Abstract
We have investigated the influence of mast cells on the barrier function of intestinal epithelium during nematode infection. Trichinella spiralis infection induces a strong type 2 cytokine-mediated inflammation, resulting in a critical mucosal mastocytosis that is known to mediate expulsion of the parasites from the intestine. The host response to infection is also characterized by an increase in mucosal leakiness. We show here that intestinal epithelial permeability is markedly elevated during infection, with kinetics that mirror the adaptive immune response to primary and secondary infection. Furthermore, we have identified degradation of the tight junction protein, occludin, thereby providing a mechanism for increased paracellular permeability during helminth infection. We further demonstrate by using anti-c-kit antibody and IL-9 transgenic mice that mast cells are directly responsible for increasing epithelial paracellular permeability and that mice deficient in a mast cell-specific protease fail to increase intestinal permeability and fail to expel their parasite burden. These results provide the mechanism whereby mucosal mast cells mediate parasite expulsion from the intestine.
The adult stage of the nematode Trichinella spiralis resides within enterocytes of the jejunum. During parasite infection characteristic changes occur in the small intestine (1). It has long been known that the gut becomes edematous and inflamed, with these responses peaking at the time of parasite expulsion from the host, but the precise mechanisms involved have remained obscure. Infection induces leakiness in the intestinal epithelium that is considered to be a host defense mechanism against the parasite (the leak-lesion hypothesis) (2).
We hypothesize that an increase in epithelial paracellular permeability resulting in the loss of parasites is a direct consequence of adaptive immunity. T. spiralis elicits a strong T helper 2 response resulting in intestinal goblet cell hyperplasia, eosinophilia, and a profound mucosal mastocytosis (3–5). Efficient parasite expulsion depends on CD4+ T cells through control of the critical mast cell response (6). In the absence of intestinal mast cells the loss of parasites is markedly delayed (7). The mechanism by which mast cells induce parasite expulsion is unknown and is the focus of this study.
Changes in epithelial paracellular permeability during the course of T. spiralis infection in mice and the role that the mast cell may play in inducing these changes were investigated. By depleting mast cells with anti-c-kit antibodies or by using IL-9 transgenic mice that overexpress mast cells (8), we present compelling evidence that mast cells are the key mediators of increased mucosal permeability. To understand further the action of mast cells on intestinal epithelium, we have infected mice deficient in mouse mast cell protease-1 (mMCP-1) that had been shown previously to delay parasite expulsion (9) and investigated whether this mast cell-specific proteinase is involved in increased epithelial permeability during T. spiralis infection.
The intestinal mucosal barrier is maintained by tight junctions (TJs), which form a continuous ring around the apices of epithelial cells and occlude the paracellular channels. TJs are composed of the transmembrane proteins claudin, occludin, and junctional adhesion molecule (10). Claudin and occludin span the plasma membrane four times, having two extracellular loops, one intracellular loop, and two cytosolic termini. The extracellular loops of adjacent cells bind to each other, generating the close membrane proximity that can be identified by transmission electron microscopy. This interaction is believed to form pores that control the selective movement of fluid and solutes through the paracellular channels (11). Associated with the carboxyl termini of both occludin and claudin are the membrane-associated guanylate kinase homologue proteins, which include ZO-1, ZO-2, and ZO-3. These link the transmembrane proteins to the actin cytoskeleton and act as a platform for a variety of signaling molecules (12). Claudin has 24 isoforms and their presence in TJs is thought to determine the “tightness,” i.e., the permeability, of a particular epithelium and the charge selectivity of its TJ channels (13, 14). The role of occludin is less well defined, although its extracellular loops are critical for regulation of paracellular permeability (15). Various stimuli including cytokines, allergens, and bacterial products have been implicated in enhancing mucosal permeability by affecting TJ integrity (16–21). Here, we have examined the effect of T. spiralis infection on intestinal epithelial TJ proteins; occludin, claudin-1, and ZO-1. We present evidence demonstrating the disruption of TJs in vivo and during an intestinal parasitic infection.
Methods
Animals and Infection. NIH and FVB strains of mice were purchased from Harlan Olac (Bicester, U.K.). IL-9 transgenic, mMCP-1-deficient mice, and BALB/c WT mice were generated as described (22, 23) and bred at University of Manchester (Manchester, U.K.) under specific pathogen-free conditions. All mice were male and infected with T. spiralis at 6–8 weeks of age. Maintenance, infection, and recovery of T. spiralis were as described (24). Mice were infected by oral gavage with 300 larvae on day 0. For challenge experiments, mice were given a further 300 larvae at day 21 after primary infection. All experiments were performed under the regulations of the Home Office Scientific Procedures Act (1986).
Worm Burdens. Small intestines were removed, opened longitudinally, and incubated in PBS at 37°C. Worms were counted after 4 h.
Antibody Treatment. NIH mice were given either 0.5 mg of anti-c-kit antibody or 0.5 mg of nonspecific rat IgG (Sigma) on days 0, 2, 4, 6, and 8 postinfection. IL-9 transgenic mice were given 1 mg of either antibody on days -1, 0, 1, and 2 postinfection.
