Abstract
Survival and differentiation of myogenic cells grafted into infarcted myocardium have raised the hope that cell transplantation becomes a new therapy for cardiovascular diseases. The approach was further supported by transplantation of skeletal myoblasts, which was shown to improve cardiac performance in several animal species. Despite the success of myoblast transplantation and its recent trial in human, the mechanism responsible for the functional improvement remains unclear. Here, we used intracellular recordings coupled to video and fluorescence microscopy to establish whether myoblasts, genetically labeled with enhanced GFP and transplanted into rat infarcted myocardium, retain excitable and contractile properties, and participate actively to cardiac function. Our results indicate that grafted myoblasts differentiate into peculiar hyperexcitable myotubes with a contractile activity fully independent of neighboring cardiomyocytes. We conclude that mechanisms other than electromechanical coupling between grafted and host cells are involved in the improvement of cardiac function.
Among the treatment options for acute myocardial infarctions, transplantation of cells that could participate to cardiac contractions and diminish the workload of the remaining myocardium represents a new strategy that could ultimately avoid the occurrence of congestive heart failure. Transplantation of contractile and noncontractile cells into infarcted myocardium has been performed in rabbit, rat, pig, dog, and sheep, and shown to improve myocardial function in vivo (for review, see ref. 1). Because of these encouraging results, transplantation of autologous skeletal myoblasts into the postinfarction scar was recently applied to human (2). Contraction was then observed in the grafted scar even though it was not possible to determine whether it originated from transplanted or host cells.
In the animal studies, the fate of skeletal myoblasts grafted into normal or injured myocardium has been a matter of debate because they were initially reported to retain skeletal muscle properties (3, 4) as well as to acquire some characteristics of cardiomyocytes (5–8). These studies did not address how electrical intrinsic membrane properties of transplanted myoblasts differentiate in the infarcted heart. Moreover, the involvement of an electromechanical coupling either among transplanted cells or between transplanted and host cells remains unclear for several reasons: (i) isolated wound strips of myoblast grafts display summation of isometric contractions, suggesting that graft cells are not all synchronously recruited and thus not electrically coupled, and (ii) transplanted myoblasts do not express N-cadherin or connexin 43, the markers of cardiomyocytes adherent and electrical junctions, respectively (9). Thus, the presence of an electromechanical coupling between grafted and host cells would require that grafted cells express new types of connexin and cell adhesion molecules. In conclusion, only direct measurements of electrical and contractile activity from single myotubes transplanted into injured heart can clarify the role of electromechanical coupling in the improvement of cardiac function. In the present study, we transplanted GFP-labeled myoblasts into infarcted tibialis muscle and myocardial muscle for several weeks, extracted grafts, and then used intracellular recordings coupled to video microscopy to analyze the electrical and contractile activity of transplanted cells. We find that the expression of electrical membrane properties of grafted cells strongly varies with the environment and that grafted and host cells are not coupled.
Materials and Methods
Myocardial and Tibialis Injury Model. Wistar rats (5-wk-old males) were anesthetized with a mixture of ketamine and xylazine injected i.p. (50 and 10 mg/kg, respectively). Through a left thoracotomy, a myocardial infarction was created by ligation of the left coronary artery, with a 6/0 polypropylene snare (Ethicon, Johnson & Johnson, Brussels). At the same time, an ischemic lesion of the left tibialis anterior muscle was created by two close ligations on the same fascicle of fibers. One week later, both these lesions were injected with 5 × 106 cells in 150 μl of culture medium. The day after cellular transplantation, an immunosuppressive treatment was started with cyclosporine (10 mg/kg/day i.p.), because of the nonisogenic origin of the cells and because of the expression of the foreign immunogenic protein enhanced GFP (eGFP). Forty-seven rats were analyzed 26–30 days after successful cell transplantation.
Cell Culture Methodology. Primary muscle cell cultures were prepared from newborn Wistar rats (Iffa-Credo). Muscles were minced and enzymatically dissociated by using collagenase IA (1.5 mg/ml; 1 h, 37°C, Sigma) for 1 h and also trypsine-EDTA (0.25%; 20 min, 37°C, GIBCO/BRL) for 20 min. After passage through a 100-μm sieve (Cell Strainer Nylon, Becton Dickinson) and centrifugation, cells were expanded in medium 199 (Earle salts, GIBCO/BRL) with 15% FCS (HyClone), 1% penicillin-streptomycin (10,000 units/ml, 10,000 mg/ml; GIBCO/BRL), and 5 ng/ml basic fibroblast growth factor (Sigma) in cell factory (Nunc).
