Abstract
Human fossils dated between 3.5 and nearly 7 million years old discovered during the last 8 years have been assigned to as many as four new genera of the family Hominidae: Ardipithecus, Orrorin, Kenyanthropus, and Sahelanthropus. These specimens are described as having morphological traits that justify placing them in the family Hominidae while creating a new genus for the classification of each. The discovery of these fossils pushed backward by >2 million years the date of the oldest hominids known. Only two or three hominid genera, Australopithecus, Paranthropus, and Homo, had been previously accepted, with Paranthropus considered a subgenus of Australopithecus by some authors. Two questions arise from the classification of the newly discovered fossils: (i) Should each one of these specimens be placed in the family Hominidae? (ii) Are these specimens sufficiently distinct to justify the creation of four new genera? The answers depend, in turn, on the concepts of what is a hominid and how the genus category is defined. These specimens seem to possess a sufficient number of morphological traits to be placed in the Hominidae. However, the nature of the morphological evidence and the adaptation-rooted concept of what a genus is do not justify the establishment of four new genera. We propose a classification that includes four well defined genera: Praeanthropus, Ardipithecus, Australopithecus, and Homo, plus one tentative incertae sedis genus: Sahelanthropus.
In his Systema Naturae, Carolus Linnaeus (1) placed the human species into the genus Homo, though such placement conveyed a taxonomic meaning different from that of present usage. Linnaeus distinguished between Homo diurnus, with different forms corresponding to European, American, Asian, and African humans, and Homo nocturnus, corresponding to the orangutan. With the passage of time, the genus Homo acquired the connotation presently associated with this taxon, which includes only one living species, Homo sapiens, and some of its near hominid relatives. Some fossil forms now included in Homo initially received different taxonomic identifications at the genus level, such as Pithecanthropus (2), Sinanthropus (3), Africanthropus (4), Telanthropus (5), and Atlanthropus (6), among others. All of these genera designated relatively recent relatives in the human family, no older than what is currently considered the middle Pleistocene. These genera referred to either ancestors or parallel lineages that shared distinctive features with modern humans, including, notably, large brains, tool-making ability, and, speculatively, at least incipient language skills.
The appearance of Pliocene specimens from the South African site of Taung made researchers aware of the existence of another type of hominid, different in relevant respects from modern humans. The large morphological differences between the Taung fossil and Homo motivated Dart (7) to create a new genus, Australopithecus. After some initial reluctance (e.g., refs. 8–11), Australopithecus became generally accepted as a separate genus that comprised hominids with a chimpanzee-sized brain who did not make stone tools. During the ensuing decades, Australopithecus and Homo seemed adequate to encompass the taxonomic range necessary to house the human lineage; thus, all other genera were abandoned [with the exception of Paranthropus (12), accepted by a significant number of authors as a genus corresponding to robust australopithecines]. Proposals for new genera, such as Zinjanthropus (13) and Paraustralopithecus (14) were also eventually discarded.§
The scenario of hominids being represented by only a few genera has critically changed in recent years with the discovery of very early hominid specimens with ages between 3.5 and 7 million years (Myr). These newly discovered specimens are sufficiently informative, according to their describers, to support the proposal of four new genera: Ardipithecus,¶ Orrorin (20), Kenyanthropus (21), and Sahelanthropus (22). The subsequent increase from three to seven hominid genera in the few years from 1995 to the present constitutes an exceptional event in hominid systematics. The questions that need to be asked are whether these recently discovered specimens are in fact hominids, and whether their different characteristics justify the creation of four new genera.
What Is a Hominid?
Three of the new genera are represented by specimens much older than previously known hominid fossils and raise questions about the timing of the split between the lineages leading to humans (Homo) and chimpanzees (Pan). The so-called “late-divergence hypothesis” (23), which is based on molecular studies, suggests that the separation may have taken place no more than 4 or 5 Myr ago. Recent analyses of genes (and other DNA sequences) support a date 5–6 Myr ago (24–27), although some authors place the divergence between Pan and Homo as early as 10.5 Myr ago (28). The genera Orrorin and Sahelanthropus support dates close to 7 Myr or older, provided that these specimens pertain to the human lineage (after the divergence). The authors who have proposed these two new genera have no doubt of their place in the human lineage (20, 22).
A commonly accepted, two-pronged criterion for including a certain specimen in the hominid family was proposed by Pilbeam (29): habitual upright bipedalism as the chief method of loco-motion and teeth that are essentially human in form.