Permeability Studies. Mice were killed, and 15 cm of proximal jejunum was quickly removed. Intestines were flushed with ice-cold Ringer's solution (121 mM NaCl/25 mM NaHCO3/0.2 mM K2HPO4/1.2 mM KH2PO4/2.6 mM KHCO3/1.2 mM CaCl2/1.2 mM MgCl2) containing 15 mM D-glucose. Intestines were mounted on a glass rod and trimmed of fat. They were opened longitudinally and divided into four pieces. Each piece was placed within an Ussing chamber (University of Manchester workshop). Both sides of the chamber were filled with 5 ml of Ringer's solution, oxygenated, and kept at 37°C throughout the experiment. After an adjustment period of 30 min, 5 μM unlabeled mannitol followed by 0.5 μCi of [14C]mannitol (Amersham Pharmacia) was added to the mucosal side of each chamber. Chambers were sampled from the serosal side after 1 h and then sampled again after another hour. Radioactivity of each sample was measured by using a scintillation β-counter. The permeability of each piece of tissue is presented as the calculated flux of [14C]mannitol during a 1-h period.
Histology. Sections of jejunum were taken at 15 cm proximal to the pylorus. They were fixed in Carnoy's solution for 4 h, then processed and embedded in paraffin. To visualize mast cells, tissue sections (5 μm) were cut, dewaxed, rehydrated, and placed in toluidine blue for 24 h. After washing, sections were counterstained with eosin and mounted. The number of mast cells per 20 villus crypt units (VCUs) was counted on each section.
mMCP-1 Assay. Blood was collected immediately from the heart of each killed animal. Serum levels of mMCP-1 were measured by using a commercially available ELISA kit (Moredun Animal Health, Penicuik, U.K.).
Immunostaining. Sections of jejunum were taken 15 cm proximal to the pylorus. They were immediately frozen in liquid nitrogen-cooled isopentane. Tissues were cut into 5-μm-thick sections and allowed to air dry. Sections were fixed in ice-cool acetone for 10 min followed by rehydration in PBS. Tissues were blocked for 30 min with 10% donkey serum (Sigma) and 1% BSA (Sigma) in PBS. Sections were incubated with rabbit anti-claudin-1 IgG (Zymed), rabbit anti-ZO-1 IgG (Zymed), or mouse anti-desmoplakin IgG (11-5F) primary antibodies for 90 min. After washing, donkey anti-rabbit Alexa 488 or donkey anti-mouse Alexa 633 secondary antibodies were left on sections for 30 min (Molecular Probes). Sections were mounted with ProLong Antifade mounting medium (Molecular Probes) and kept in the dark at -20°C until used.
Western Blotting. Epithelial cells from jejuna of infected or naïve mice were isolated by collagenase/dispase digestion (25). In brief, jejuna were removed, flushed with ice-cold Hanks' balanced salt solution (GIBCO), and cut into 1-mm3 cubes. Tissues were shaken vigorously in 1 mg/ml collagenase type 1 (Sigma) and 1 mg/ml dispase II (Roche, Basel) for 30 min at room temperature.
Epithelial cells were boiled for 8 min in sample buffer (0.5 M Tris, pH 6.8/10% SDS/30% glycerol/10% 2-mercaptoethanol/ 0.1 mg/ml bromophenol blue). After SDS/PAGE, proteins were transferred by electrophoresis to nitrocellulose membranes (Bio-Rad). Membranes were probed with rabbit anti-occludin primary antibody (Zymed) followed by donkey anti-rabbit secondary antibody conjugated to horseradish peroxidase (Amersham Pharmacia). Bands were detected by using enhanced chemiluminescence (ECL plus, Amersham Pharmacia). Band density was measured by using a Bio-Rad GS-700 imaging densitometer.
Confocal Microscopy. TJ proteins were visualized by using an Axiovert 100M confocal microscope (Zeiss). Images were captured by using LSM 510 software (Zeiss).
Statistics. Significance was determined by using Student's t test.
Results
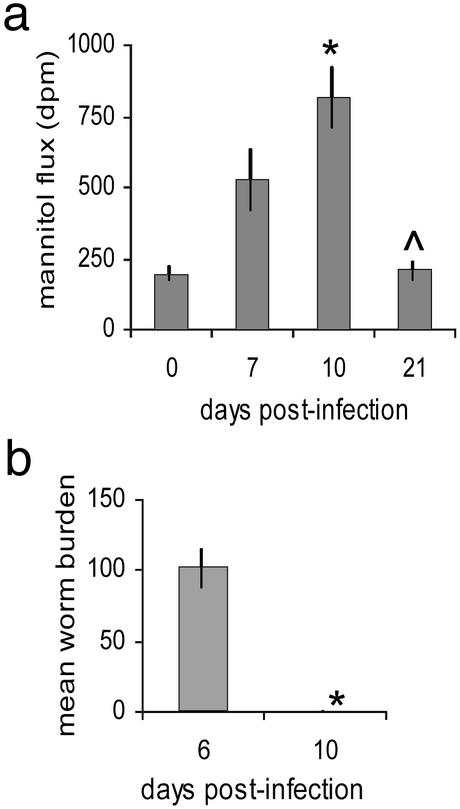
Mucosal Permeability Increases After Infection. Considerable swelling of the small intestine occurred a few days after infection with T. spiralis. Intestines became opaque, yellowish, and filled with fluid, indicating an increase in mucosal permeability. During the course of a primary T. spiralis infection the intestine became significantly more permeable to the paracellular permeability indicator, mannitol (Fig. 1a). Permeability increased up until day 10 postinfection, corresponding to the time of worm expulsion from the intestine (Fig. 1b). After expulsion, the tissue began to recover until by day 21 after infection, permeability returned to naïve levels (Fig. 1a).
