Abstract
Vascular endothelial growth factor (VEGF) is a critical promoter of blood vessel growth during embryonic development and tumorigenesis. To date, studies of VEGF antagonists have primarily focused on halting progression in models of minimal residual cancer. Consistent with this focus, recent clinical trials suggest that blockade of VEGF may impede cancer progression, presumably by preventing neoangiogenesis. However, VEGF is also a key mediator of endothelial–vascular mural cell interactions, a role that may contribute to the integrity of mature vessels in advanced tumors. Here, we report that high-affinity blockade of VEGF, using the recently described VEGF-Trap, abolishes mature, preexisting vasculature in established xenografts. Eradication of vasculature is followed by marked tumor regression, including regression of lung micrometastases. Thus, the contribution of relatively low levels of VEGF to vessel integrity may be critical to maintenance of even very small tumor masses. Potent blockade of VEGF may provide a new therapeutic option for patients with bulky, metastatic cancers.
Expression of vascular endothelial growth factor (VEGF) is nearly ubiquitous in human cancer, consistent with its role as a key mediator of tumor neoangiogenesis. Blockade of VEGF function, by binding to the molecule or its VEGFR-2 receptor, inhibits growth of implanted tumor cells in multiple different xenograft models (1–3). Moreover, recent clinical testing seems to validate the choice of VEGF as a new target for cancer (4). However, previous studies have focused on the role of VEGF in models of minimal residual disease, in which inhibitors are used with the goal of preventing tumor growth rather than treating large lesions with established vasculature and distant metastases. In support of this approach has been the observation that established vascular networks in normal tissues, in which recruited smooth muscle-like perivascular cells adhere to endothelium, do not seem to become destabilized when VEGF is withdrawn or antagonized (5, 6).
Tumors engineered to stop VEGF production after growth and development of a vascular network exhibit regression primarily of those vessels that lack vascular mural cells (6, 7). However, we reasoned that the apparent susceptibility of endothelial-only tumor vessels to VEGF withdrawal might be relative, rather than absolute, and that this pathological vasculature may remain globally dependent on VEGF. Withdrawal of tumor-derived VEGF might still allow for survival of vessels whose endothelium requires only the low levels of VEGF provided by associated stromal cells. Such tumor vessels, when compared with the vasculature of normal tissues, might still be relatively immature and pathological, and thus vulnerable to VEGF blockade. This vulnerability may be reflected in recent findings that pericytes in tumor vessels can appear to be morphologically abnormal, displaying a looser association with endothelial cells and altered immunoreactivity compared with those in normal tissues (8). Thus, we hypothesized that blockade of both tumor and stromal VEGF might potentially disrupt endothelial–perivascular cell signaling in at least some tumors, leading to destabilization of vasculature and frank tumor regression.
A previous comparative study of antiangiogenic agents in experimental tumors suggests that molecules targeting VEGF are the most effective in up-front tumor inhibition (9). The most efficient anti-VEGF blocking strategy reported to date involves using soluble forms of the VEGF receptor 1 (VEGFR-1) (2). Therefore, we tested the effect of a recently described soluble decoy receptor, the VEGF-Trap (10, 11). This construct incorporates domains of both VEGFR-1 and VEGFR-2 and binds VEGF with significantly higher affinity than previously reported VEGF antagonists (10). To investigate whether blocking the additional VEGF in the tumor vessel microenvironment would produce disruption of preexisting vasculature, we examined the results of administering VEGF-Trap to animals with established xenografts and metastases.
Methods
Xenograft Model. SK-NEP-1 cells (American Type Culture Collection) were maintained in culture with McCoy's 5A medium (Mediatech, Fisher Scientific), supplemented with 15% FBS and 1% penicillin-streptomycin (GIBCO). Cells were grown at 37°C in 5% CO2 until confluent, harvested, counted with trypan blue staining, and washed and resuspended in sterile PBS at a concentration of 107 cells per ml. Xenografts were established in 4- to 6-week-old female NCR nude mice (National Cancer Institute–Frederick Cancer Research and Development Center) by intrarenal injection of 106 SK-NEP-1 cultured human Wilms tumor cells and allowed to grow. After 5 weeks, large tumors were palpable in all mice, and a cohort was randomly selected (n = 10) to provide day-0 controls. Remaining mice were divided into two groups and injected twice weekly with VEGF-Trap (500 μg; Regeneron Pharmaceuticals, Tarrytown, NY) or an equal amount of human Fc protein in the same volume of vehicle. Mice (n = 5 control and treated animals at each time point) were killed at days 1, 5, 8, 15, and 27 after initiation of injections, and tumors were excised and weighed. Only treated mice survived until day 36 (n = 10).