Sahelanthropus, Orrorin, and Ardipithecus have been described or inferred as having been bipedal, at least to a certain extent. The holotype of Sahelanthropus, TM 266-01-060-1, is an almost complete cranium. Although it is damaged in the occipital area, the foramen magnum is longer than it is wide and not at all rounded as is typical of Pan (22). The basioccipital is described as “correlatively short and shaped like a truncated triangle as in Ardipithecus” (22), but not as triangular as in other early hominids. The available information seems insufficient to infer reliably whether Sahelanthropus was a habitual biped, but, the authors claim, “such an inference would not be unreasonable given the skull's other basic cranial and facial similarities to later fossil hominids that were clearly bipedal” (22).∥
The two left femora described for Orrorin tugenensis (BAR 1002-00 and BAR 1003-00) have been interpreted as indicating that it “was already adapted to habitual or perhaps even obligate bipedalism when on the ground” (20). The shortened basioccipital component of the cranial bone for Ardipithecus from Aramis is similar to that of other hominids and unlike that of chimpanzees (18). This interpretation is corroborated by a proximal foot phalange, AME-VP-1/71, later found in Middle Awash (Ethiopia), described as “derived relative to all known apes and... consistent with an early form of terrestrial bipedality” (32).
The second hominid feature identified by Pilbeam (29) is a distinctive dentition. The small canines of Sahelanthropus, such as TM 266-01-060-1 (holotype) and TM 266-02-154-2, are derived features bringing the specimen close to later hominids; the thickness of the postcanine enamel of Sahelanthropus is intermediate, thicker than that of chimpanzees but thinner than that of later australopithecines (22).
The molars of Orrorin tugenensis “are smaller than those of australopithecines and are closer in size to those of Ardipithecus” (20). The molar enamel of Orrorin in the upper central incisor and in the lower cheek teeth is “thick,” as in other hominids, but other features of the incisors and canines, and the lower pre-molar P4, are less hominid-like and more ape-like.
The dentition of Ardipithecus is ambiguous and has revived the argument of whether enamel thickness is a defining feature of hominid lineages. The Aramis specimens have thin molar enamel (18), whereas the other hominids show thick tooth enamel. Chimpanzees and gorillas have thin enamel, whereas orangutans show an intermediate thickness (33). Moreover, the Ardipithecus fossils from Middle Awash, which are 0.8–1.4 Myr older than those found at Aramis, have challenged the enamel characteristics attributed to the genus. The enamel characteristics of Ardipithecus ramidus kadabba are incomplete, but it has been proposed that the available broken and little-worn teeth suggest a molar enamel thickness similar to or slightly greater than those of the younger Aramis samples of Ar. ramidus (32). In any case, the significance of tooth enamel is not definitive, but should be considered together with tooth shape and consideration of diet.
The features related to bipedalism and those of the dentition follow a mosaic evolutionary trend, showing different mixtures of primitive and derived morphological features in all early hominids from Orrorin and Sahelanthropus to the Rift Valley (Praeanthropus africanus) and the South African (Australopithecus africanus) australopithecines. This mixture is hardly unexpected, because evolution is a gradual process (albeit rate-variable) and these Miocene specimens are close to the proposed time of divergence of chimpanzees and humans. But a mixture of primitive and derived characters poses a problem. The phylogenetic definition of a certain taxon will depend on whether we emphasize the primitive features or the derived features. Thus, the authors who documented the new Miocene findings claim “hominidness” for their specimens and at the same time cast doubt on the hominid status of specimens discovered by others. Haile-Selassie (32), who asserts the hominidness of Ardipithecus, writes about Orrorin: “There is nothing to preclude Orrorin from representing the last common ancestor, and thereby antedating the cladogenesis of hominids. It is equally plausible that it represents a previously unknown African hominoid with no living descendants, or an exclusive precursor of chimpanzees, gorillas or humans.” Brunet et al. (22), who promote Sahelanthropus as a hominid, note the similarity between the upper canine of Orrorin “and that of a female chimpanzee.” But the discoverers of Orrorin argue that “small, thick enameled molars are an archaic feature for the hominid lineage, which is retained in Homo,” whereas Ardipithecus “has thin enameled cheek teeth, which may be a derived feature for the Gorillidae” (20).
The important issue at hand is not primarily whether we may want to classify a particular taxon as being a hominid or not, but rather whether the specimens included in that taxon are more recently related to present humans than to chimpanzees. For the nominalist taxonomist, it is quite arbitrary to include or exclude apes from the family Hominidae, as first defined by Gray in 1825. But the historically substantive issues, and the ones cladists or other modern taxonomists want to answer, are as follows: Is Ardipithecus part of the chimpanzee lineage? Is Orrorin a common ancestor of both humans and chimpanzees? Is Sahelanthropus an early hominid that lived outside the Rift Valley, or is it an ape, as proposed by Wolpoff et al. (30)?
There are additional meaningful taxonomic questions, such as whether it is appropriate to create a new genus for specimens that are not particularly different from those included in an already established genus. The issues relevant to such questions, namely how to reconstruct (and test) a phylogeny and what should constitute a new genus, are related but distinct.