Fig. 1.
Kinetics of intestinal permeability during primary T. spiralis infection. Permeability of jejunum sections to mannitol was measured on days (d) 0, 7, 10, and 21 postinfection; *, P < 0.003 d0 vs. d10, Λ, P < 0.003 d10 vs. d21 (a). Parasite numbers in the jejunum were counted on days 6 and 10 postinfection, *, P < 0.005 (b).
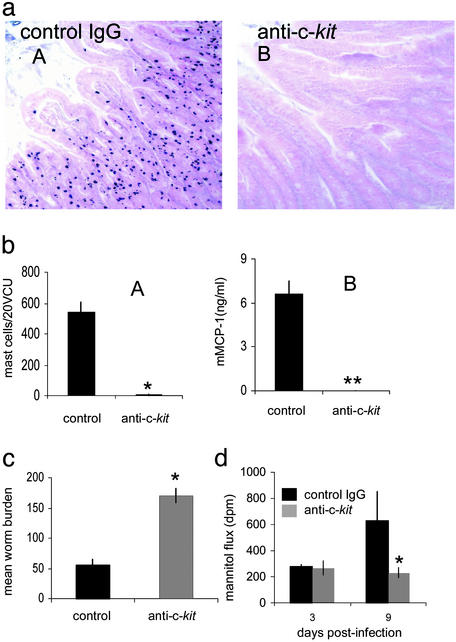
Abrogation of Mast Cells Blocks Increased Mucosal Permeability. Mast cells are critical for efficient nematode expulsion in this model (7, 26). Intestinal permeability changes in infected animals mirrored previously described kinetics of mast cell accumulation in the intestine and serum levels of mMCP-1 before and after worm expulsion (27). We have previously shown that inhibition of mucosal mastocytosis during infection prevents worm expulsion (26). By using blocking antibody against c-kit mast cell accumulation in the small intestine and mMCP-1 secretion was prevented (Fig. 2 a and b). Control mice that had received nonspecific rat IgG developed normal mastocytosis and were expelling their worm burden by day 9 after infection. However, anti-c-kit-treated animals still had a full worm burden at this stage (Fig. 2c). Failure to expel the parasite in the absence of mast cells was related to changes in intestinal permeability. Fig. 2d shows that intestines from mice that received anti-c-kit antibody were significantly less permeable than control animals on day 9 after infection.
Fig. 2.
Abrogation of mucosal mast cells reduces intestinal permeability. Mast cells were absent in jejuna from d9 postinfected mice treated with anti-c-kit (aB) compared with control mice treated with rat IgG (aA), magnification ×200. Numbers of mast cells in 20 VCUs from infected mice treated with rat IgG or anti-c-kit *, P < 0.005 (bA). mMCP-1 was absent in sera of infected mice treated with anti-c-kit compared with control mice **, P < 0.003 (bB). Worm burdens in jejunum from anti-c-kit-treated mice were compared with control mice on day 9 postinfection; *, P < 0.05 (c). Increased intestinal permeability at day 9 postinfection was blocked by anti-c-kit treatment. *, P < 0.05 (d).
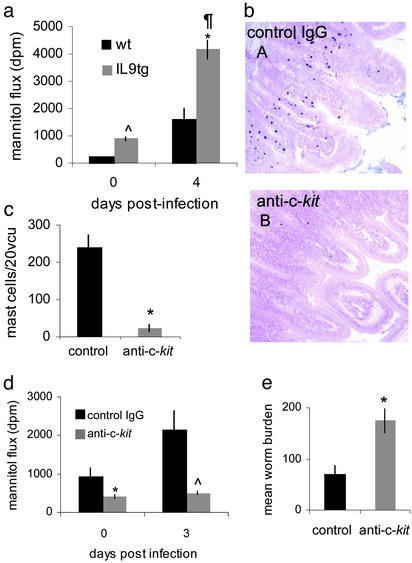
Overabundance of Mast Cells Increases Epithelial Permeability. The influence of mast cells on intestinal epithelium was further examined by using a transgenic strain of mice that overexpresses IL-9. We have previously shown that these mice have an abundance of mast cells in their small intestine at an earlier timepoint after infection by T. spiralis that results in faster expulsion of the parasite than WT animals (FVB) (28). Intestines from IL-9 transgenic mice were significantly more permeable to mannitol than those of WT animals on day 4 after infection (Fig. 3a). Uninfected tissue from IL-9 overexpressing mice was also significantly more permeable than uninfected WT tissue. This coincides with the elevated numbers of mast cells in the intestine and elevated serum mMCP-1 in these mice compared with WTs even in the absence of infection (8, 28). To rule out the possibility that IL-9 itself, rather than the mast cells, was inducing a highly permeable state after infection, we treated the mice with anti-c-kit antibodies. Our previous studies on IL-9 transgenic mice have shown that this inhibits efficient worm expulsion (28). Infected mice treated with anti-c-kit antibodies did not mount a mucosal mastocytosis compared with animals that received a control antibody (Fig. 3 b and c). Anti-c-kit treatment significantly blocked the increase in permeability after infection, reduced permeability in naïve animals (Fig. 3d), and inhibited worm expulsion (Fig. 3e).