Lectin Perfusion. Before death, selected mice at each time point underwent intravascular injection of fluorescein-labeled Lycopersicon esculentum lectin (100 μg in 100 μl of saline, Vector Laboratories) into the left ventricle. The vasculature was fixed by infusion of 1% paraformaldehyde (pH 7.4) in PBS and then washed by perfusion of PBS, as described (12).
Digital Image Analysis. Digital images from the fluorescein-labeled lectin studies were captured from a Nikon E600 fluorescence microscope (×10 objective) with a Spot RT slider digital camera (Diagnostic Instruments, Sterling Heights, MI) and stored as TIFF files. Quantitative assessment of angiogenesis and tumor vessel architecture was performed by computer-assisted digital image analysis as described by Wild et al. (13), except that fluorescein-labeled lectin was substituted for phycoerythrin-conjugated mAb to CD-31. The fraction of fluorescein-labeled lectin-positive pixels per total field was quantified by a computer-assisted method as described (13). Changes in vessel architecture were evaluated by quantifying branch points (nodes), end points, and total vessel length. Images were analyzed after application of a common threshold value, inversion of the image, morphological erosion, and skeletonization, using a combination of PHOTOSHOP (Adobe Systems, Mountain View, CA) and IMAGE PROCESSING TOOL KIT (Reindeer Graphics, Raleigh, NC) as described (13).
Platelet-Endothelial Cell Adhesion Molecule 1 (PECAM-1) Immunostaining. Control and VEGF-Trap-treated tumors were immunostained with a rat anti-mouse PECAM-1 mAb (Research Diagnostics, Flanders, NJ) and a rabbit anti-rat biotinylated secondary antibody (Zymed). Enhanced horseradish peroxidase-conjugated streptavidin and a substrate chromogen, AEC (3-amino-9-ethyl carbazole), were used to visualize the signal (HISTOSTAIN-PLUS kit, Zymed); slides were examined with a Nikon Eclipse E600 microscope.
α-Smooth Muscle Actin (αSMA) Immunostaining. Monoclonal anti-αSMA antibody (Sigma) was incubated at room temperature for 30 min. Specimens were then incubated with a 1:400 rabbit anti-mouse biotinylated secondary antibody. Fluorescein-labeled avidin was used to develop a green fluorescent signal. Specimens were analyzed and photographed by fluorescence microscopy.
Confocal Microscopy. Serial optical sections of lectin-perfused tumor were acquired by using a Zeiss LSM 410 confocal laser-scanning microscope. A computerized algorithm was used to assign color codes to fluorescein-labeled vessels by depth of field.
PECAM-1, αSMA, and Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick End Labeling (TUNEL) Double Staining. Apoptosis was determined by TUNEL staining. Immunofluorescent double-staining for PECAM-1/apoptosis and αSMA/apoptosis was performed on frozen sections by using the ApopTag red in situ apoptosis detection kit (Intergen, Purchase, NY) and either rat anti-mouse PECAM-1 or anti-αSMA mAb. A biotinylated secondary antibody was used in combination with fluorescein-labeled avidin to visualize endothelial and vascular mural cells, respectively. Slides were examined and photographed by fluorescence microscopy.
In Situ Hybridization. Tissue was initially preserved in 4% paraformaldehyde overnight, transferred to 17% sucrose, and embedded in OCT compound and frozen. Tissue sections were then probed with 35S-labeled cRNA with probes hybridizing to human VEGF, Ang-2, or mouse VEGFR-2 as described (14).
Analysis of Metastases. Three different levels of hematoxylin and eosin-stained sections through the entire lung of each tumor-bearing animal were examined for metastasis. Cells per metastasis were counted, metastasis diameters were measured independently by two observers, and the numbers were averaged. Volume was calculated by the standard formula (length) × (width)2 × 0.5.
Statistical Analysis. Comparisons of image analysis measurements, tumor weights, and metastasis measurements (cell count, largest diameter, and volume) were performed by using Kruskal–Wallis analysis.