One day later, skeletal myoblasts were transfected with a eGFP-adenoviral vector (cytomegalovirus promoter, kind gift of Genethon) by using a multiplicity of infection of 100 (2 h without FCS). The day after, skeletal myoblasts were harvested by trypsination and frozen in medium 199 with 20% FCS and 10% DMSO (Sigma).
The day of transplantation, cells were thawed and washed three times in medium 199 with 0.5% BSA (fraction V, Sigma), the viability (88.1% ± 3.1) was assessed with trypan blue (GIBCO/BRL). Samples of 5 × 106 cells were prepared and kept on ice until transplantation.
The proportion of skeletal myoblasts (33.9% ± 1.3) was estimated, as described, by desmin immunolabeling (10). Briefly, cells were fixed in methanol cooled at -20°C for 5 min and incubated with antibody directed against desmin (clone D33, 1/300, 1 h, 37°C; DAKO). The secondary antibody (anti-mouse Ig) was conjugated to A-568 (1/1,000, 1 h, 37°C; Alexa Fluor). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Immunohistofluorescence Studies. Immunohistofluorescence studies were performed as described (10). Briefly, heart explants were frozen in isopentane cooled with nitrogen. Serial cryosections 10 μm thick were fixed in methanol cooled at -20°C for 5 min, and immunostained by using antibodies directed against skeletal fast myosin heavy chain (clone My32, 1/400, 1 h, 37°C; Sigma) or directed against skeletal and cardiac slow myosin heavy chain (clone NOQ7, 1/1,500, 1 h, 37°C, Sigma). The secondary antibody (anti-mouse Ig) was conjugated to A-568 (1/1,000, 1 h, 37°C; Alexa Fluor).
Cardiac and Tibialis Muscle Explants. During anesthesia, the heart was quickly removed and bathed in ice-cold oxygenated saline containing (in mM): 129 NaCl, 16 KCl, 0.9 NaH2PO4, 0.5 MgSO4, 1.8 CaCl2, 20 NaHCO3, and 5.5 glucose. A block of the left ventricle containing the infarcted zone was isolated and placed on the stage of a vibratome. A thin slice of the fibrous scar was removed to facilitate micropipette penetration in the tissue. No tissue slicing was performed in control myocardium. The explant was then slightly stretched, glued on a platinum frame, and kept 1 h at 35°C to recover, before its transfer in the recording chamber. There, it was continually perfused (at ≈5 ml/min) with heated and oxygenated saline (35°C) containing a lower concentration of KCl (4 mM). A similar approach was used when removing the entire tibialis muscle.
Electrophysiology and Fluorescent Microscopy. Myocardium explants, tibialis muscles, and myotube cultures were recorded in a chamber placed on the stage of an upright microscope (BX 50, Olympus, New Hyde Park, NY). Intracellular signals were recorded with a Neurodata amplifier (Cygnus Technology, Delaware Water Gap, PA), digitized and stored on a PC (Digidata 1200A, Clampex 8, Axon Instruments, Foster City, CA). Recording quartz micropipettes contained 1 mM KCl and 0.5% Alexa Fluor 568 (Molecular Probes). They were aimed at eGFP myotubes under visual control by using a video camera (Hamamatsu Photonics, Hamamatsu City, Japan) and FITC excitation and emission filters. Diffusion of Alexa Fluor from the micropipette occurred rapidly after cell penetration and was observed by using rhodamine excitation and emission filters. Fluorescence was generally observed by using one-photon epifluorescence microscopy. However, in a couple of cases the general organization of eGFP transplanted myotubes was observed with a custom-built two-photon microscope (11).
