Abstract
IFN-γ is well known as the signature cytokine of CD4+ T helper 1, CD8+, and natural killer cells, but recent studies demonstrate that antigen-presenting cells, in particular dendritic cells (DCs), are another potent source for this proinflammatory cytokine. T-bet, a transcription factor that controls IFN-γ expression in CD4+ T cells, was reported recently to be expressed in human monocytes and myeloid DCs. In this study we investigate the role of T-bet in this important cell type. The development, differentiation, and activation of bone marrow and splenic DCs were unimpaired in mice lacking T-bet. However, T-bet was essential for the optimal production of IFN-γ by both CD8α+ and CD8α- DCs. T-bet-deficient DCs were significantly impaired in their capacity to secrete IFN-γ after both stimulation with IL-12 alone or in combination with IL-18. Further, T-bet-/- DCs were impaired in their ability to activate the T helper 1 program of adoptively transferred antigen-specific T cells in vivo. The rapid up-regulation of T-bet by IFN-γ in DCs coupled with a function for DC-derived IFN-γ in T cell activation may constitute a positive feedback loop to maximize type 1 immunity.
Dendritic cells (DCs) are professional antigen-presenting cells with a remarkable capacity to activate naive T cells (1, 2). They are widely distributed in both lymphoid and nonlymphoid sites and have evoked much interest by their potent capacity to capture antigen and present it as diverse peptides to CD4+ and CD8+ T cells to initiate a primary immune response. This ability to specifically recognize, acquire, and deliver antigen from the periphery to secondary lymphoid organs makes DCs an important link between innate and adaptive immunity.
Both human and mouse DCs have been divided into subsets, on the basis of phenotypic markers, that carry out different functions in driving the expansion of T helper (Th) subsets and achieving tolerance of self-antigens (3–5). One mechanism by which they accomplish the former is the differential secretion of cytokines such as IL-12 and IL-10 as well as the differential expression of surface receptors such as Toll-like receptor 4 (6). It was discovered recently that DCs also produce IFN-γ (7–13), a pleiotropic cytokine essential for both innate and adaptive immunity that acts by binding to a widely expressed IFN-γ receptor (14, 15). Most IFN-γ responses are coupled to the Janus tyrosine kinase (Jak)-signal transducers and activators of transcription (STAT) signaling pathway via the protein tyrosine kinases Jak1 and Jak2 and the transcription factor Stat1 (16). Mice lacking IFN-γ, the IFN-γ receptor, or Stat1 have pro-foundly impaired innate and adaptive immunity leading to death from infection (17–19), whereas humans with inactivating mutations in components of the IFN-γ signaling pathway die from overwhelming atypical mycobacterial infection in childhood (20).
T-bet, a T-box family member, is a transcription factor essential for lineage commitment of CD4+ Th lymphocytes (21, 22). Retroviral gene transduction of T-bet into primary T cells or developing Th2 cells results in activation of IFN-γ production (21), whereas mice lacking T-bet fail to develop Th1 cells and display a dramatic reduction of IFN-γ production by CD4+ T cells (22). In this study we demonstrate that T-bet is expressed in DCs at levels comparable with Th1 cells and is necessary for the optimal production of IFN-γ and optimal activation of antigen-specific Th1 cells.
Materials and Methods
Mice. C57BL/6 (B6), BALB/c, 129/S6, and Stat1-/- mice (4–8 weeks old) were purchased from Taconic Farms. The generation and screening of T-bet-deficient mice have been described (22, 23), and mice used here have been backcrossed at least six generations onto the B6 and BALB/c backgrounds.
Cell Lines and Cytokines. Granulocyte/macrophage colony-stimulating factor (GM-CSF) to generate bone marrow-derived DCs (bmDCs) was produced as culture supernatant from a mouse macrophage cell line (J558L) transfected with the mouse GM-CSF gene (gift of I. Mellman, Yale University, New Haven, CT). L-929 cells (gift of M. Starnbach, Harvard University, Boston) were used as the source of L-cell medium to produce bone marrow (BM)-derived macrophages. The cell lines 3T3-CD40L and 3T3-Samen were gifts from P. Hwu (National Cancer Institute, Bethesda), and the melanomas B16-FLT3L and B16-GM-CSF were provided by G. Dranoff (Harvard Medical School, Boston) (24).
Purification and Isolation of DC and Macrophages. Briefly, BM cells were cultured in GM-CSF-containing DMEM-10 medium (25). At day 8, floating cells were collected and purified with anti-CD11c magnetic beads (Miltenyi Biotec, Auburn, CA). Fluorescence-activated cell sorter (FACS) analysis with Abs to I-Ab and CD11c revealed >95% purity. Similarly, macrophages were derived from BM cells cultured in L-cell medium (26). At day 8, adhesive cells were scraped off gently and purified with anti-CD11b magnetic beads. FACS analysis with Abs to F4/80 and CD14 (PharMingen) revealed >95% macrophages.