What Is a Genus? The Cladistic Criterion
Even before the recent discovery of hominid specimens and the proposal of four new genera for them (Ardipithecus, Orrorin, Kenyanthropus, and Sahelanthropus), the simple, classic three-genus scheme (Australopithecus, Paranthropus, Homo) encountered difficulties with regard to adequately including all known specimens assigned to the Hominidae. As far as possible, a genus is supposed to be monophyletic; that is to say, it must contain only species that form a clade (a complete lineage sharing a common ancestor within the family's evolutionary tree). This is an unrealistic taxonomic expectation, given the fact that phylogeny is a continuum, with the exception of terminal twigs or extant taxa (34). Furthermore, there is evidence already available that indicates, at least to some authors, that all three genera are paraphyletic (containing species that belong to different lineages): Australopithecus (15), Paranthropus (34), and Homo (16).
Cladistic analyses seeking to determine the branching relationships of given taxa follow a series of ordered steps. For example, in their reappraisal of early hominid phylogeny, Strait et al. (15) proceeded in this way: (i) nine species (from Australopithecus afarensis to H. sapiens) were selected; (ii) in each taxon, all specimens that formed the hypodigm (i.e., the entire fossil record considered for a given taxon) were included; (iii) the characters of each hypodigm were analyzed; and (iv) the best cladogram was selected on the basis of computer algorithms that use a parsimony criterion that chooses the one with the fewest steps and that minimizes the number of convergent traits that evolve independently in each clade. This procedure, of course, assumes that all of the taxa examined are the results of lineage splitting and do not represent stages in a lineage (35).
Claims of objectivity notwithstanding, the matter of which cladogram is “best” depends largely on the decisions made at each step. But no step is free from difficulties. For example, does the fragmentary cranial vault and face KNM-ER 2602 of Koobi Fora (Kenya) pertain to the hypodigm of A. afarensis or to that of Australopithecus boisei? Do Homo habilis and Homo rudolfensis represent one or two species? Does H. habilis exist at Sterkfontein (South Africa) or must the Stw 53 and Sts 19 specimens be regarded as A. africanus?
The way in which the species are chosen and the hypodigms are constructed leads to very different cladograms. In their revision of the genus Homo, Wood and Collard (16) evaluated six different cladistic studies to elucidate the monophyly of Homo. Three studies concluded that Homo is monophyletic (i.e., holophyletic), whereas the remaining three suggested that it is paraphyletic. The authors (16) concluded on the basis of their own morphological, functional, and developmental analyses that H. habilis and H. rudolfensis cannot reliably be assumed to be more closely related to H. sapiens than to the australopiths, and thus proposed that H. habilis and H. rudolfensis should be removed from Homo if monophyly is to be preserved. This proposal, however, leads to another problem. If H. habilis and H. rudolfensis cannot be classified in the genus Homo, the only alternatives are to include them in Australopithecus or to create a new genus for them. The first option would increase the paraphyly in Australopithecus even more than already occurs, whereas the second alternative would excessively increase the number of hominid genera.
Other cladistic analyses often lead to a similar dilemma in human phylogeny. However, one way out of the dilemma might be to rely on the assumption that the first species of any new genus conserves some primitive features close to those of the previous ancestral genus. We will adopt this convention in this article to avoid (or, at least, minimize) the two horns of the dilemma: too many genera and paraphyly. Our proposal is to identify the early species of a new genus as the species germinalis of the genus, even though these species do not meet all of the defining characteristics of the genus, thus eliminating the need to include them in the previous genus or to create a new one. Species germinalis, because of their intermediate features, must not be included in computational cladistic analyses (17).
Genus as Single Adaptive Zone
Beyond cladistic issues, the matter of a genus being a taxon that occupies a “single adaptive zone,” as defined by Mayr (36), has been applied to hominid taxa (e.g., ref. 16). This evolutionary and ecological concept of the genus leads to identification of three hominid genera, corresponding to three distinctive adaptive zones: (i) Australopithecus, encompassing the first hominids that gradually developed bipedalism; (ii) Paranthropus, the evolutionary branch (incorporating the robust australopithecines) that colonized the open spaces of the savanna with specialized feeding on hard vegetables (12); and (iii) Homo, the branch that evolved large brains and retained from Australopithecus gracile features, used stone tools, and developed a more carnivorous diet. Presently, we encounter four newly proposed genera for recently discovered fossils: Ardipithecus, Orrorin, Kenyanthropus, and Sahelanthropus. Do these taxa represent new ancestors of modern humans that occupied distinctive adaptive zones?