Results
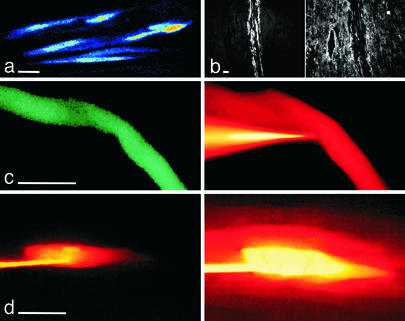
We used intracellular recordings in heart explants to determine the differentiation status of rat myoblasts transplanted for 4 wk into infarcted myocardium. To follow their fate and distinguish them from host cardiomyocytes, myoblasts were labeled before transplantation by using an adenovirus encoding for eGFP (see Materials and Methods). They were visualized in a recording chamber by using infrared and fluorescence microscopy. After 4 wk in situ, eGFP-expressing myoblasts had fused into myotubes organized in small bundles (Fig. 1a). These myotubes coexpressed the heavy chain of the fast myosin isoform, indicating that they retained some skeletal muscle contractile properties (Fig. 1b). Due to light scattering of the explant scar surrounding grafted cells, we found it difficult to ascertain that the recording pipette was precisely placed in the observed eGFP-myotube. We thus added in the pipette the fluorescent dye Alexa Fluor, which colabeled the recorded cell (Fig. 1b). Dye coupling was never observed between eGFP-expressing myotubes or between myotubes and cardiomyocytes. In contrast, dye coupling was the rule between cardiomyocytes (n = 8, Fig. 1c), suggesting that, if some electrical coupling occurred between grafted and host cells, it would involve a connexin subtype impermeant to Alexa Fluor.
Fig. 1.
Differences in dye coupling between transplanted myoblasts and host cells. (a) eGFP-expressing myotubes form bundles when transplanted in infarcted myocardium. A 2D projection of a stack of 20 images (each separated by 2 μm) obtained with two-photon microscopy. (b) Immunostaining of the heavy chain of the fast (Left) and the slow (Right) myosin isoforms. (c) A myotube expressing eGFP recorded intracellularly with an Alexa Fluor 568-containing micropipette (Left, FITC filters to reveal eGFP; Right, rhodamine filters to reveal Alexa Fluor). Thirty minutes after the impalement (Right), no dye coupling was observed between the myotube and neighboring myocytes. (d) A myocyte recorded intracellularly with an Alexa Fluor-containing micropipette (rhodamine filters). Labeling of the myocyte 30 s (Left) and 8 min (Right) after penetration. Dye coupling and labeling of neighboring myocytes occurred within 1–2 min. Scale bars = 20 μm.
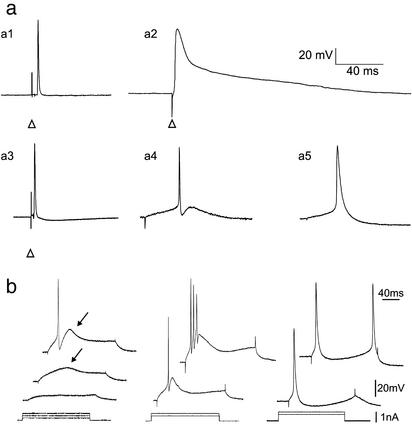
We analyzed the intrinsic membrane properties of eGFP-expressing cells either maintained in culture for 4 wk (n = 9), or 4 wk after their transplantation in infarcted myocardium (n = 12) or in infarcted tibialis muscle (n = 8). The properties of these cells were compared with those of native cardiomyocytes (n = 22) and tibialis skeletal fibers (n = 15). In all conditions, eGFP-expressing myoblasts fused into myotubes; however, they expressed different intrinsic membrane properties according to their environment. Fig. 2a illustrates that the action potential duration (APD 50% and APD 75%, corresponding to the action potential duration at mid height and at a membrane potential recovery of 75% from the overshoot; mean ± SEM) of eGFP-myotubes, whether transplanted into infarcted tibialis muscle (0.61 ± 0.06 ms and 0.90 ± 0.08 ms, n = 8), into infarcted myocardium (0.77 ± 0.06 ms and 1.17 ± 0.10 ms, n = 12), or maintained in culture (3.24 ± 0.37 ms and 5.57 ± 0.72 ms, n = 9), was much shorter than that of cardiomyocytes (8.56 ± 0.70 ms and 32.46 ± 3.08 ms, n = 22) (P < 0.001 in all comparisons with cardiomyocytes). This difference was not correlated to the resting membrane potential nor to the action potential size but was due to the presence of a plateau potential in cardiomyocytes. At first, eGFP-myotubes thus seemed to retain “normal” skeletal muscle electrical properties. However, the action potential shape of eGFP-myotubes was different in all three experimental conditions. eGFP-myotubes fired fast action potentials, followed by a slow afterhyperpolarization when transplanted in the tibialis muscle (Fig. 2a3), fast action potentials followed by a fast and a slow afterhyperpolarization when transplanted in the infarcted heart (Fig. 2a4), and slower action potentials followed by a slow afterhyperpolarization when maintained in culture (Fig. 2a5).