Fig. 3.
IL-9 transgenic mice overexpress mucosal mast cells and enhance intestinal permeability. Intestinal permeability was compared between IL-9 transgenic (tg) mice and WT (wt) mice at days 0 and 4 postinfection. Λ, P < 0.002 wt vs. IL-9tg d0; *, P < 0.001 IL9tg d0 vs. d4 postinfection; ¶, P < 0.002 wt vs. IL-9tg d4 postinfection (a). Anti-c-kit antibody blocked mucosal mastocytosis in IL-9tg mice at d4 postinfection (bB) compared with infected control IgG-treated mice (bA), magnification ×200. Numbers of mast cells per 20 VCUs were counted in anti-c-kit-treated IL-9 tg mice compared with control IgG-treated mice; *, P < 0.03 (c). Anti-c-kit blocked enhanced mucosal permeability at d0 and d3 postinfection compared with IL-9 tg mice treated with control IgG antibodies, *, P < 0.03 control IgG vs. anti-c-kit d0; Λ, P < 0.05 control IgG vs. anti-c-kit d3 (d). Anti-c-kit antibody delayed worm expulsion compared with mice treated with control antibody; *, P < 0.02 (e).
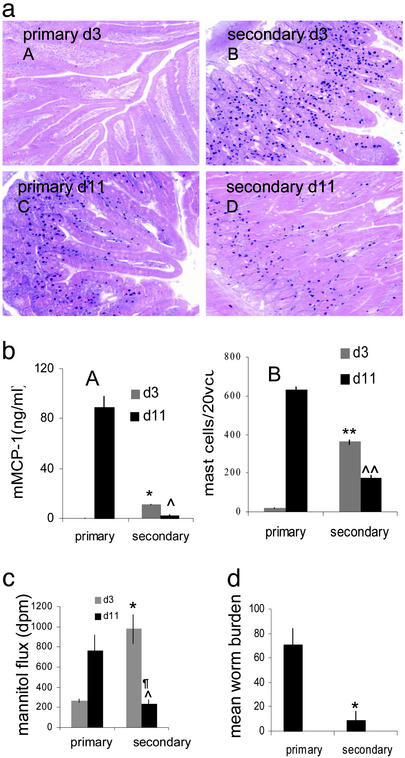
Epithelial Permeability Is Rapidly Enhanced During Secondary Infection. Mice that have previously experienced a T. spiralis infection will respond in a classical way to a challenge infection by mounting an accelerated and amplified immune response (29). Mast cell numbers and serum mMCP-1 levels were significantly higher at day 3 after infection in secondary infected animals than numbers at day 3 after primary infection. By day 11 after infection, numbers of intestinal mast cells and serum mMCP-1 of primary infected mice had risen, whereas mast cells and protease had already begun to decline in challenged animals (Fig. 4 a and b). Fig. 4c shows that intestines from challenged mice increased in permeability significantly faster than primary infected mice. This reflected worm expulsion, which occurred between days 3 and 4 in challenged animals, compared with primary infected mice that still had a full worm burden at this stage (Fig. 4d). After secondary worm expulsion from challenged mice intestinal permeability returned to preinflammatory levels (Fig. 4c).
Fig. 4.
Intestinal permeability is enhanced during secondary T. spiralis infection. Mast cell numbers were greater in jejuna of secondary infected mice on day 3 postinfection (aB) than in primary infected animals (aA). On day 11 postinfection mast cell numbers were greater in primary infected mice (aC) than in secondary infected mice (aD), magnification ×200. Mast cells were counted in 20 VCUs from primary and secondary infected mice on days 3 and 11 postinfection. **, P < 0.002 prim vs. sec d3; ΛΛ, P < 0.003 prim vs. sec d11 (bB). Sera levels of mMCP-1, *, P < 0.001 prim vs. sec d3; Λ, P < 0.001 prim vs. sec d11 (bA). Permeability was greater in secondary infected mice on day 3 postinfection compared with primarily infected mice but decreased by day 11 postinfection. *, P < 0.02 prim vs. sec d3; Λ, P < 0.05 prim vs. sec d11; ¶, P < 0.03 sec d3 vs. d11 (c). Worm burden of primary infected mice compared with secondary infected mice at d3 postinfection. *, P < 0.05 (d).