Results
Regression of Established Tumors During VEGF-Trap Injection. Orthotopically implanted SK-NEP-1 human Wilms tumor cells were allowed to grow for 5 weeks, forming large retroperitoneal tumors (mean weight, 5.8 ± 1.1 g; Fig. 1A). Injections of VEGF-Trap (500 μg) or Fc control protein were then given i.p. biweekly. Subsets of treated and control mice were killed at intervals. By day 36, mean tumor weight had decreased by 79.3% (day 36, 1.2 ± 0.3 g, P < 0.0002; Fig. 1A). On gross examination, the VEGF-Trap-treated tumors were markedly pale as compared with control tumors (Fig. 1B), with strikingly diminished vasculature by day 15 (Fig. 1C) and virtual absence of vessels by day 36 (Fig. 1D). The kidney, which was grossly replaced by tumor tissue, reemerged as the tumor tissue receded, returning to a remarkably normal appearance by day 36 (Fig. 1D).
Fig. 1.
VEGF-Trap causes involution of xenograft vessels, followed by regression of tumors. Xenografts were established in NCR nude mice and were allowed to grow for 5 weeks. (A) A random cohort of mice was killed (n = 10) to provide day-0 controls (mean weight 5.8 ± 1.1 g). The remaining mice were divided into two groups and injected twice weekly with VEGF-Trap (500 μg) or an equal amount of human Fc protein. Mice were killed at days 1, 5, 8, 15, and 27 after initiation of injections (mean tumor weights ± SEM: 5.5 ± 1.02, 4.2 ± 0.66, 3.9 ± 0.87, 3.5 ± 0.91, and 2.7 ± 0.8 g, respectively). Only treated mice survived until day 36 (mean tumor weight ± SEM: 1.2 g ± 0.3 g, P < 0.0002 vs. day-0 controls). Error bars represent standard error of the mean. (B) Xenografts were initially abundantly vascular at day 0. (C) Surface vessels disappeared and tumors became pale and poorly perfused by day 15. Arrows indicate the contralateral, non-tumor-bearing kidney (approximate size: 10 × 5 mm) in B and C. (D) The native ipsilateral kidney (arrowhead) subsequently reemerged as tumors regressed (day 36).
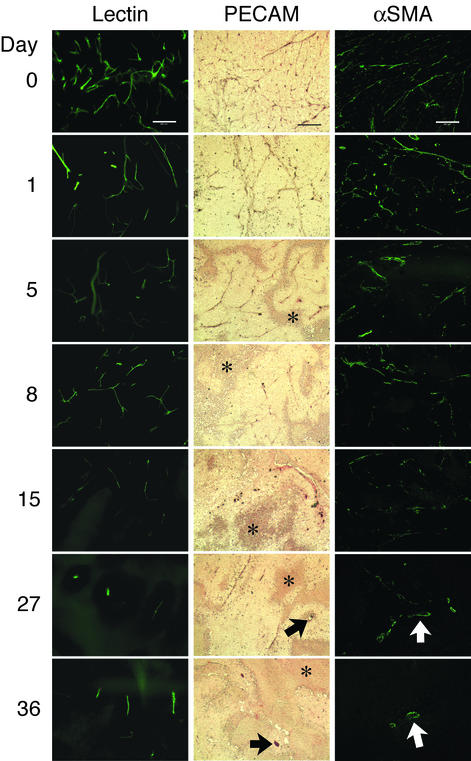
Involution of Existing Vasculature During VEGF-Trap Injection. We next examined in detail the vascular alterations caused by VEGF-Trap. To outline the vessel lumens, we injected fluorescein-labeled L. esculentum lectin intravascularly in tumor-bearing animals. One day after the first injection of VEGF-Trap (day 1), we observed a marked decrease in lectin-outlined vessels (Fig. 2, lectin). In a separate experiment, we used quantitative image analysis to compare microvessel density, total length of lectin-perfused vessels, vessel ends, and branch points/nodes in tumors 1 day after VEGF-Trap injection. Tumor weights were unchanged as compared with controls at the same time point. VEGF-Trap-treated tumor vasculature showed significant decreases in all parameters measured as compared with untreated controls: microvessel density decreased by 54% (37,599 ± 23,428 vs. 81,167 ± 39,363, mean white pixel count ± SD, P = 0.037), total vessel length decreased by 42% (3,340 ± 1,244 vs. 5,725 ± 1,438, P = 0.01), vessel ends decreased by 63% (127 ± 22 vs. 347 ± 178, P < 0.004), and branches points/nodes decreased by 80% (17 ± 6 vs. 85 ± 40, P < 0.004). Vasculature progressively disappeared, resulting in almost complete absence of vessels by day 15. No changes in vessel architecture were observed in normal tissues in VEGF Trap-treated animals (data not shown).