Fig. 2.
Emerging intrinsic membrane properties of eGFP-expressing myotubes. (a) Action potential characteristics of 5 types of muscle cell. (a1) Tibialis skeletal muscle cell; (a2) ventricular myocyte; (a3) eGFP-expressing myotube grafted for 28 days in the tibialis muscle; (a4) eGFP-expressing myotube grafted for 28 days in infarcted myocardium; (a5) eGFP-expressing myotube maintained in culture for 28 days. Whether grafted or in culture, eGFP-expressing myotubes fired brief action potentials (a3, a4, a5) in comparison with myocytes (a2). Note that the action potential of the myotubes grafted in infarcted myocardium (a4) is followed by a fast afterhyperpolarization. The action potentials were evoked either with extracellular stimulations (arrow heads point to the stimulation artifact) or with intracellular depolarizing current injections. (b) Firing properties of eGFP-expressing myotubes recorded in infarcted myocardium (Left and Center) or in culture (Right). (Left) Subthreshold depolarizing current pulses evoked a slow voltage-dependent depolarizing hump (arrow, middle trace) on top of which an action potential was triggered on higher stimulation (top trace). (Center) In another cell, a burst of action potentials was emitted on the slow depolarizing hump and followed by a slow afterhyperpolarization. (Right) Myotubes in culture do not fire bursts of action potentials.
We further investigated the intrinsic membrane properties of eGFP-moytubes transplanted into infarcted myocardium or maintained in culture (Fig. 2b). Direct injection of DC current pulses through the recording electrode evoked a surprising firing pattern in myotubes transplanted in infarcted myocardium: depolarization evoked bursts of action potentials crowned on a slow voltage-dependent hump (Fig. 2b Left and Center), reminiscent of thalamic neuron discharges (12) rather than of cardiomyocyte activity (Fig. 2a2). This discharge of multiple action potentials could create cardiac hyperexcitability if electromechanical coupling between grafted and host cells were present.
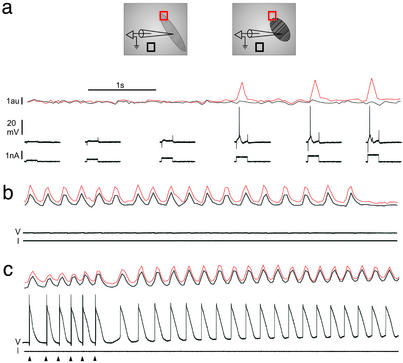
The relationship between eGFP myotubes and neighboring cardiomyocytes was thus investigated by using intracellular recordings and detection of movements within the myocardium explant. Spontaneous contractions of eGFP myotubes were observed only occasionally (n = 3) whereas the explant remained still. However, action potentials evoked intracellularly in eGFP myotubes were systematically accompanied by myotube contractions (n = 12), but these contractions never spread to neighboring cardiomyocytes. However, this negative observation could result from the incapacity of cardiomyocytes to contract in the explant. We thus repeated the experiments in conditions where spontaneous and synchronous contractions of the entire myocardium explant occurred. In the presence of isoprenaline chlorhydrate (4 × 10-9 M), these contractions appeared spontaneously or after entrainment with extracellular electrical stimulations (see Fig. 3c). Under these conditions of excitability, no mechanical coupling was observed between individual myotubes and cardiomyocytes (Fig. 3a; n = 4). Furthermore, when stable myotube recordings were maintained during myocardium explant contractions (n = 3), no electrical activity was observed in myotubes during contractions (Fig. 3b). These results were consistent with the observation that dye coupling did not occur between grafted and host cells. As expected, cardiomyocytes systematically fired with each myocardium explant contraction (Fig. 3c; n = 8).
Fig. 3.
Absence of electromechanical coupling between eGFP-expressing myotubes and myocytes. (Inset) Schematic representation of the experimental protocol used to detect contractions: movements of the recorded cell (red box) [i.e., an eGFP-expressing myotube (a and b) or a myocyte (c)] and of the neighboring myocardium (black box) were detected as local fluctuations in transilluminated light intensity. (a) Each action potential evoked in the myotube was accompanied by a local contraction (continuous red trace) that did not spread to the myocardium (continuous black trace). (b) The same myotube was electrically silent during spontaneous contractions of the entire explant. Light fluctuations detected in the red trace reflect only passive movements. (c) In opposition to the myotube, an intracellularly recorded myocyte fired with each contraction of the explant. The arrowheads point to the extracellular stimulations used to entrain the explant spontaneous contractions. The same calibration bars apply for a–c.