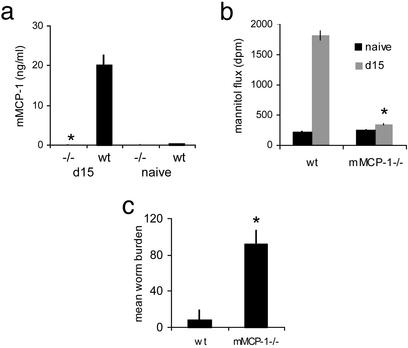
Increased Epithelial Permeability Is Blocked in mMCP-1-Deficient Mice. Previous work has shown that a key component of mast cell activity is the secretion of mMCP-1. Similar to mast cell numbers, the levels of mMCP-1 measured in the serum increases after parasite infection and declines after worm expulsion (27). Mice deficient for this protease are unable to expel their parasite load efficiently (9). Intestines from T. spiralis-infected mMCP-1 null mice were significantly less permeable than WT mice at day 15 after infection (Fig. 5b) and unable to expel their worm burden as compared with WT mice (Fig. 5c). Surprisingly, mast cell numbers in the intestine of infected null mice (374 ± 36 per 20 VCUs) were higher than the WT (193 ± 18 per 20 VCUs). This suggests (i) that the protease plays a regulatory role in development of mucosal mastocytosis during infection (30) and (ii) that it is secretion of mMCP-1 that is critical for increasing intestinal permeability during infection rather than the number of mast cells present in the intestine (although these correlate under normal conditions).
Fig. 5.
Delayed worm expulsion and reduced intestinal permeability of mMCP-1-deficient mice. Levels of mMCP-1 were measured in sera of WT and mMCP-1-deficient mice (mMCP-1-/-) at d0 and d15 postinfection. *, P < 0.02 -/- vs. wt d15 postinfection (a). Permeability of jejunum at d0 and d15 postinfection. *, P < 0.005 (b) and worm burdens at d15 postinfection. *, P < 0.04 (c) were compared between WT and mMCP-1-/- mice.
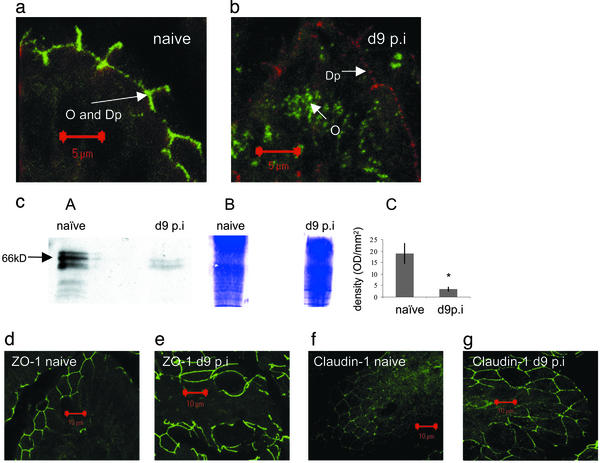
T. spiralis Infection Induces Disruption of TJs. TJs were examined in intestinal epithelium lining the villi of jejuna from naïve or infected mice. A marked change was detected in integrity of occludin, with infected mice showing a disorganized pattern of expression. Jejuna were dual-stained for occludin and the desmosomal protein, desmoplakin (Fig. 6 a and b). Occludin and desmoplakin were colocalized in the cell membrane of enterocytes of naïve mice (Fig. 6a). In infected tissue, however, desmoplakin (stained red) remained in the cell membrane whereas occludin (stained green) appeared to have translocated from the plasma membrane and into the cytoplasm (Fig. 6b). Diminished levels of occludin in infected epithelium compared with naïve were confirmed by Western blotting (Fig. 6c). Bands <66 kDa may correspond to degradation products resulting from spontaneous turnover. Equal loading of the gel was confirmed by Coomassie blue staining. The mean density of the 66-kDa bands from four different experiments is shown. Levels of expression and integrity of both the intracellular TJ protein ZO1 (Fig. 6 d and e) and claudin-1 (Fig. 6 f and g) in intestinal villi were comparable between infected and uninfected tissue.
Fig. 6.
TJ expression by jejunal epithelial cells from naïve or d9 post-T. spiralis-infected mice. Shown are TJ proteins in epithelium of intestinal villi visualized by confocal microscopy. (a) Dual staining of occludin (O) and desmoplakin (Dp) shows that both localize to the plasma membrane of naïve enterocytes. (b) Occludin (green) is internalized by enterocytes from d9 postinfected (p.i.) mice whereas desmoplakin (red) remains within the plasma membrane. (cA) Representative Western blot of occludin expression in epithelial tissue from naïve mice compared with d9 postinfection mice. (cB) A Coomassie blue-stained gel shows equal loading of total protein. (cC) Four Western blots of occludin from naïve or infected mice were analyzed by densitometry and the mean density of the 66-kDa band ± SEM is shown. *, P < 0.05. (d–g) Jejunal villi from naïve or infected mice were stained for ZO-1 (d and e) or claudin-1 (f and g).