The Aramis specimens of Ardipithecus have been described as exhibiting incipient bipedalism while retaining a tree-climbing ability, so that they were still largely “ecological apes” (37). Other specimens of Ardipithecus also fit this description. The humerus of Ar. ramidus kadabba has “a relatively sharp lateral trochlear crest” similar to that of modern apes and some A. afarensis, whereas the proximal foot phalanx from Amba, dated at 5.2 Myr, is clearly derived relative to all known apes and suggests an early form of terrestrial bipedality (32). The lack of Sahelanthropus postcranial remains prevents establishment of the degree of its bipedalism, though the cranium clearly shows a mosaic of derived and primitive ape-like features. The most informative anatomical features of the Sahelanthropus tchadensis cranium are in the face, which shows a mosaic of primitive and derived features. “The face is tall with a massive brow ridge, yet the mid-face is short (in the superoinferior dimension) and less prognathic than in Pan or Australopithecus. This unusual combination of features is in turn associated with a relatively long braincase, comparable in size to those of extant apes” (22).
The authors describing the Miocene exemplars of Ardipithecus and Sahelanthropus claim that the mosaic combination of their specimens' features is unique, thus justifying the proposal of a new genus in each case. The issue here is to determine to what extent these differences justify the naming not only of a new species, but also of a new genus. In fact, none of the new Miocene specimens clearly reflects an exclusive and peculiar single adaptive zone. The most significant difference between them and the later, smaller Australopithecus is size. Indeed, the specimens ARA-VP-7/2 (Ar. ramidus ramidus) and ASK-VP-3/78 (Ar. ramidus kadabba) are larger than the nonrobust australopithecines, such as A. afarensis AL 288-1m and AL 322-1 (32). Consideration, in particular, of Ar. ramidus kadabba specimens supports, in our view, the inclusion of Ardipithecus in the family Hominidae, although its evolutionary relationships with Praeanthropus and Australopithecus remain obscure. We tentatively propose placing Sahelanthropus in a new genus (incertae sedis), but without including it in the same subfamily (or genus) as the more definitively bipedal Ardipithecus, Orrorin, and Australopithecus.
The humerus and femur of Orrorin are 1.5 times larger than those of A. afarensis AL 288-1 (Lucy), which confirms that early human ancestors were larger than previously believed (20). Orrorin's femora indicate that it was a biped when on the ground, while its humerus and manual phalanx show that it retained some arboreal adaptations (20). We propose that the specimens described as Orrorin may be classified within the genus Praeanthropus, which also includes A. afarensis of Johanson et al. (38) and other late, nonrobust australopithecines, as belonging to the same adaptive zone.
Kenyanthropus platyops (21) of Lomekwi (West Turkana, Kenya), aged at 3.5 Myr, represents a different case. The holotype KNM-WT 40000 combines a cranium with a capacity in the range of the australopithecines, a flat transverse facial contour at a level just below the nasal bones, and a tall malar region with a zygomaticoalveolar crest low and curved. The result is an orthognathic (flat) face, very different from the prognathic (protruding) face of the nonrobust australopithecines, and similar to that of Paranthropus and H. rudolfensis. But in the case of robust hominids (Paranthropus), the flattened face is associated with molar megadonty, whereas Kenyanthropus has small cheek teeth. More generally, Kenyanthropus lacks almost all of the derived features of Paranthropus, showing a unique pattern of facial and dental morphology that suggests a distinct dietary adaptive zone (21). Consequently, Kenyanthropus is best placed in a genus different from Paranthropus. It has been suggested that the particular morphology of the KNM-WT 40000 specimen could be the result of a distortion caused by “artifacts of postmortem fossilization processes” (39). However, there seem to be other reasons to distinguish KNM-WT 40000 from the australopithecines. The thinner enamel and smaller teeth of Kenyanthropus bring it closer to the Homo exemplars of the Plio-Pleistocene (H. habilis and, mainly, H. rudolfensis), although it lacks the more advanced features of Homo that appear with Homo erectus and Homo ergaster. Not surprisingly, Lieberman (40) asserts that this new taxon “will act as a sort of party spoiler, highlighting the confusion that confronts research into evolutionary relationships.” We propose, conservatively, to include K. platyops in the genus Homo, but as species germinalis (see below), on account of its lacking some of the distinctive features of later Homo.
As noted by Tobias (41), there has recently been an inclination toward creating new hominid species and genera based on samples from just one or two sites. Indeed, recent finds bridge the gap between taxa that were, until now, considered as different. KGA10-525 (42) supposedly links Paranthropus aethiopicus, Paranthropus robustus, and Paranthropus boisei. The “Daka” calvaria (43) purportedly brings H. ergaster closer to H. erectus. OH 65, the latest Olduvai discovery of H. habilis, is claimed to cast “doubts on Homo rudolfensis as a biologically valid taxon” (44). In any case, for the purpose of determining the number and definition of hominid genera, there is no need to decide whether one or more species should be distinguished in each of these cases.
H. ergaster and H. erectus raise no cladistic problems about including them in the same genus, but morphological (and cultural) considerations may favor considering them as separate species. The “robust” Australopithecus/Paranthropus lineage raises some difficulties about including all of the taxa in a single, monophyletic group (34), though Grine and Martin (45) have offered morphological reasons for their inclusion in a single lineage.