Fig. 2.
Progressive decrease in luminal perfusion and in endothelial and vascular mural compartments of vasculature during the course of VEGF-Trap injection. Day 0 represents tumor vasculature before VEGF-Trap treatment. Before death, selected mice at each time point underwent intravascular injection of fluorescein-labeled L. esculentum lectin. Fluorescein-labeled vasculature decreased progressively, detectable 1 day after the first injection of VEGF-Trap. For immunostaining for endothelial cells (middle column), PECAM-1-stained vasculature decreased after the first dose of VEGF-Trap, with rare remaining immunopositive cells by 27 and 36 days (arrows). Areas of necrosis (asterisks) surround viable regions, which have surviving vessels centrally. For immunostaining for vascular mural cells (right column), decrease in αSMA-positive staining was observed after one dose of VEGF-Trap, and scant remaining αSMA-positive vessels were seen at 27 and 36 days with relatively large diameters (arrows). (Scale bars, 200 μm.)
We compared these perfusion studies with the status of endothelial and recruited perivascular cells in tumors by performing specific immunostaining for these populations in the same samples. We demonstrated a similarly timed decrease in endothelial cells: PECAM-1-immunopositive vasculature diminished after one injection of VEGF-Trap (day 1), with abolition of endothelium by day 15 (Fig. 2, PECAM). Necrosis of tumor cells was evident by day 5. It has been proposed that recruitment of vascular mural cells protects tumor endothelium from apoptosis during withdrawal of VEGF. If this were the case, it might be predicted that αSMA-immunopositive vasculature (8) would not regress during VEGF blockade, or would do so more slowly than endothelial cells alone. However, immunostaining for αSMA demonstrated that this population of cells decreased after one injection of VEGF-Trap and was absent by day 15 (Fig. 2, αSMA), in parallel with endothelium.
To examine vascular anatomic changes resulting from this rapid involution of endothelium and perivascular cells in detail, we performed confocal microscopic analysis with pseudodepth coloring through sections of lectin-perfused tumor 1 day after the initial injection of VEGF-Trap (Fig. 3B). These studies demonstrate that VEGF-Trap causes not only a rapid decrease in vascularity but abrupt truncation of vessels, consistent with luminal collapse (green or pale blue vessel termini indicate location in midsection).
Fig. 3.
Truncation of vasculature during treatment with VEGF-Trap. (A) Day-0 tumor sections during confocal microscopy of lectin-perfused sections. Colors assigned by computer algorithm indicate the relative spatial orientation of vessels. (B) Truncation of vessels is observed at day 1, with abrupt termination of branches (arrows) in midfield. A decrease in branching off large vessels is also evident. (Scale bars, 100 μm.)
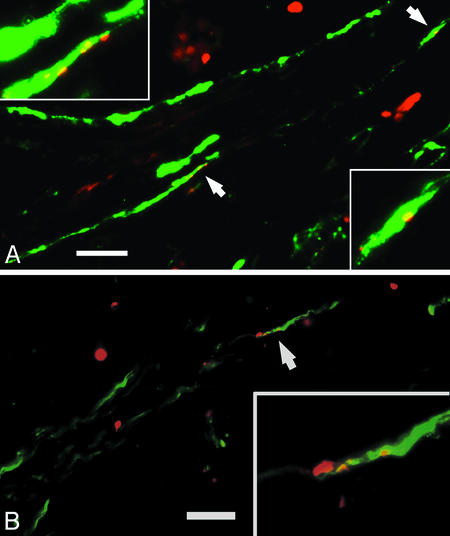
Apoptosis in Endothelial and Vascular Mural Cells. If VEGF-mediated signaling is critical to the survival of both the endothelial and vascular mural cells of mature tumor vessels, we reasoned that apoptosis should be detectable concurrently in both cell populations. Double-labeling using the TUNEL assay combined with PECAM-1 (Fig. 4A) and αSMA (Fig. 4B) immunostaining demonstrated apoptosis in both components of xenograft vessels 1 day after the initial injection of VEGF-Trap. We observed more widespread apoptosis in endothelial and recruited perivascular cells at day 5 (data not shown). These observations suggest that potent blockade of VEGF rapidly interrupts the endothelial–vascular mural cell signaling, which protects both components of tumor vessels from apoptosis. Thus, a certain level of VEGF may be critical to stability even in “mature” tumor vasculature.