Discussion
We provide here a compelling link between mucosal mast cells and intestinal epithelium during parasite infection. We show that an increase in intestinal epithelial paracellular permeability occurs during the course of both a primary and secondary T. spiralis infection and that this effect is induced by mast cells. In addition, we demonstrate that the kinetics of permeability changes parallels the kinetics of nematode expulsion. It has been postulated for many years that an influx of solutes and water into the gut during enteric nematode infection may be a method by which the host attempts to expel the parasite (2).
Enhanced intestinal permeability is also a feature of infection by another small intestine-dwelling nematode, Nippostrongylus brasiliensis. King and Miller (31) challenged previously infected rats with soluble N. brasiliensis antigen and identified rat MCP-II in the intestinal perfusate. However, unlike T. spiralis, expulsion of N. brasiliensis does not depend on mast cells (32), and the cell type or factors that increase enteric leakiness during this infection remained undefined. Shea-Donohue et al. (33) stimulated tissue from Heligmosomoides polygyrus-challenged mice with prostaglandin E2 and observed a rise in chloride ion secretion. This response was blocked when mice were treated with anti-IL-4 receptor antibodies. N. brasiliensis infection induces a strong IL-4 response, which is critical for resolution of infection (34), which may be a factor responsible for increasing enteric permeability in both these models. T. spiralis also induces a strong T helper 2 response and high levels of IL-4 production (3) but it is the mast cell that is crucial for efficient worm expulsion (7, 26, 27). In the absence of mast cells and mMCP-1 [but with IL-4 still present (35)] we have shown that infection-induced permeability increase is blocked. This block may account for the inability of these mice to expel parasites efficiently. Further proof of the mast cell's effect on intestinal epithelium is shown by the present data from T. spiralis-infected IL-9 transgenic mice. These mice overexpress mast cells and accordingly have highly permeable intestines.
Our data support the results of Harari et al. (36), who demonstrated increased intestinal chloride ion secretion in T. spiralis-infected tissue in response to T. spiralis antigen challenge in vitro. Their further work (37) revealed an increase in chloride ion secretion by infected tissue in response to externally applied histamine, serotonin, and prostaglandin E2, three factors produced by mast cells. Equally, Madden et al. (38) showed that WT mice but not mast cell-deficient mice respond to IL-4 by increasing chloride secretion after either histamine or prostaglandin E2 stimulation in vitro. Their data support our demonstration that mast cells are crucial for increased mucosal permeability during T. spiralis infection. We show here that mice deficient in the homologous β-chymase mMCP-1 fail to increase mucosal permeability, explaining their inability to resolve T. spiralis infection efficiently. This finding is supported by the work of Scudamore et al. (39), who treated rat jejunum with purified rat MCP-II in the absence of infection and observed an increase in mucosal permeability.
Application of rat MCP-II to a dog kidney epithelial cell line induces permeability and alters the distribution of TJ proteins surrounding the apex of the cells (40). Because T. spiralis infection increased mucosal permeability, we hypothesized that TJ integrity may be compromised. We observed dramatic disruption of occludin localization with protein translocating from the membrane into the cytosol. This observation, along with diminished levels of occludin protein, suggests degradation by a protease. Because we have shown that mMCP-1, a serine protease, is critical for increased paracellular permeability in this infection model, we postulate that its action may involve occludin degradation. Wan et al. showed that occludin could be cleaved by both a cysteine protease, Der p1 (21), and serine proteases extracted from house dust mite fecal pellets (41). Because mMCP-1 is also a serine protease, mMCP-1 may be able to cleave occludin. Disassociation of occludin from F-actin may also result in increased paracellular permeability, an effect demonstrated by the deletion of the COOH terminus of occludin in vitro (42). Moreover, mMCP-1 may indirectly disrupt assembly of occludin by interfering with normal rapid turnover by ubiquitination (43). Despite the effect on occludin, there was no discernible change in the cytoplasmic protein ZO-1 or claudin-1. ZO-1 is also known to associate with claudin molecules, thereby maintaining its position in the junction despite the demise of occludin. However, it is possible that the function of TJs may be compromised because of altered localization of other TJ proteins. The full complement of TJ proteins in the small intestine has not been characterized. For example, 23 other isoforms of claudin exist, and it remains to be determined which are expressed by intestinal epithelium under normal conditions and during inflammation. The arrangement of pore-forming claudin proteins between enterocytes, rather than the quantity of protein, may be a critical factor in conferring “tightness.” Moreover, there may be subtle signaling effects on the regulation of TJs including phosphorylation of occludin (44, 45).
In conclusion, we have shown that T. spiralis infection increases paracellular permeability of the jejunum and decreases the expression of occludin in the TJs of enterocytes. In the absence of mucosal mast cells or mMCP-1 this enhanced intestinal permeability is blocked. These data finally explain the mechanism mediating the expulsion of this parasite from the intestine and furthermore provide a compelling link between mast cells and epithelium during T helper 2-dominated disease states.