Splenic DCs (spDCs) and macrophages were isolated by collagenase treatment (27) and enriched by centrifugation in Accudenz cell-separation medium (Accurate Chemicals). T cells and natural killer (NK) cells were subsequently depleted by using anti-CD90 and anti-NK1.1 magnetic beads, and DCs then were positively selected with anti-CD11c magnetic beads. In some experiments, DCs first were positively selected with anti-CD11c magnetic beads from collagenase-treated spleen and FACS-sorted into subpopulations by staining with anti-MHC II (I-E/I-A), anti-B220, anti-CD11c, anti-CD8α, or anti-CD4 (PharMingen). Large numbers of CD8α- and CD8a+ DCs were obtained by injecting mice with 106 B16-FLT3L (s.c.) and harvesting spleens 10 days later. Macrophages were FACS-sorted with Abs to CD11b and F4/80 or CD11b and CD14. Activated peritoneal macrophages were generated with 3% thioglycolate (Difco).
DC and Macrophage Stimulation. DCs and macrophages were cultured in DMEM-10 at a concentration of 1 × 106 per ml. Cells were stimulated with 10 ng/ml recombinant mouse IL-12 or IL-18 (R & D systems), 1–100 units/ml recombinant IFN-α/β (National Institutes of Health, Bethesda), 20 ng/ml IL-15 or IL-1 (PeproTech, Rocky Hill, NJ), 1–100 ng/ml rIFN-γ (PeproTech), 10 ng/ml IL-21 (R & D Systems), and lipopolysaccharide (LPS; Sigma–Aldrich) at 100 ng/ml. To induce maturation of DCs in vivo, we injected (i.p.) LPS (25 μg per mouse).
Real-Time PCR, ELISA, and Western Blot Analysis. RNA was isolated with Trizol (Sigma–Aldrich) from unstimulated and stimulated DCs and macrophages, and cDNA synthesis was performed with 1 μg of total RNA by using oligo(dT)15 primer, 20 nM each dNTP, 0.1 M DTT, 1× first-stranded buffer, SuperScript II, and RNaseOUT (Invitrogen). Quantitative RT-PCR to determine the levels for T-bet, IFN-γ, tumor necrosis factor α, IL-12 subunits p40 and p35, and other inflammatory cytokines was performed as described (28). TaqMan universal PCR master mix was used for all reactions (Applied Biosystems). Sequences of primers and TaqMan probe for most cytokines including β-actin are as described (29, 30). Expression levels for the gene of interest are reported relative to β-actin abundance. Protein levels of IFN-γ, tumor necrosis factor α, IL-12p70, and IL-12p40 were detected by ELISA from harvested supernatants of stimulated DCs and macrophages (PharMingen). To measure T-bet protein, whole extracts were collected from DCs stimulated for different time periods, and immunoblot analysis was performed by using the 4B10 mAb performed as described (21).
In Vivo Function of DCs Lacking T-bet. We used the adoptive transfer assay described by others (31–34). Briefly, 1 × 106 purified, carboxyfluorescein diacetate-succinimidyl ester (CFSE)-labeled BALB/c DO11.10 T cell antigen receptor transgenic T cells were injected (i.p.) into BALB/c recipients. Two days later, mice were challenged in the footpads with 3.5 × 105 ovalbumin peptide [ISQAHAAHAEINEAGR]-pulsed wild-type (wt) or T-bet-/- splenic CD11c+ DCs per hind footpad. After 5 days of priming, popliteal lymph node (LN) cells were harvested from recipients, and proliferation was assessed by CFSE on FACS. LN cells, 5 × 105 per well, were cultured with varying numbers of wt ovalbumin peptide-pulsed DCs, and cytokine secretion was assessed at 96 h as described above.
Results and Discussion
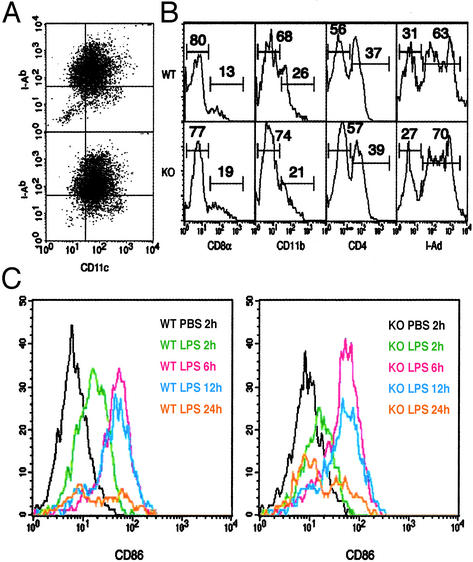
Normal Development and Activation of Murine DCs in T-bet-/- Mice. We investigated the role of T-bet in the development and maturation of the DC lineage. DCs from both myeloid and lymphoid origin usually reside in the secondary lymphoid organs and can be distinguished by the surface expression of CD11b, CD4, and CD8α (10, 35–39). bmDCs of myeloid origin are obtained by culturing BM with GM-CSF (25, 40), and stages of development were monitored over time by assessing surface expression of MHC class II and CD11c. Precursor DCs (CD11chiMHC IIlo) are apparent at day 4, immature DCs (CD11chiMHC IIint) at days 4–8, and mature DCs (CD11cintMHC IIhi) at days 8–10 followed by DC apoptosis. There was no significant difference in the yield of DCs at any stage of development in mice lacking T-bet and similar proportions of precursor, immature, and mature DCs as determined by surface phenotype were obtained from T-bet-/- and control BM (day 8 shown in Fig. 1A). Similarly, FACS analysis of wt and T-bet-/- spleens revealed no obvious difference in spDC composition based on expression of CD11c, I-Ab, CD11b, CD4, and CD8α (Fig. 1B), and comparable numbers of CD11c+ DCs were isolated from multiple independent preparations of spleen. Plasmacytoid DCs were also present at normal levels based on intermediate levels of CD11c and high B220 expression (data not shown). Last, we examined whether T-bet plays a role in spDC maturation in vivo. After LPS injection (i.p.), up-regulation of CD86 (B7-2) in T-bet-/-spDCs was normal (Fig. 1C). Similar results were obtained for the up-regulation of CD80 (B7-1), CD40, and MHC II (data not shown). Taken together, we conclude that T-bet does not play a noticeable role in the development, differentiation, or activation of DCs.