Fig. 4.
Endothelial and vascular mural cell populations undergo apoptosis concurrently, detectable 1 day after initiation of VEGF-Trap injections. Double-labeling studies were performed to identify endothelial and recruited vascular mural cells (using anti-PECAM an anti-αSMA antibodies, respectively) and apoptosis (using TUNEL assays). TUNEL-positive nuclei (red) colocalize with endothelial cells (A, gray signal) and vascular mural cells (B, gray signal) in regions of intact vessels 1 day after start of VEGF-Trap treatment. (Scale bars, 25 μm.)
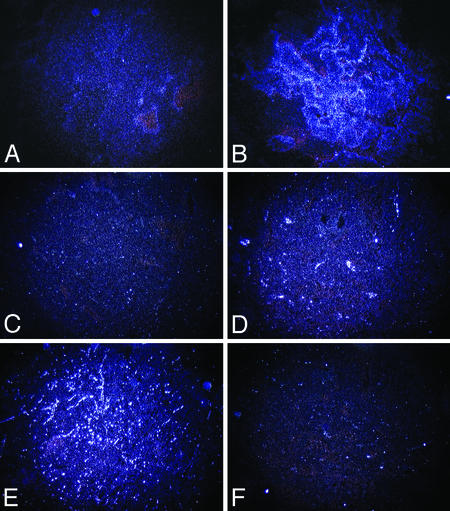
Alteration in Expression of Angiogenic Factors in Tumors Exposed to VEGF-Trap. Expression of VEGF is exquisitely regulated by hypoxia (15, 16), whereas angiopoietin-2 (Ang-2) is regulated both by VEGF and by hypoxia (17). Concurrent expression of VEGF and Ang-2 may therefore serve as an indication of the physiologic response of tumor cells to hypoxia, which normally promotes angiogenic remodeling and new capillary sprouting (18). In addition, Ang-2 can cause vessel involution when VEGF is deficient (14). We reasoned that tumors regressing solely as a result of vascular involution should exhibit global up-regulation of these factors but decreased expression of VEGFR-2, a marker for growing vasculature. We investigated VEGF, Ang-2, and VEGFR-2 expression by in situ hybridization. Expression of VEGF and Ang-2 increased markedly between day 0 and day 36 (Fig. 5 A–D). Conversely, expression of VEGFR-2 in tumors decreased over the same period (Fig. 5 E and F), consistent with the disappearance of endothelial cells expressing this receptor.
Fig. 5.
Increased xenograft expression of VEGF and Ang-2 over the course of VEGF-Trap-induced regression, with concurrent decrease in VEGFR-2 expression. VEGF expression is low at day 0 (A) but increases markedly by day 36 (B). Expression of Ang-2, which can cause vessel involution when VEGF is deficient, similarly increases from day 0 (C) to day 36 (D). VEGFR-2 expression decreases from day 0 (E) to day 36 (F). (Original magnification, ×4.)
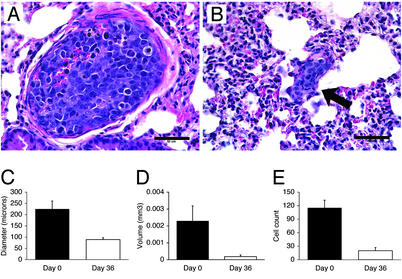
Regression of Established Lung Metastases During VEGF-Trap Administration. Blockade of VEGF has previously been shown to decrease subsequent formation of lung micrometastases in our model (19). However, the role of VEGF in maintenance of lung metastases is unknown. We found that 60% of mice at day 0 and 50% of VEGF-Trap treated mice at day 36 had lung metastases, and that the number of established metastases had not significantly changed. However, pulmonary tumor deposits were strikingly smaller in the VEGF-Trap-treated lungs (Fig. 6B) in comparison with controls (Fig. 6A). We quantified the size of the pulmonary lesions at days 0 and 36 by diameter (Fig. 6C), volume (Fig. 6D), and individual cell count (Fig. 6E). There was a significant decrease in the size of the pulmonary metastases by all three measurements. Mean diameter of metastases decreased by 80% (225.2 ± 35.4 μm vs. 89.2 ± 8.4 μm, P = 0.0005), mean volume decreased by 78% (0.0023 ± 0.0009 mm3 to 0.00018 ± 0.0001 mm3, P = 0.0004), and mean cell count per metastasis decreased by 83% (115.3 ± 16.9 to 20.1 ± 7.2, P = 0.0002). TUNEL assay demonstrated apoptosis in lung metastases after one dose of VEGF-Trap (data not shown), whereas apoptotic cells were rare in day-0 control metastases. Day-0 metastases were adjacent to lung capillaries, rather than surrounding new vessels, a pattern that was not changed in day 36 metastases. These results suggest that the maximum size of tumor deposit sustainable without perfusion may be even smaller than previous estimates (20), or that such micrometastases depend on other properties of VEGF (such as enhanced endothelial permeability).