Acknowledgments
We thank Drs. Jacques Van Snick and Jean Christophe Renauld (Ludwig Institute for Cancer Research, Catholic University of Louvain, Brussels) for the IL-9 transgenic mice and Steve Bagley (Paterson Institute for Cancer Research, Manchester, U.K.) for help with confocal microscopy. Anti-c-kit antibody was a kind gift of Dr. S Nishikawa (Kyoto University, Kyoto). This work was funded by the Medical Research Council, United Kingdom (to J.R.M., R.E.B., D.R.G., and R.K.G.) and Wellcome Trust Grant 060312 (to P.A.K. and H.R.P.M.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: mMCP-1, mouse mast cell protease-1; TJ, tight junction; VCU, villus/crypt unit.
References
- 1.Despommier, D. (1995) in Enteric Infection, eds. Farthing, M. J. G., Keutsch G. T. & Wakelin, D. (Chapman & Hall, London), Vol. 2, pp. 107-113. [Google Scholar]
- 2.Murray, M., Jarrett, W. F. & Jennings, F. W. (1971) Immunology 21, 17-31. [PMC free article] [PubMed] [Google Scholar]
- 3.Grencis, R. K., Hultner, L. & Else, K. J. (1991) Immunology 74, 329-332. [PMC free article] [PubMed] [Google Scholar]
- 4.Lammas, D. A., Wakelin, D., Mitchell, L. A., Tuohy, M., Else, K. J. & Grencis, R. K. (1992) Parasitology 105, 117-124. [DOI] [PubMed] [Google Scholar]
- 5.Alizadeh, H. & Wakelin, D. (1982) Clin. Exp. Immunol. 49, 331-337. [PMC free article] [PubMed] [Google Scholar]
- 6.Garside, P., Grencis, R. K. & Mowat, A. M. (1992) Parasite Immunol. (Oxf.) 14, 217-225. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya, M., Oku, Y., Itayama, H. & Obayashi, M. (1985) J. Helminthol. 59, 233-239. [DOI] [PubMed] [Google Scholar]
- 8.Godfraind, C., Louahed, J., Faulkner, H., Vink, A., Warnier, G., Grencis, R. K. & Renauld, J.-C. (1998) J. Immunol. 160, 3989-3996. [PubMed] [Google Scholar]
- 9.Knight, P. A., Wright, S. H., Lawrence, C. E., Paterson, Y. Y. & Miller, H. R. P. (2000) J. Exp. Med. 192, 1849-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson, J. M. (2001) News Physiol. Sci. 16, 126-130. [DOI] [PubMed] [Google Scholar]
- 11.Tsukita, S., Furuse, M. & Itoh, M. (2002) Nat. Rev. Mol. Cell Biol. 2, 285-293. [DOI] [PubMed] [Google Scholar]
- 12.Tsukita, S., Furuse, M. & Itoh, M. (1999) Curr. Opin. Cell Biol. 11, 628-633. [DOI] [PubMed] [Google Scholar]
- 13.Tsukita, S. & Furuse, M. (2002) Curr. Opin. Cell Biol. 14, 531-536. [DOI] [PubMed] [Google Scholar]
- 14.Colegio, O. R., Van Itallie, C. M., McCrea, H. J., Rahner, C. & Anderson, J. M. (2002) Am. J. Physiol. 283, C142-C147. [DOI] [PubMed] [Google Scholar]
- 15.Balda, M., Flores-Maldonado, C., Cereijido, M. & Matter, K. (2000) J. Cell. Biochem. 78, 85-96. [PubMed] [Google Scholar]
- 16.Ahdieh, M., Vandenbos, T. & Youakim, A. (2001) Am. J. Physiol. 281, C2029-C2038. [DOI] [PubMed] [Google Scholar]
- 17.Ceponis, P. J. M., Botelho, F., Richards, C. D. & McKay, D. M. (2000) J. Biol. Chem. 275, 29132-29137. [DOI] [PubMed] [Google Scholar]
- 18.Wu, Z., Milton, D., Nybom, P., Sjo, A. & Magnusson, K.-E. (1996) Microb. Pathog. 21, 111-123. [DOI] [PubMed] [Google Scholar]
- 19.Wells, C. L., van de Westerlo, E. M., Jechorek, R. P., Feltis, B. A., Wilkins, T. D. & Erlandsen, S. L. (1996) Gastroenterology 110, 1429-1437. [DOI] [PubMed] [Google Scholar]
- 20.Simonovic, I., Rosenberg, J., Koutsouris, A. & Hecht, G. (1999) Cell Microbiol. 2, 305-315. [DOI] [PubMed] [Google Scholar]
- 21.Wan, H., Winton, H. L., Soeller, C., Tovey, E. R., Gruenert, D. C., Thompson, P. J., Stewart, G. A., Taylor, G. W., Garrod, D. R., Cannell, M. B. & Robinson, C. (1998) J. Clin. Invest. 104, 123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renauld, J. C., van der Lugt, N., Vink, A., van Roon, M., Godfraind, C., Warnier, G., Merz, H., Feller, A., Berns, A. & Van Snick, J. (1994) Oncogene 9, 1327-1332. [PubMed] [Google Scholar]