Fig. 1.
Normal development and activation of DCs in T-bet-/- mice. (A and B) DCs were isolated from wt (Upper) and T-bet-/- (Lower) mice on a B6 background. DCs derived from BM cultures (A) or spleen-gated on the CD11c+ population (B) were stained with the indicated Abs. KO, knockout. (C)wt(Left) and T-bet-/- (Right) mice were injected i.p. with LPS and spDCs collected at the indicated times. DC maturation was assessed by FACS analysis of CD11c+ cells stained with CD86 (B7-2). The data shown are representative of at least four independent experiments.
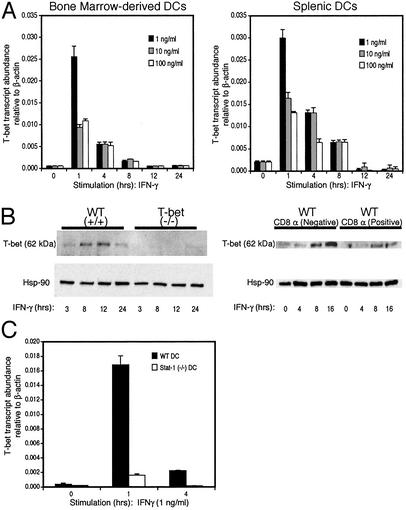
T-bet Expression in Murine DCs. A recent study demonstrated that T-bet expression is almost exclusively restricted to the hematopoietic system during mouse development, the only exception being the olfactory bulb. T-bet was expressed in several blood lineages including progenitors/stem cells found in human BM and cord blood (41). In the adult animal, expression of T-bet is primarily evident in lymphoid organs. We have reported that T-bet is expressed in Th1, CD8T, NK, and B cells, and others have noted T-bet expression in human monocytes and myeloid DCs (21–23, 42). We therefore examined constitutive and regulated T-bet expression in mouse DCs and macrophages. Using BM-derived myeloid DCs isolated to >95% purity by selection on CD11c-coated magnetic beads, we failed to detect T-bet mRNA in unstimulated precursor, immature, or mature cells (Fig. 2A Left and data not shown). However, CD11c+ bmDCs displayed a rapid up-regulation of T-bet mRNA after stimulation with IFN-γ (Fig. 2 A Left). Spleen cells from B6 mice were FACS-sorted with Abs to CD11c and I-Ab to avoid contamination with CD8 T and NK cells (>99% pure), sorted spDCs were cultured for various time periods in the presence or absence of IFN-γ, and RNA was isolated for real-time PCR analysis. These experiments revealed low levels of T-bet expression in unstimulated spDCs and a rapid up-regulation of T-bet transcript levels after treatment with IFN-γ (Fig. 2 A Right). Similar to bmDCs, T-bet mRNA expression in spDCs peaked during the first hour, remained high up to 4 h, and declined dramatically after 8 h (Fig. 2 A Right). Of note, the levels of T-bet transcripts in DCs were very comparable with that observed in Th1 cells and ranged between 10-3 and 10-2 molecules of T-bet per 1 molecule of β-actin (Fig. 2 A). The expression of T-bet protein in spDCs treated with IFN-γ mirrored RNA expression with a rapid induction of T-bet protein beginning at 3 h, peaking at 12 h, and decreasing 24 h after stimulation with IFN-γ (Fig. 2B Left). Similar to what has been demonstrated for CD4 Th1 cells (42), DCs from mice lacking the IFN-γR signaling molecule, Stat1, had a marked defect in expression of T-bet (Fig. 2c). Other stimuli known to either activate DCs (LPS, tumor necrosis factor α, and IL-1) or recently described to increase T-bet expression in NK cells (IL-21 and IL-15) (43) did not induce T-bet expression (data not shown).
Fig. 2.