H. rudolfensis could be reduced to “a taxonomic junior synonym of Homo habilis” (41), but one must, then, take into account the strong similarities of KNM-ER 1470 (H. rudolfensis) with KNM-WT 40000 (K. platyops). One possibility would be placing all three taxa (H. habilis, H. rudolfensis, and K. platyops) into the genus Australopithecus, as has been proposed for H. habilis and H. rudolfensis (16, 46). However, this solution would greatly increase the paraphyly of Australopithecus. A second alternative would be to include the three taxa within a separate genus, Kenyanthropus. But the current state of knowledge makes it difficult to assign KNM-ER 1470 (H. rudolfensis) to this genus unless H. habilis is also moved to Kenyanthropus (21, 47).
We agree with the view that K. platyops, H. habilis, and H. rudolfensis should be classified together, but we propose to include them within the genus Homo. H. habilis and H. rudolfensis share the morphological traits that, in 1964, led Leakey, Tobias, and Napier to define Homo as a new taxon. Although K. platyops (KNM-WT 40000) “does not show the derived features associated with Homo (excluding H. rudolfensis and H. habilis)” (21), it may be considered as the earliest member (i.e., species germinalis) of the genus, which would also include H. habilis and H. rudolfensis as early forms of the lineage that adapted to the savanna by means of a nonspecialized diet, eventually developing lithic tools for scavenging and hunting purposes. This proposal places the appearance of the genus Homo as early as 3.5 Myr ago.
Phylogeny of the Hominidae
The reconstruction of human phylogeny is fraught with seemingly insurmountable obstacles, especially if one seeks to employ only a reasonable number of hominid genera while abiding by the cladistic objective of a classification to encompass only monophyletic lineages. The obstacles come, first and foremost, from the great gaps in the fossil record. Our lack of knowledge of the ancestors in the chimpanzee lineage, for example, handicaps the task of establishing the accurate place for taxa close to the divergence of humans and chimpanzees, such as Orrorin, Sahelanthropus, and Ardipithecus. But other obstacles exist that cannot be overcome simply by the discovery of new exemplars. Our present methods do not permit an unambiguous determination of the ancestor–descendant relationship between any two given taxa. We can only identify, within certain limits, sister groups, i.e., taxa immediately proceeding from a common ancestor (which, again, cannot be identified). An additional difficulty lies in identifying similarity among taxa that are close to any point of divergence. It seems impossible at present to determine beyond any doubt where to fit the new Miocene specimens. The best we can do is to assign them tentative places in the phylogeny, subject to eventual revision with the discovery of new specimens.
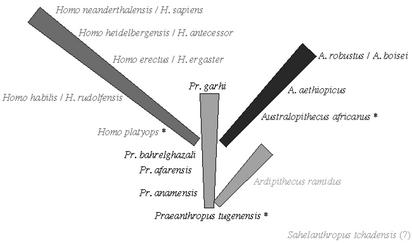
The new fossil findings warrant two conclusions: first, the diversity of lineages among very early hominids is greater than previously believed; moreover, their geographical range has been expanded with the discovery of Sahelanthropus from Chad, west-central Africa. Nevertheless, and until new evidence makes it possible to decide on the degree of bipedalism of Orrorin, Sahelanthropus, and Ardipithecus, a parsimonious option would be to retain only four genera in Hominidae: Praeanthropus, Ardipithecus, Australopithecus, and Homo (plus one genus incertae sedis, whose definitive status can only later be determined) (Fig. 1). A tentative outline of hominid evolution involving four major (genus-determining) episodes is shown in Table 1.
Fig. 1.
Phylogeny of the genera belonging to the Hominid family. Four different genera are proposed, corresponding to four kinds of adaptation. The genus Praeanthropus (formerly named Australopithecus) evolved incipient bipedalism on the ground of tropical forests. The genus Ardipithecus evolved a dietary adaptation that developed thin molar enamel, similar to that of the African great apes. The genus Australopithecus (formerly also named Paranthropus) exploited hard vegetal resources of the savanna by developing a robust masticatory apparatus. The genus Homo retained gracile maxillae and dentition and later initiated the development of larger crania and cultural adaptation to the savanna by means of lithic industries. The proposed names follow the rules of taxonomy, favoring the names given initially, when the first taxon of the genus was established. The phylogenetic location and taxonomic classification of Sahelanthropus are uncertain. Pairs of Homo taxa separated by a slash represent closely related (or, according to some authors, the same) species. An asterisk marks each species germinalis, i.e., originating a genus.