- 23.Wastling, J. M, Knight, P., Ure, J., Wright, S., Thornton, E. M., Scudamore, C. L., Mason, J., Smith, A. & Miller, H. R. P. (1998) Am. J. Pathol. 153, 491-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakelin, D. & Wilson, M. M. (1977) Parasitology 74, 215-234. [DOI] [PubMed] [Google Scholar]
- 25.Evans, G. S., Flint, N., Somers, A. S., Eyden, B. & Potten, C. S. (1992) J. Cell Sci. 101, 219-231. [DOI] [PubMed] [Google Scholar]
- 26.Grencis, R. K., Else, K. J., Huntley, J. F. & Nishikawa, S. I. (1993) Parasite Immunol. (Oxf.) 15, 55-59. [DOI] [PubMed] [Google Scholar]
- 27.Tuohy, M., Lammas, D. A., Wakelin, D., Huntley, J. F., Newlands, G. F. J. & Miller, H. R. P. (1990) Parasite Immunol. (Oxf.) 12, 675-685. [DOI] [PubMed] [Google Scholar]
- 28.Faulkner, H., Humphreys, N., Renauld, J.-C., Van Snick, J. & Grencis, R. K. (1997) Eur. J. Immunol. 27, 2536-2540. [DOI] [PubMed] [Google Scholar]
- 29.Wakelin, D. & Lloyd, M. (1976) Parasitology 72, 173-182. [DOI] [PubMed] [Google Scholar]
- 30.Miller, H. R. P. & Pemberton, A. D. (2002) Immunology 105, 375-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King, S. J. & Miller, H. R. P. (1984) Immunology 51, 653-660. [PMC free article] [PubMed] [Google Scholar]
- 32.Crowle, P. K. & Reed, N. D. (1981) Infect. Immun. 33, 54-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shea-Donohue, T., Sullivan, C., Finkelman, F. D., Madden, K. B., Morris, S. C., Goldhill, J., Pineiro-Carrero, V. & Urban, J. F., Jr. (2001) J. Immunol. 167, 2234-2239. [DOI] [PubMed] [Google Scholar]
- 34.Finkelman, F. D., Shea-Donohue, T., Goldhill, J., Sullivan, C. A., Morris, S. C., Madden, K. B., Gause, W. C. & Urban, J. F., Jr. (1997) Annu. Rev. Immunol. 15, 505-533. [DOI] [PubMed] [Google Scholar]
- 35.Donaldson, L. E., Schmitt, E., Huntley, J. F., Newlands, G. F. & Grencis, R. K. (1996) Int. Immunol. 8, 559-567. [DOI] [PubMed] [Google Scholar]
- 36.Harari, Y., Russell, D. A. & Castro, G. A. (1987) J. Immunol. 138, 1250-1255. [PubMed] [Google Scholar]
- 37.Castro, G. A., Harari, Y. & Russell, D. (1987) Am. J. Physiol. 253, G540-G548. [DOI] [PubMed] [Google Scholar]
- 38.Madden, K. B., Whitman, L., Sullivan, C., Gause, W. C., Urban, J. F., Jr., Katona, I. M., Finkelman, F. D. & Shea-Donohue, T. (2002) J. Immunol. 169, 4417-4422. [DOI] [PubMed] [Google Scholar]
- 39.Scudamore, C. L., Thornton, E. M., McMillan, L., Newlands, G. F. & Miller, H. R. P. (1995) J. Exp. Med. 182, 1871-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scudamore, C. L., Jepson, M. A., Hirst, B. H. & Miller, H. R. P. (1998) Eur. J. Cell Biol. 75, 321-330. [DOI] [PubMed] [Google Scholar]
- 41.Wan, H., Winton, H. L., Soeller, C., Taylor, G. W., Gruenert, D. C., Thompson, P. J., Cannell, M. B., Stewart, G. A., Garrod, D. R. & Robinson, C. (2001) Clin. Exp. Allergy 31, 279-294. [DOI] [PubMed] [Google Scholar]
- 42.Balda, M. S., Whitney, J. A., Flores, C., Gonzalez, S., Cereijido, M. & Matter, K. (1996) J. Cell Biol. 134, 1031-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Traweger, A., Fang, D., Liu, Y. C., Stelzhammer, W., Krizbai, I. A., Fresser, F., Bauer, H. C. & Bauer, H. (2002) J. Biol. Chem. 277, 10201-10208. [DOI] [PubMed] [Google Scholar]
- 44.Walsh, S. V., Hopkins, A. M., Chen, J., Narumiya, S., Parkos, C. A. & Nusrat, A. (2001) Gastroenterology 121, 566-579. [DOI] [PubMed] [Google Scholar]
- 45.Wong, V. (1997) Am. J. Physiol. 273, C1859-C1867. [DOI] [PubMed] [Google Scholar]