomas, M. B. & Luke, B. M. (1984) Zool. J. Linn. Soc. 82, 335–343. [Google Scholar]
- 11.Kathirithamby, J. (2000) Zool. J. Linn. Soc. 128, 269–287. [Google Scholar]
- 12.Neville, C. (1975) Biology of Arthropod Cuticle (Springer, Berlin).
- 13.Mitchenson, N. A. (1984) Parasitology 88, 575–577.6238273 [Google Scholar]
- 14.Dawkins, R. & Krebs, J. R. (1979) Proc. R. Soc. London Ser. B 205, 489–511. [DOI] [PubMed] [Google Scholar]
- 15.Salt, G. (1968) Biol. Rev. Camb. Philos. Soc. 43, 200–232. [DOI] [PubMed] [Google Scholar]
- 16.Salt, G. (1971) Nature 232, 639. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence, P. O. (1990) Arch. Insect Biochem. Physiol. 13, 199–216. [Google Scholar]
- 18.Jackson, D. J. (1935) Nature 135, 1040–1041. [Google Scholar]
- 19.Smith, O. J. (1952) Univ. Calif. Publ. Entomol. 9, 315–344. [Google Scholar]
- 20.Trembly, E. (1966) Boll. Lab. Ent. Agr. Filippo Silvestri 24, 209–225. [Google Scholar]
- 21.Strand M. R., Quarles, J. M., Meola, S. M. & Vinson, S. B. (1985) In Vitro Cell. Dev. Biol. 21, 361–367. [Google Scholar]
- 22.Dahlman, D. H. (1991) Biol. Control 1, 118–186. [Google Scholar]
- 23.Stoltz, D. B. & Vinson, S. B. (1979) Adv. Virus Res. 24, 125–171. [DOI] [PubMed] [Google Scholar]
- 24.Edson, K. M., Vinson, S. B., Stoltz, D. B. & Summers, M. D. (1981) Science 211, 582–583. [DOI] [PubMed] [Google Scholar]
- 25.Stoltz, D. B., Krell, P., Summers, M. D. & Vinson, S. B. (1984) Intervirology 21, 1–4. [DOI] [PubMed] [Google Scholar]
- 26.Stoltz, D. B. & Guzo, D. (1986) J. Insect Physiol. 32, 377–388. [Google Scholar]
- 27.Beckage, N. E. (1998) Parasitology 116, S57–S64. [DOI] [PubMed] [Google Scholar]
- 28.Madhaven, K. & Schneiderman, H. A. (1968) J. Insect Physiol. 14, 777–781. [Google Scholar]