Table 1. Modification of previous taxonomies (32, 33), including formal taxonomic designations and reference to geological and geographical ranges.
| Family Hominidae Gray, 1825. Miocene to the present, worldwide. |
| Genus †SahelanthropusBrunet et al., 2002, incertae sedis. Miocene, Central Africa. |
| Species †Sahelanthropus tchadensisBrunet et al., 2002. Miocene, Central Africa. |
| Subfamily Preanthropinae Cela-Conde and Altaba, 2002. Miocene-Pliocene, Africa. |
| Genus †PraeanthropusSenyürek 1955 (includes OrrorinSenut et al., 2001). Miocene-Pliocene, Africa. |
| Species †Praeanthropus tugenensis*Senut et al., 2001. Miocene, East Africa. |
| Species †Praeanthropus africanus Weinert, 1950 (= Australopithecus afarensisJohanson et al. 1978). Pliocene, East Africa. |
| Species †Praeanthropus bahrelghazaliBrunet et al., 1996. Pliocene, Subsahara. |
| Species †Praeanthropus anamensisM. G. Leakey et al., 1995. Pliocene, East Africa. |
| Species †Praeanthropus garhiAsfaw et al., 1999. Pliocene, East Africa. |
| Genus †ArdipithecusWhite et al., 1995. Miocene-Pliocene, East Africa. |
| Species †Ardipithecus ramidusWhite et al., 1994. Miocene-Pliocene, East Africa. |
| Subfamily Australopithecinae Gregory and Hellman, 1939. Pliocene, Africa. |
| Genus †AustralopithecusDart 1925 (includes PlesianthropusBroom, 1938; ParanthropusBroom, 1938; Zinjanthropus L. S. B. Leakey, 1959; and Paraustralopithecus Arambourg and Coppens, 1967). Pliocene, Africa. |
| Species †Australopithecus africanus*Dart, 1925. Pliocene, Africa. |
| Species †Australopithecus aethiopicusArambourg and Coppens, 1968. Pliocene, East Africa. |
| Species †Australopithecus boisei L. S. B. Leakey, 1959. Pliocene-Pleistocene, East Africa. |
| Species †Australopithecus robustusBroom, 1938. Pleistocene, southern Africa. |
| Subfamily Homininae Gray, 1825. Pliocene to the present, worldwide. |
| Genus HomoLinnaeus, 1758 (includes PithecanthropusDubois, 1894; Protanthropus Haeckel, 1895; SinanthropusBlack, 1927; Cyphanthropus Pycraft, 1928; AfricanthropusDreyer, 1935; TelanthropusBroom and Robinson, 1949; Atlanthropus Arambourg, 1954; Tchadanthropus Coppens, 1965; and KenyanthropusM. G. Leakey et al., 2001). Pliocene to the present, worldwide. |
| Species †Homo platyops*M. G. Leakey et al., 2001. Pliocene, East Africa. |
| Species †Homo rudolfensis Alexeev, 1986. Pliocene, Africa. |
| Species †Homo habilis L. S. B. Leakey et al., 1964. Pliocene, Africa. |
| Species †Homo ergaster Groves and Mazák, 1975. Plio-Pleistocene, Africa and ? Eurasia. |
| Species †Homo erectus Dubois, 1892. Pleistocene, Africa and Eurasia. |
| Species †Homo antecessor Bermúdez de Castro et al., 1997. Plio-Pleistocene, Europe and ? Africa. |
| Species †Homo heidelbergensis Schoetensack, 1908. Pleistocene, Africa and Eurasia. |
| Species †Homo neanderthalensis King, 1864. Pleistocene, western Eurasia. |
| Species Homo sapiensLinnaeus, 1758. Pleistocene to the present, worldwide. |
| ?, uncertainty about the taxon's presence in that region |
, Taxon is extinct
, taxon considered species germinalis (32), i.e., a species originating a genus
The divergence of the chimpanzee and human lineages occurred close to 7 Myr ago. The early hominid species occupy a single adaptive zone, which involves gradual colonization of the ground by bipedalism, while preserving notable tree-climbing abilities. These species are included in a single genus, which according to the preference rules of taxonomy, would be Praeanthropus (48). East and central African australopithecines (A. afarensis, ref. 38; A. anamensis, ref. 49; A. bahrelghazali, ref. 50; A. garhi, ref. 51) are included in this genus, as is Orrorin, which, despite some doubts about its bipedalism (32), possesses enough derived features to be included in Praeanthropus. With regard to other Miocene specimens, the alternatives are to keep them in Praeanthropus if their bipedalism is confirmed, or to place them in a genus incertae sedis if they are understood to be too close to the divergence process. The absence of Sahelanthropus postcranial remains (together with its geographic location) necessitates recognition of this taxon as incertae sedis. Regarding Ardipithecus, bipedalism inferred from the postcranial remains of Ar. ramidus kadabba favors placing it in Hominidae, but the presence of thin tooth enamel in the later Ar. ramidus sets this taxon apart from the australopithecines.