T-bet expression in murine DCs. DCs were sorted by staining with Abs to CD11c and I-Ab and stimulated with IFN-γ as indicated. (A) T-bet mRNA expression was analyzed by real-time PCR in DCs derived from BM cultures (Left) or spleen (Right). (B) spDCs were purified with CD11c magnetic beads (Left) or by three-color FACS with CD8α (Right) and stimulated with IFN-γ (10 ng/ml). Whole extracts were prepared at the indicated time points. T-bet protein levels were detected by immunoblot analysis. The data shown are representative of at least three independent experiments. (C) T-bet mRNA expression analyzed by real-time PCR in spDCs isolated from wt (black) and STAT-/- (white) mice.
spDCs have been subdivided on the basis of expression of the surface marker CD8α into “lymphoid” and “myeloid” subsets that subsume different functions in driving Th polarization. However, recent work suggests that the phenotype and function of these subsets may be more fluid than fixed and may alter depending on environmental stimuli (3–5, 44, 45). We purified CD8α+ and CD8α- subpopulations of wt and T-bet-/- DCs by three-color FACS sorting, stimulated them for various time points with IFN-γ, and measured expression of T-bet. Both subsets up-regulated T-bet mRNA (data not shown) and protein (Fig. 2B Right) after stimulation with IFN-γ. We found that T-bet was expressed in both subsets but at significantly lower levels in the CD8α+ subset.
We conclude that DCs of both myeloid and lymphoid origin express T-bet and that this expression is controlled by IFN-γ in a positive feedback loop similar to what has been observed in T cells. These data confirm and extend earlier observations on the ability of IFN-γ to control T-bet expression in human monocytes and myeloid DCs (42). One difference emerged, however. In contrast to the findings of Lighvani et al. (42), where human monocytes treated with IFN-γ expressed substantial levels of T-bet, we did not detect T-bet expression in peritoneal, splenic, or BM-derived macrophages after treatment with IFN-γ or after phagocytosis of latex beads (data not shown), leading us to conclude that the production of IFN-γ from these cells is controlled by transcription factors other than T-bet. One of these factors may well be Stat4 (46–48). Substantial differences in lineage commitment and subset profiles of DCs and macrophages have been observed between human and mouse species (5).
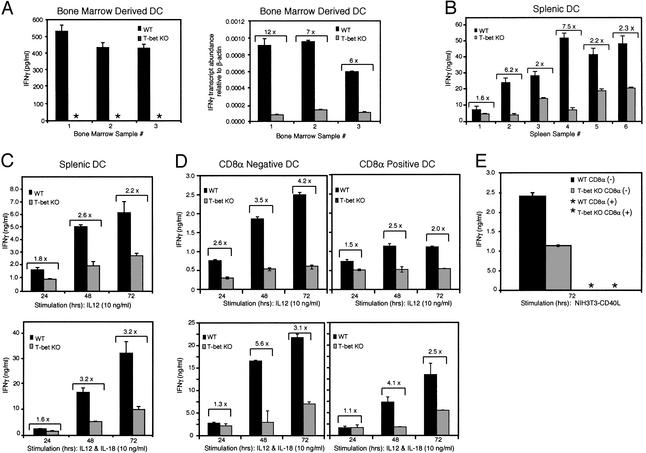
T-bet Is Essential for Optimal Production of IFN-γ by DCs. Recent studies have established DCs as an important source of IFN-γ. After a 72-h stimulation with IL-12 and IL-18, DCs secrete substantial amounts of IFN-γ, ranging between 10 and 300 ng·ml-1 (8–13). To date, Stat4 is the only transcription factor known to control the production of IFN-γ in myeloid cells (11). In both DCs and macrophages, the IL-12-dependent secretion of IFN-γ is severely diminished in the absence of Stat4 (11). In addition, Stat4-/- macrophages exhibited defective production of nitric oxide in response to IL-12 and are susceptible to Toxoplasma gondii infection (11). Because T-bet controls the transcription of the IFN-γ gene in CD4+ T cells but not, for example, in B cells, we asked whether it did so in DCs.
A marked impairment in IFN-γ secretion was observed in bmDCs derived from T-bet-/- B6 mice after treatment with IL-12 and IL-18. Although IFN-γ production by bmDCs is typically lower than from spDCs, ranging from 100 to 5,000 pg·ml-1 (11–13), T-bet-/- bmDCs produced no detectable IFN-γ at all (Fig. 3A Left). wt B6 bmDCs produced levels that ranged from 100 to 500 pg·ml-1 (Fig. 3A Left). Unlike spDCs, which die in culture after 48–72 h, bmDCs survive beyond 72 h, allowing us to measure IFN-γ transcripts as well. Survival rates were similar in bmDCs from wt and knockout mice after stimulation for 72 h (data not shown), and real-time PCR analysis confirmed a marked decrease (6- to 12-fold) in IFN-γ transcripts in T-bet-/- bmDCs (Fig. 3A Right).
Fig. 3.