Discussion
Our results show that rat neonatal eGFP-labeled myoblasts transplanted in injured myocardium differentiate into excitable and contractile myotubes. These myotubes express intrinsic membrane properties that underlie a bursting behavior. They remain electrically uncoupled from cardiomyocytes after 4 wk of transplantation.
Transplantation of skeletal muscle fiber precursors in normal and injured heart was initially motivated by the hope that transplanted cells would actively participate to and improve heart contractions. To fulfill such a function, grafted cells should first organize in fiber fascicles with the right orientation such that their synchronous contraction increases the heart contraction strength. Furthermore, grafted cells should also express the appropriate electromechanical properties required for contraction. Finally, some type of coupling should occur between grafted and host cells. Improvement of cardiac function after transplantation of adult satellite cells or of neonatal myoblasts in injured heart was reported in rabbit (5), rat (10) and sheep (13, 14). In keeping with a direct involvement of the graft in improving the contraction strength, several studies, including ours, reported that the main orientation of the graft is parallel to that of the surrounding myocardium. In their original study, Taylor et al. (5) even reported the presence of intercalated disk-like structures in grafted cells, a morphological differentiation strongly supporting the electromechanical coupling hypothesis. Murry and colleagues (4, 9), however, using specific markers of grafted and host cells, demonstrated that grafted neonatal and adult satellite cells did not express cadherin or connexin 43 and did not present intercalated disks. Thus, to hold valid, the electromechanical coupling hypothesis requires that grafted cells express another type of connexin or get excited in synchrony through another mechanism such as a field effect.
Our results rule out these possibilities. Molecules of the Alexa Fluor family, which have already been used to permeate through liver and lens epithelial cells (15, 16), did not diffuse from the recorded eGFP myotubes to other myotubes or to cardiomyocytes. Although we cannot rule out that grafted cells express connexins impermeable to Alexa Fluor, it is unlikely that functional gap junctions link grafted and host cells. Furthermore, we found no evidence for an electrical field effect; i.e., the synchronous activity of all cardiomyocytes from the explants did not affect the electrical activity of transplanted myotubes (17). Thus, it is most likely that transplanted cells are functionally isolated from their host.
This absence of coupling contrasts with in vitro observations that skeletal cells cocultured with cardiomyocytes can express cadherin and connexin 43 and contract in synchrony with cardiomyocytes through the activation of functional gap junctions (4). In situ, such junctional channels do not seem to be present or are not functional. Grafted myotubes nevertheless could contract in response to electrical stimuli, but the shape of their action potentials was clearly of the “skeletal” type. However, grafted myotubes underwent a particular differentiation, and their ability to fire bursts of action potentials was more reminiscent of the firing behavior of thalamic neurons than that of skeletal muscle cells. In view of this latter peculiar membrane property, the absence of coupling may in fact be beneficial to the grafted heart: indeed, if coupled to neighboring cardiomyocytes, the subthreshold voltage-dependent behavior of transplanted myotubes could favor the generation of deleterious myocardial extrasystoles. In the absence of coupling, the mechanism underlying the episodes of ventricular tachycardia recently observed after myoblast transplantation in human remains unclear (18).
Our observations demonstrate that the improvement of cardiac function after grafting of myoblasts into infarcted myocardium does not rely on the electromechanical properties acquired by the grafted myotubes. Rather, our results favor the hypothesis that a factor released by the graft may instruct neighboring cardiomyocytes to maintain their replicative potential (19) or favor differentiation of cardiac stem cells (20, 21) in neocardiomyocytes.
Acknowledgments
Support was provided by the Institut National de la Santé et de la Recherche Médicale, the Ministère de l'Education Nationale de la Recherche et de la Technologie, the Centre National de la Recherche Scientifique, the European Community [5th Programme Cadre de Recherche et de Développement; QLG3-CT-2000-00934], the Fondation pour la Recherche Médicale (ICP20001222128), and the Fondation de l'Avenir (ET1-231).
Abbreviation: eGFP, enhanced GFP.