A divergence episode close to 3.5 Myr ago separates robust and gracile lineages, which are also characterized by different dietary adaptive zones. The robust lineage entails the appearance of Australopithecus, whereas the genus Homo corresponds to the gracile lineage. The most parsimonious solution consists of placing all gracile specimens in the same genus, which according to the taxonomic rules, must be Homo. H. habilis and H. rudolfensis (formerly considered also as Kenyanthropus rudolfensis; ref. 21) would represent an anagenetic evolutionary advance relative to the species Kenyanthropus platyops, which would also be included in Homo, as the stem species (species germinalis) of the genus.
Finally, there was an increase of the robust and gracile tendencies ≈2.5 Myr ago. The robust lineage evolved megadonty adapted to eating hard vegetable materials, while the gracile lineage incorporated a more carnivorous diet with the development of stone-tool manufacture and large crania. Both tendencies can be considered evolutionary events that do not, at present, call for an assessment of either a cladistic or an anagenetic differentiation of genera. The classification we favor is conservative, and new discoveries may make it advisable to recognize the existence of additional genera, particularly with regard to our very ample, anagenetically stretched genus Homo.
Acknowledgments
We thank Profs. Jeffrey H. Schwartz and Frederick S. Szalay for valuable comments.
Abbreviation: Myr, million years.
Footnotes
A minority of authors prefer to use Praeanthropus as the name for the Rift and Tchad specimens grouped in Australopithecus (15–17), in accordance with the preference rules of taxonomy, but this does not change the current prevailing consideration of only three genera.
White and colleagues first classified their Aramis fossils as Australopithecus ramidus (18), but later (19) proposed a new generic classification as Ardipithecus, on the grounds that ramidus is likely to be the sister taxon of the hominid clade rather than a direct ancestor of Homo.
Wolpoff et al. (30), based on a consideration of the morphology of the masticatory apparatus, face, and skull, differ on the alleged bipedalism of Sahelanthropus. They opine that Sahelanthropus “was an ape living in an environment that was later inhabited by australopithecines.” For a response defending the phylogenetic position of Sahelanthropus as a hominid, see ref. 31.
References
- 1.Linnaeus, C. (1735; 10th and definitive edition, 1758) Systema Naturae Naturae Regna Tria, Secundum Classes, Ordines, Genera, Species cum Characteribus, Synonymis, Locis (Laurentii Sylvii, Stockholm).
- 2.Dubois, E. (1894) Pithecanthropus erectus: Eine menschanähnliche Übergangs-form aus Java (Landsdruckerei, Batavia).
- 3.Black, D. (1927) Paleontol. Sin. Ser. D 7, 1-29. [Google Scholar]
- 4.Dreyer, T. F. (1935) Kon. Ned. Akad. Wet. 38, 118-128. [Google Scholar]
- 5.Broom, R. & Robinson, J. T. (1949) Nature 164, 322-323. [DOI] [PubMed] [Google Scholar]
- 6.Arambourg, C. (1955) Am. J. Phys. Anthropol. 13, 191-202. [Google Scholar]
- 7.Dart, R. (1925) Nature 115, 195-199. [Google Scholar]
- 8.Elliot Smith, G. (1925) Nature 115, 235. [Google Scholar]
- 9.Keith, A. (1925) Nature 115, 234-235. [Google Scholar]
- 10.Woodward, A. (1925) Nature 115, 235-236. [Google Scholar]
- 11.Duckworth, W. J. H. (1925) Nature 115, 236. [Google Scholar]
- 12.Broom, R. (1938) Nature 142, 377-379. [Google Scholar]
- 13.Leakey, L. (1959) Nature 184, 491-493.13850382 [Google Scholar]
- 14.Arambourg, C. & Coppens, Y. (1968) S. Afr. J. Sci. 64, 58-59. [Google Scholar]
- 15.Strait, D. S., Grine, F. E. & Moniz, M. A. (1997) J. Hum. Evol. 32, 17-82. [DOI] [PubMed] [Google Scholar]
- 16.Wood, B. & Collard, M. (1999) Science 284, 65-71. [DOI] [PubMed] [Google Scholar]
- 17.Cela-Conde, C. J. & Altaba, C. R. (2002) S. Afr. J. Sci. 98, 229-232. [Google Scholar]