Impaired IFN-γ production in T-bet-/- DCs. B6 wt and T-bet-/- DCs were seeded at a concentration of 1 × 106 cells per ml and stimulated with IL-12 alone, or in combination with IL-18, at 10 ng/ml. Supernatants were collected after 72 h, and IFN-γ secretion was measured by ELISA. (A) DCs were derived from three independent BM cultures and purified at day 8 by using CD11c magnetic beads. (Left) ELISA results. (Right) IFN-γ mRNA expression measured by real-time PCR from cells collected at 72 h. (B) DCs were isolated from six independent preparations of spleens and sorted (CD11c+ and I-Ab). (C) Time-course analysis of IFN-γ secretion from CD11c+ I-Ab-sorted DCs from eight pooled spleens per genotype. These results are representative of at least three independent experiments. (D) CD11c+CD8α+ and CD8α- subpopulations from wt and T-bet-/- mice were isolated and stimulated with IL-12 or IL-12 plus IL-18 as described above, and IFN-γ was measured by ELISA. The data are representative of at least three independent experiments. (E) CD11c+CD8α+ and CD8α- subpopulations from wt and T-bet-/- mice were isolated and stimulated with CD40L-transfected NIH 3T3 cells for 72 h, and IFN-γ in supernatants was measured by ELISA. Brackets indicate the fold difference of IFN-γ levels between wt and T-bet-/- samples. *, not detected; KO, knockout.
Similarly, a significant reduction in IFN-γ secretion, ranging from 40% to 80% from six independent preparations of spDCs stimulated for 72 h with IL-12 or IL-12 plus IL-18, was observed in T-bet-/- as compared with control mice (Fig. 3B). T-bet functions early in naive T helper progenitor cells to regulate IFN-γ gene transcription. Optimal production of IFN-γ by murine DCs has been reported to occur between 3 and 5 days (8–13). To investigate whether T-bet plays a role during the early production of IFN-γ by spDCs, a time-course analysis was performed for IFN-γ secretion during the first 3 days after stimulation with IL-12 alone or in combination with IL-18. In the absence of T-bet, spDCs displayed a 40–45% reduction in IFN-γ production during the first 24 h, which decreased further over the next 48 h to between 60% and 70% (Fig. 3C). Similar patterns of T-bet expression and impairment in IFN-γ production were observed in mice of the BALB/c as well as B6 strains (data not shown).
We also examined IFN-γ production after IL-12 and IL-12 plus IL-18 stimulation by CD8α+ and CD8α- wt and T-bet-/- DCs. T-bet was required for optimal production of IFN-γ by both subsets (Fig. 3D). Interestingly, CD8α+ DCs also produced significantly less IFN-γ than CD8α- DCs, thus providing a correlation between levels of T-bet (Fig. 2B Right) and production of IFN-γ. Further, coculture of DCs with a more physiologic stimulus, CD40L-transfected or control untransfected 3T3 cells rather than recombinant cytokines, led to similar results for CD8α- DCs (Fig. 3E). In this experiment, neither wt nor T-bet-/- CD8α+ DCs produced detectable IFN-γ, again demonstrating that it is the CD8α- subset that is the major producer of this cytokine, as discovered recently by others (49), although levels of IL-12p40 and IL-12p70 were equivalent in the two subsets (data not shown).
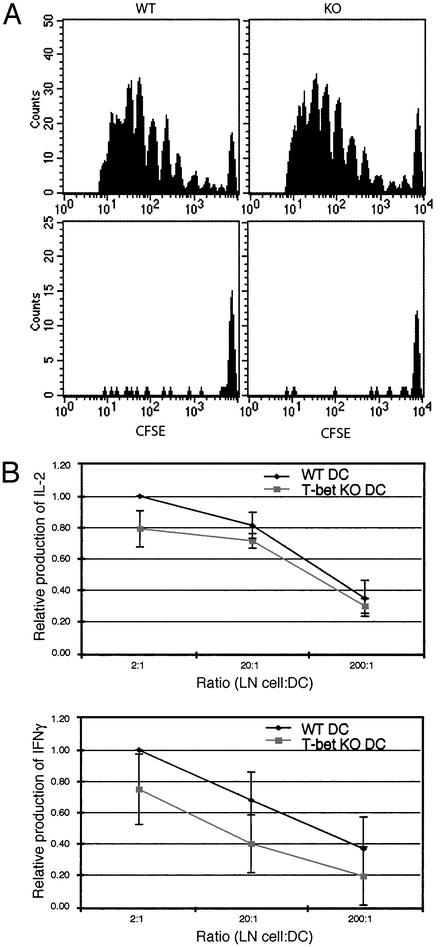
In Vivo Function of T-bet in DCs. IFN-γ-deficient DCs fail to prime antigen-specific T cells in vivo (33). To examine the in vivo function of T-bet in DCs, as opposed to its function in other cell types, we therefore used the adoptive transfer assay previously described. This assay measures the activation of adoptively transferred T cell antigen receptor transgenic T cells that have been primed in vivo with peptide-pulsed DCs by using proliferation and secretion of Th cytokines as a read-out (31–34). Briefly, BALB/c DO11.10 T cell antigen receptor transgenic T cells were purified, labeled with CFSE, and injected (i.p.) into BALB/c recipients. Two days later, mice were challenged in the footpads with ovalbumin peptide-pulsed or unpulsed wt or T-bet-/- CD11c+DCs. After 5 days of priming, popliteal LN cells were harvested from recipients, and both proliferation and cytokine secretion was assessed. Fig. 4A demonstrates no difference between proliferation of BALB/c wt T cells primed with either wt or T-bet-/- DCs as assessed by CFSE. Mice injected with unpulsed DCs did not undergo proliferation and did not recruit additional DO11.10 T cells to LN. LN cells from primed mice were stimulated in culture with varying numbers of peptide-loaded wt DCs for 96 h, and cytokine production was measured by ELISA. The results of four independent experiments, each using three mice per condition, are summarized and shown in Fig. 4B. An antigen-specific response was observed as indicated by the increasing cytokine secretion observed with increasing numbers of peptide-pulsed wt DCs. Both IL-2 and IFN-γ were produced by T cells that had been primed with pulsed DCs. There was a minimal decrease in amounts of IL-2 produced from LN T cells in mice primed with T-bet-/- as compared with wt DCs (Fig. 4B Upper), which was not a consequence of impaired proliferation as demonstrated above. Notably, a moderate to marked decrease in the production of the Th1 cytokine, IFN-γ, was observed in mice primed with T-bet-/- DCs as compared with wt DCs (Fig. 4B Lower), consistent with a role for DC-derived IFN-γ in Th1 polarization. There was no difference in cytokine production from cultures containing LN cells harvested from mice that had been primed with either unpulsed wt or T-bet-/- DCs (data not shown), demonstrating that only primed antigen-specific T cell activation was being assessed. No IL-4 was detected in supernatants from cultures of either wt- or T-bet-/--primed LN cells, consistent with the tendency of the DO11.10 transgenic T cell antigen receptor to generate Th1 but not Th2 cells.