Fig. 6.
Pulmonary metastases decrease in size 36 days after initiation of VEGF-Trap injections. Pulmonary micrometastases in day-0 control (A) and tumor 36 days (B) after initiation of VEGF-Trap treatment (arrow). (Scale bars, 50 μm.) The incidence of pulmonary metastasis and the pattern of adjacent lung microvessels in tumor-bearing animals did not change significantly during VEGF-Trap administration, but diameter (C), volume (D), and cell count (E) significantly decreased.
Discussion
Previous investigators have reported that those tumor vessels in which a layer of vascular mural cells lies adjacent to endothelium are protected from the effects of tumor-derived VEGF withdrawal (6, 7). We reasoned that the effect of VEGF produced locally by endothelial or stromal cells should not be altered by cessation of tumor VEGF production. In addition, a low level of VEGF might not be captured by agents with less affinity for this factor than the soluble receptor decoy construct we tested (10, 11). If the role of VEGF in endothelial–vascular mural cell trafficking is critical to tumor vessel integrity, even mature tumor vasculature might be susceptible to disruption by such a high-affinity anti-VEGF agent. In support of this hypothesis, in our studies VEGF-Trap caused concurrent apoptosis of both endothelial and recruited perivascular cells in preexisting tumors, without the apparent protective effect of the vascular mural cell layer. Expression of Ang-2 by hypoxic tumors, in conjunction with VEGF depletion by VEGF-Trap, is likely to have further promoted vessel involution (14). It is also possible that VEGF-Trap may bind other VEGF family members with affinity for VEGFR-1 or VEGFR-2, such as PlGF (21), and thus may also disrupt the additional contributions of these cytokines to vascular stability.
A variety of antiangiogenic agents prevent growth of implanted xenografts, a setting that mimics the status of minimal residual disease in human cancer patients. However, many patients with resistant cancers have bulky primary lesions or metastases. These individuals are at high risk of dying from their disease and would benefit greatly if antiangiogenic drugs that regressed preexisting tumors and metastases could be developed. VEGF-Trap almost completely abolished tumor vasculature in experimental animals with established tumors, causing rapid progressive disappearance of both endothelial and vascular mural components. Vessel involution was followed by significant regression of large preexisting xenografts. In addition, preexisting lung micrometastases markedly decreased in both size and cell number, displaying apoptosis after one dose of VEGF-Trap, suggesting a role for VEGF-dependent homeostasis in these lesions as well. Because the pattern of lung microvessels adjacent to micrometastases did not seem to be altered by exposure to VEGF-Trap, regression may be linked to disruption of other VEGF functions (such as permeability); such micrometastases may be supplied by diffusion before reaching a size where tumor cell hypoxia stimulates neoangiogenesis. Our results lend support to the evidence for the importance of VEGF as a target in cancer therapy and provide evidence that anti-VEGF strategies may not only halt tumor growth but produce actual regression. We postulate that potent blockade of VEGF may be effective in the treatment of patients with metastatic, bulky cancers, as well as those with minimal residual disease.
Acknowledgments
We thank Dr. Judah Folkman for his insightful discussion of our metastasis studies. This work was supported by the Pediatric Cancer Foundation (J.J.K. and D.J.Y.), the Sorkin Fund (J.J.K.), and National Cancer Institute Grant 1R01-CA088951-01A1 (to D.J.Y.).
Abbreviations: VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; αSMA, α-smooth muscle actin; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling; Ang-2, angiopoietin 2; PECAM-1, platelet-endothelial cell adhesion molecule 1.