- 18.White, T. D., Suwa, G. & Asfaw, B. (1994) Nature 371, 306-312 [DOI] [PubMed] [Google Scholar]
- 19.White, T. D., Suwa, G. & Asfaw, B. (1995) Nature 375, 88. [DOI] [PubMed] [Google Scholar]
- 20.Senut, B., Pickford, M., Gommery, D., Mein, P., Cheboi, K. & Coppens, Y. (2001) C. R. Acad. Sci. Ser. IIa 332, 137-144. [Google Scholar]
- 21.Leakey, M. G., Spoor, F., Brown, F. H., Gathogo, P. N., Kiarie, C., Leakey, L. N. & McDougall, I. (2001) Nature 410, 433-440. [DOI] [PubMed] [Google Scholar]
- 22.Brunet, M., Guy, F., Pilbeam, D., Mackaye, H. T., Likius, A., Ahounta, D., Beauvilain, A., Blondel, C., Bocherens, H., Boisserie, J. R., et al. (2002) Nature 418, 145-151. [DOI] [PubMed] [Google Scholar]
- 23.Sarich, V. & Wilson, A. C. (1967) Science 158, 1200-1203. [DOI] [PubMed] [Google Scholar]
- 24.Horai, S., Hayasaka, K., Kondo, R., Tsugane, K. & Takahata, N. (1995) Proc. Natl. Acad. Sci. USA 92, 532-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen, F. C. & Li, W. H. (2001) Am. J. Hum. Genet. 68, 444-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stauffer, R. L., Walker, A., Ryder, O. A., Lyons-Weiler, M. & Hedges, S. B. (2001) J. Hered. 92, 469-474. [DOI] [PubMed] [Google Scholar]
- 27.Nei, N. & Glakko, G. V. (2002) J. Hered. 93, 157-164. [DOI] [PubMed] [Google Scholar]
- 28.Arnason, U., Gullberg, A., Burguete, A. S. & Janke, A. (2000) Hereditas 133, 217-228. [DOI] [PubMed] [Google Scholar]
- 29.Pilbeam, D. R. (1968) Nature 219, 1335-1338. [DOI] [PubMed] [Google Scholar]
- 30.Wolpoff, M. H., Senut, B., Pickford, M. & Hawks, J. (2002) Nature 419, 581-582. [DOI] [PubMed] [Google Scholar]
- 31.Brunet, M. (2002) Nature 419, 582 (lett.). [Google Scholar]
- 32.Haile-Selassie, Y. (2001) Nature 412, 178-181. [DOI] [PubMed] [Google Scholar]
- 33.Martin, L. B. (1985) Nature 314, 260-263. [DOI] [PubMed] [Google Scholar]
- 34.Wood, B. A. (1988) in Evolutionary History of the “Robust” Australopithecines, ed. Grine, F. E. (de Gruyter, New York), pp. 269-284.
- 35.Szalay, F. S. (2001) Ludus Vitalis 15, 143-169. [Google Scholar]
- 36.Mayr, E. (1950) Cold Spring Harbor Symp. Quant. Biol. 15, 109-118. [DOI] [PubMed] [Google Scholar]
- 37.Andrews, P. (1995) Nature 376, 555-556. [DOI] [PubMed] [Google Scholar]
- 38.Johanson, D., White, T. & Coppens, Y. (1978) Kirtlandia 28, 1-14. [Google Scholar]
- 39.White, T. (2003) Science 299, 1994-1997. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman, D. E. (2001) Nature 410, 419-420. [DOI] [PubMed] [Google Scholar]
- 41.Tobias, P. V. (2003) Science 299, 1193-1194. [DOI] [PubMed] [Google Scholar]
- 42.Suwa, G., Asfaw, B., Beyene, Y., White, T. D., Katoh, S., Nagaoka, S., Nakaya, H., Uzawa, K., Renne, P. & WoldeGabriel, G. (1997) Nature 389, 489-492. [DOI] [PubMed] [Google Scholar]
- 43.Asfaw, B., Glibert, W. H., Beyene, Y., Hart, W. K., Renne, P. R., WoldeGabriel, G., Vrba, E. S. & White, T. D. (2002) Nature 416, 317-320. [DOI] [PubMed] [Google Scholar]
- 44.Blumenschine, R. J., Peters, C. R., Masao, F. T., Clarke, R. J., Deino, A. L., Hay, R. L., Swisher, C. C., Stanistreet, I. G., Ashley, G. M., McHenry, L. J., et al. (2003) Science 299, 1217-1221. [DOI] [PubMed] [Google Scholar]
- 45.Grine, F. E. & Martin, L. B. (1988) in Evolutionary History of the “Robust” Australopithecines, ed. Grine, F. E. (de Gruyter, New York), pp. 3-42.
- 46.Wood, B. & Collard, M. (1999) Evol. Anthropol. 8, 195-207. [Google Scholar]
- 47.Aiello, L. & Collard, M. (2001) Nature 410, 526-527. [DOI] [PubMed] [Google Scholar]
- 48.Senyürek, M. (1955) Belleten 19, 1-57. [Google Scholar]
- 49.Leakey, M. G., Feibel, C. S., McDougall, I. & Walker, A. (1995) Nature 376, 565-572. [DOI] [PubMed] [Google Scholar]
- 50.Brunet, M., Beauvilain, A., Coppens, Y., Heintz, E., Moutaye, A. H. E. & Pilbeam, D. (1996) C. R. Acad. Sci. Ser. IIa 322, 907-913. [Google Scholar]
- 51.Asfaw, B., White, T., Lovejoy, O., Latimer, B., Simpson, S. & Suwa, G. (1999) Science 284, 629-635. [DOI] [PubMed] [Google Scholar]