Fig. 4.
In vivo function of T-bet in DCs. (A) In vivo proliferation of DO11.10 T cells adoptively transferred into BALB/c recipients. These cells were stained with PE-coupled anticlonotypic KJ126 Ab and propidium iodide. The plots represent levels of CFSE on KJ126-positive and propidium iodide-negative cells. (Upper and Lower) Proliferation of D011.10 T cells primed with peptide-pulsed or unpulsed DCs, respectively. DCs were isolated from wt (Left) and T-bet-/- (Right) mice. (B) Cytokine secretion from LN cells were harvested from the mice described above and cultured for 96 h with varying numbers of peptide-pulsed wt DCs. Results are expressed as the ratio of IL-2 or IFN-γ produced by DO11.10 T cells from mice primed in vivo with T-bet-/- as compared with wt peptide-pulsed DCs, with the wt value set arbitrarily at 1.0. KO, knockout.
In summary, we demonstrate that T-bet is expressed by and has a functional role in murine DCs. As in CD4 cells, the expression of T-bet is controlled by IFN-γ. We did not observe an obvious role for T-bet in DC development, differentiation, or activation. We found rather that T-bet was essential for the optimal secretion of IFN-γ by DCs. This role is quite selective for IFN-γ in DCs, because the expression of other DC cytokines such as IL-12p70 and IL-12p40, tumor necrosis factor α, and IL-1 after stimulation with IL-12 and IL-18 or LPS was not affected by the absence of T-bet (data not shown). Although it is clear that Stat4 is absolutely required for the initiation of IL-12-dependent production of IFN-γ in myeloid cells, our data demonstrate that T-bet also participates in the control of this cytokine. One possible scenario is that after the interaction of a pathogen with Toll receptor family members, the DC is stimulated to secrete IL-12 and IFN-γ, thereby activating both the Stat1 and Stat4 signaling pathways. Stat1 may control both the expression of T-bet and Stat4, thus simultaneously polarizing the T helper precursor cell along a Th1 pathway and maximizing its production of IFN-γ. Characterization of DC populations from mice lacking both T-bet and Stat4 or T-bet and Stat1 should provide further insight into these molecular circuits.
We conclude that T-bet influences the generation of type I immunity not only by controlling Th1 lineage commitment in the adaptive immune system but also by a direct influence on the transcription of the IFN-γ gene in DCs. The impairment in activating the Th1 program by T-bet-/- DCs we observed might be partly attributed to defective IFN-γ production (33). However, it is likely that T-bet will participate in the expression of other genes in DCs that are required for optimal antigen-specific T cell responses. T-bet therefore is a transcription factor that may be a molecular bridge between the innate and adaptive immune systems.
Acknowledgments
We are grateful to all Glimcher and Grusby laboratory members for thoughtful review of the manuscript and valuable technical assistance, and we offer special thanks to Neal Iwakoshi and Adrian Erlebacher; C. McCall for manuscript preparation; and Betty Tang and Jacobo Ramirez for excellent animal care. G.L.-V. thanks A. C. Hernandez, S. M. Lugherna, C. J. Lugherna, family, and friends for pura vida. This work was supported by a National Science Foundation graduate research fellowship (to G.L.-V.), an Irvington Institute Postdoctoral Fellowship (to R.M.-L.); a Howard Hughes Predoctoral Fellowship (to R.P.); a Harvard School of Public Health summer fellowship (to C.P.), and National Institutes of Health Grants AI32412 and AI29673 (to L.H.G.).
Abbreviations: DC, dendritic cell; Th, T helper; STAT, signal transducers and activators of transcription; B6, C57BL/6; GM-CSF, granulocyte/macrophage colony-stimulating factor; BM, bone marrow; bmDC, BM-derived DC; FACS, fluorescence-activated cell sorter; spDC, splenic DC; NK, natural killer; LPS, lipopolysaccharide; CFSE, carboxyfluorescein diacetate-succinimidyl ester; wt, wild type; LN, lymph node.