References
- 1.Taylor, D. A. (2001) Curr. Control Trials Cardiovasc. Med. 2, 208-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menasche, P., Hagege, A. A., Scorsin, M., Pouzet, B., Desnos, M., Duboc, D., Schwartz, K., Vilquin, J. T. & Marolleau, J. P. (2001) Lancet 357, 279-280. [DOI] [PubMed] [Google Scholar]
- 3.Murry, C. E., Wiseman, R. W., Schwartz, S. M. & Hauschka, S. D. (1996) J. Clin. Invest. 98, 2512-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinecke, H., MacDonald, G. H., Hauschka, S. D. & Murry, C. E. (2000) J. Cell Biol. 149, 731-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor, D. A., Atkins, B. Z., Hungspreugs, P., Jones, T. R., Reedy, M. C., Hutcheson, K. A., Glower, D. D. & Kraus, W. E. (1998) Nat. Med. 4, 929-933. [DOI] [PubMed] [Google Scholar]
- 6.Chiu, R. C., Zibaitis, A. & Kao, R. L. (1995) Ann. Thorac. Surg. 60, 12-18. [PubMed] [Google Scholar]
- 7.Yoon, P. D., Kao, R. L. & Magovern, G. J. (1995) Tex. Heart Inst. J. 22, 119-125. [PMC free article] [PubMed] [Google Scholar]
- 8.Dorfman, J., Duong, M., Zibaitis, A., Pelletier, M. P., Shum-Tim, D., Li, C. & Chiu, R. C. (1998) J. Thorac. Cardiovasc. Surg. 116, 744-751. [DOI] [PubMed] [Google Scholar]
- 9.Reinecke, H., Poppa, V. & Murry, C. E. (2002) J. Mol. Cell. Cardiol. 34, 241-249. [DOI] [PubMed] [Google Scholar]
- 10.Pouzet, B., Vilquin, J. T., Hagege, A. A., Scorsin, M., Messas, E., Fiszman, M., Schwartz, K. & Menasche, P. (2000) Circulation 102, III210-III215. [DOI] [PubMed] [Google Scholar]
- 11.Charpak, S., Mertz, J., Beaurepaire, E., Moreaux, L. & Delaney, K. (2001) Proc. Natl. Acad. Sci. USA 98, 1230-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steriade, M., Jones, E. G. & McCormick, D. A. (1997) in Thalamus, eds. Steriade, M., Jones, E. G. & McCormick, D. A. (Elsevier Science, Oxford), pp. 339-392.
- 13.Rajnoch, C., Chachques, J. C., Berrebi, A., Bruneval, P., Benoit, M. O. & Carpentier, A. (2001) J. Thorac. Cardiovasc. Surg. 121, 871-878. [DOI] [PubMed] [Google Scholar]
- 14.Ghostine, S., Carrion, C., Souza, L. C., Richard, P., Bruneval, P., Vilquin, J. T., Pouzet, B., Schwartz, K., Menasche, P. & Hagege, A. A. (2002) Circulation 106, I131-I136. [PubMed] [Google Scholar]
- 15.Romualdi, A., Niessen, H., Dombrowski, F., Willecke, K. & Ott, T. (2002) Cell Tissue Res. 307, 315-320. [DOI] [PubMed] [Google Scholar]
- 16.Churchill, G. C., Lurtz, M. M. & Louis, C. F. (2001) Am. J. Physiol. 281, C972-C981. [DOI] [PubMed] [Google Scholar]
- 17.Faber, D. S. & Korn, H. (1989) Physiol. Rev. 69, 821-863. [DOI] [PubMed] [Google Scholar]
- 18.Menasche, P., Hagege, A. A., Vilquin, J. T., Desnos, M., Abergel, E., Pouzet, B., Bel, A., Sarateanu, S., Scorsin, M., Schwartz, K., et al. (2003) J. Am. Coll. Cardiol. 41, 1078-1083. [DOI] [PubMed] [Google Scholar]
- 19.Kajstura, J., Leri, A., Finato, N., Di Loreto, C., Beltrami, C. A. & Anversa, P. (1998) Proc. Natl. Acad. Sci. USA 95, 8801-8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laflamme, M. A., Myerson, D., Saffitz, J. E. & Murry, C. E. (2002) Circ. Res. 90, 634-640. [DOI] [PubMed] [Google Scholar]
- 21.Quaini, F., Urbanek, K., Beltrami, A. P., Finato, N., Beltrami, C. A., Nadal-Ginard, B., Kajstura, J., Leri, A. & Anversa, P. (2002) N. Engl. J. Med. 346, 5-15. [DOI] [PubMed] [Google Scholar]