References
- 1.Kim, K. J., Li, B., Winer, J., Armanini, M., Gillett, N., Phillips, H. S. & Ferrara, N. (1993) Nature 362, 841-844. [DOI] [PubMed] [Google Scholar]
- 2.Gerber, H. P., Kowalski, J., Sherman, D., Eberhard, D. A. & Ferrara, N. (2000) Cancer Res. 60, 6253-6258. [PubMed] [Google Scholar]
- 3.Prewett, M., Huber, J., Li, Y., Santiago, A., O'Connor, W., King, K., Overholser, J., Hooper, A., Pytowski, B., Witte, L., et al. (1999) Cancer Res. 59, 5209-5218. [PubMed] [Google Scholar]
- 4.Yang, J. C., Haworth, L., Steinberg, S. M., Rosenberg, S. A. & Novotny, W. (2002) Proc. Am. Soc. Clin. Oncol. 21, 5. [Google Scholar]
- 5.Benjamin, L. E., Hemo, I. & Keshet, E. (1998) Development (Cambridge, U.K.) 125, 1591-1598. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin, L. E., Golijanin, D., Itin, A., Pode, D. & Keshet, E. (1999) J. Clin. Invest. 103, 159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramovitch, R., Dafni, H., Smouha, E., Benjamin, L. E. & Neeman, M. (1999) Cancer Res. 59, 5012-5016. [PubMed] [Google Scholar]
- 8.Morikawa, S., Baluk, P., Kaidoh, T., Haskell, A., Jain, R. K. & McDonald, D. M. (2002) Am. J. Pathol. 160, 985-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo, C. J., Farnebo, F., Yu, E. Y., Christofferson, R., Swearingen, R. A., Carter, R., von Recum, H. A., Yuan, J., Kamihara, J., Flynn, E., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 4605-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, E. S., Serur, A., Huang, J., Manley, C. A., McCrudden, K. W., Frischer, J. S., Soffer, S. Z., Ring, L., New, T., Zabski, S., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 11399-11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holash, J., Davis, S., Papadopoulos, N., Croll, S. D., Ho, L., Russell, M., Boland, P., Leidich, R., Hylton, D., Burova, E., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 11393-11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurston, G., Baluk, P., Hirata, A. & McDonald, D. M. (1996) Am. J. Physiol. 271, H2547-H2562. [DOI] [PubMed] [Google Scholar]
- 13.Wild, R., Ramakrishnan, S., Sedgewick, J. & Griffioen, A. W. (2000) Microvasc. Res. 59, 368-376. [DOI] [PubMed] [Google Scholar]
- 14.Holash, J., Maisonpierre, P. C., Compton, D., Boland, P., Alexander, C. R., Zagzag, D., Yancopoulos, G. D. & Wiegand, S. J. (1999) Science 284, 1994-1998. [DOI] [PubMed] [Google Scholar]
- 15.Shweiki, D., Itin, A., Soffer, D. & Keshet, E. (1992) Nature 359, 843-845. [DOI] [PubMed] [Google Scholar]
- 16.Levy, A. P., Levy, N. S., Wegner, S. & Goldberg, M. A. (1995) J. Biol. Chem. 270, 13333-13340. [DOI] [PubMed] [Google Scholar]
- 17.Oh, H., Takagi, H., Suzuma, K., Otani, A., Matsumura, M. & Honda, Y. (1999) J. Biol. Chem. 274, 15732-15739. [DOI] [PubMed] [Google Scholar]
- 18.Maisonpierre, P. C., Suri, C., Jones, P. F., Bartunkova, S., Wiegand, S. J., Radziejewski, C., Compton, D., McClain, J., Aldrich, T. H., Papadopoulos, N., et al. (1997) Science 277, 55-60. [DOI] [PubMed] [Google Scholar]
- 19.Rowe, D. H., Huang, J., Kayton, M. L., Thompson, R., Troxel, A., O'Toole, K. M., Yamashiro, D., Stolar, C. J. & Kandel, J. J. (2000) J. Pediatr. Surg. 35, 30-33. [DOI] [PubMed] [Google Scholar]
- 20.Folkman, J. (1972) Ann. Surg. 175, 409-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyden, D., Hattori, K., Dias, S., Costa, C., Blaikie, P., Butros, L., Chadburn, A., Heissig, B., Marks, W., Witte, L., et al. (2001) Nat. Med. 7, 1194-1201. [DOI] [PubMed] [Google Scholar]