References
- 1.Mellman, I. & Steinman, R. M. (2001) Cell 106, 255-258. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., Briere, F., Caux, C., Davoust, J., Lebecque, S., Liu, Y. J., Pulendran, B. & Palucka, K. (2000) Annu. Rev. Immunol. 18, 767-811. [DOI] [PubMed] [Google Scholar]
- 3.Liu, Y. J. (2001) Cell 106, 259-262. [DOI] [PubMed] [Google Scholar]
- 4.Shortman, K. & Liu, Y. J. (2002) Nat. Rev. Immunol. 2, 151-161. [DOI] [PubMed] [Google Scholar]
- 5.Moser, M. & Murphy, K. M. (2000) Nat. Immunol. 1, 199-205. [DOI] [PubMed] [Google Scholar]
- 6.Dabbagh, K., Dahl, M. E., Stepick-Biek, P. & Lewis, D. B. (2002) J. Immunol. 168, 4524-4530. [DOI] [PubMed] [Google Scholar]
- 7.Frucht, D. M., Fukao, T., Bogdan, C., Schindler, H., O'Shea, J. J. & Koyasu, S. (2001) Trends Immunol. 22, 556-560. [DOI] [PubMed] [Google Scholar]
- 8.Ohteki, T., Fukao, T., Suzue, K., Maki, C., Ito, M., Nakamura, M. & Koyasu, S. (1999) J. Exp. Med. 189, 1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukao, T. & Koyasu, S. (2000) Eur. J. Immunol. 30, 1453-1457. [DOI] [PubMed] [Google Scholar]
- 10.Hochrein, H., Shortman, K., Vremec, D., Scott, B., Hertzog, P. & O'Keeffe, M. (2001) J. Immunol. 166, 5448-5455. [DOI] [PubMed] [Google Scholar]
- 11.Fukao, T., Frucht, D. M., Yap, G., Gadina, M., O'Shea, J. J. & Koyasu, S. (2001) J. Immunol. 166, 4446-4455. [DOI] [PubMed] [Google Scholar]
- 12.Fukao, T., Matsuda, S. & Koyasu, S. (2000) J. Immunol. 164, 64-71. [DOI] [PubMed] [Google Scholar]
- 13.Stober, D., Schirmbeck, R. & Reimann, J. (2001) J. Immunol. 167, 957-965. [DOI] [PubMed] [Google Scholar]
- 14.Bach, E. A., Aguet, M. & Schreiber, R. D. (1997) Annu. Rev. Immunol. 15, 563-591. [DOI] [PubMed] [Google Scholar]
- 15.Boehm, U., Klamp, T., Groot, M. & Howard, J. C. (1997) Annu. Rev. Immunol. 15, 749-795. [DOI] [PubMed] [Google Scholar]
- 16.Leonard, W. J. & O'Shea, J. J. (1998) Annu. Rev. Immunol. 16, 293-322. [DOI] [PubMed] [Google Scholar]
- 17.Dalton, D. K., Pitts-Meek, S., Keshav, S., Figari, I. S., Bradley, A. & Stewart, T. A. (1993) Science 259, 1739-1742. [DOI] [PubMed] [Google Scholar]
- 18.Huang, S., Hendriks, W., Althage, A., Hemmi, S., Bluethmann, H., Kamijo, R., Vilcek, J., Zinkernagel, R. M. & Aguet, M. (1993) Science 259, 1742-1745. [DOI] [PubMed] [Google Scholar]
- 19.Meraz, M. A., White, M., Sheehan, K. C. F., Bach, E. A., Rodig, S. J., Dighe, A. S., Kaplan, D. H., Riley, J. K., Greenlund, A. C., Campbell, D., et al. (1996) Cell 84, 431-442. [DOI] [PubMed] [Google Scholar]
- 20.Casanova, J. L. & Abel, L. (2002) Annu. Rev. Immunol. 20, 581-620. [DOI] [PubMed] [Google Scholar]
- 21.Szabo, S. J., Kim, S. T., Costa, G. L., Zhang, X., Fathman, G. C. & Glimcher, L. H. (2000) Cell 100, 655-669. [DOI] [PubMed] [Google Scholar]
- 22.Szabo, S. J., Sullivan, B. M., Stemmann, C., Satoskar, A. R., Sleckman, B. P. & Glimcher, L. H. (2002) Science 295, 338-342. [DOI] [PubMed] [Google Scholar]
- 23.Peng, S. L., Szabo, S. J. & Glimcher, L. H. (2002) Proc. Natl. Acad. Sci. USA 99, 5545-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasare, C. & Medzhitov, R. (2003) Science 299, 1033-1036. [DOI] [PubMed] [Google Scholar]
- 25.Lutz, M. B., Kukutsch, N., Ogilvie, A. L., Rossner, S., Koch, F., Romani, N. & Schuler, G. (1999) J. Immunol. Methods 223, 77-92. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich, W. F., Damron, D. M., Isberg, R. R., Lander, E. S. & Swanson, M. S. (1995) Genomics 26, 443-450. [DOI] [PubMed] [Google Scholar]
- 27.Crowley, M., Inaba, K., Witmer-Pack, M. & Steinman, R. M. (1989) Cell. Immunol. 118, 108-125. [DOI] [PubMed] [Google Scholar]
- 28.Overbergh, L., Valckx, D., Waer, M. & Mathieu, C. (1999) Cytokine 11, 305-312. [DOI] [PubMed] [Google Scholar]
- 29.Grogan, J. L., Mohrs, M., Harmon, B., Lacy, D. A., Sedat, J. W. & Locksley, R. M. (2001) Immunity 14, 205-215. [DOI] [PubMed] [Google Scholar]
- 30.Erlebacher, A., Lukens, A. K. & Glimcher, L. H. (2002) Proc. Natl. Acad. Sci. USA 99, 16940-16945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonifaz, L., Bonnyay, D., Mahnke, K., Rivera, M., Nussenzweig, M. C. & Steinman, R. M. (2002) J. Exp. Med. 196, 1627-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawiger, D., Inaba, K., Dorsett, Y., Guo, M., Mahnke, K., Rivera, M., Ravetch, J. V., Steinman, R. M. & Nussenzweig, M. C. (2001) J. Exp. Med. 194, 769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maldonado-Lopez, R., Maliszewski, C., Urbain, J. & Moser, M. (2001) J. Immunol. 167, 4345-4350. [DOI] [PubMed] [Google Scholar]
- 34.Maldonado-Lopez, R., DeSmedt, T., Michel, P., Godfroid, J., Pajak, B., Heirman, C., Thielemans, K., Leo, O., Urbain, J. & Moser, M. (1999) J. Exp. Med. 189, 587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu, L., Li, C. L. & Shortman, K. (1996) J. Exp. Med. 184, 903-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vremec, D. & Shortman, K. (1997) J. Immunol. 159, 565-573. [PubMed] [Google Scholar]
- 37.Leenen, P. J., Radosevic, K., Voerman, J. S., Salomon, B., van Rooijen, N., Klatzmann, D. & van Ewijk, W. (1998) J. Immunol. 160, 2166-2173. [PubMed] [Google Scholar]
- 38.Kamath, A. T., Pooley, J., O'Keeffe, M. A., Vremec, D., Zhan, Y., Lew, A. M., D'Amico, A., Wu, L., Tough, D. F. & Shortman, K. (2000) J. Immunol. 165, 6762-6770. [DOI] [PubMed] [Google Scholar]
- 39.Henri, S., Vremec, D., Kamath, A., Waithman, J., Williams, S., Benoist, C., Burnham, K., Saeland, S., Handman, E. & Shortman, K. (2001) J. Immunol. 167, 741-748. [DOI] [PubMed] [Google Scholar]
- 40.Inaba, K., Inaba, M., Romani, N., Aya, H., Deguchi, M., Ikehara, S., Muramatsu, S. & Steinman, R. M. (1992) J. Exp. Med. 176, 1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faedo, A., Ficara, F., Ghiani, M., Aiuti, A., Rubenstein, J. L. & Bulfone, A. (2002) Mech. Dev. 116, 157-160. [DOI] [PubMed] [Google Scholar]
- 42.Lighvani, A. A., Frucht, D. M., Jankovic, D., Yamane, H., Aliberti, J., Hissong, B. D., Nguyen, B. V., Gadina, M., Sher, A., Paul, W. E. & O'Shea, J. J. (2001) Proc. Natl. Acad. Sci. USA 98, 15137-15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strengell, M., Sareneva, T., Foster, D., Julkunen, I. & Matikainen, S. (2002) J. Immunol. 169, 3600-3605. [DOI] [PubMed] [Google Scholar]
- 44.Edwards, A. D., Manickasingham, S. P., Sporri, R., Diebold, S. S., Schulz, O., Sher, A., Kaisho, T., Akira, S. & Reis e Sousa, C. (2002) J. Immunol. 169, 3652-3660. [DOI] [PubMed] [Google Scholar]
- 45.Reis e Sousa, C. (2001) Immunity 14, 495-498. [DOI] [PubMed] [Google Scholar]
- 46.Frucht, D. M., Aringer, M., Galon, J., Danning, C., Brown, M., Fan, S., Centola, M., Wu, C. Y., Yamada, N., Gabalawy, H. E. & O'Shea, J. J. (2000) J. Immunol. 164, 4659-4664. [DOI] [PubMed] [Google Scholar]
- 47.Lawless, V. A., Zhang, S., Ozes, O. N., Bruns, H. A., Oldham, I., Hoey, T., Grusby, M. J. & Kaplan, M. H. (2000) J. Immunol. 165, 6803-6808. [DOI] [PubMed] [Google Scholar]
- 48.Kuroda, E., Kito, T. & Yamashita, U. (2002) J. Immunol. 168, 5477-5482. [DOI] [PubMed] [Google Scholar]
- 49.Fujii, S., Shimizu, K., Smith, C., Bonifaz, L. & Steinman, R. M. (2003) J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]