Abstract
Tissue transglutaminase (TGase2) is a protein-crosslinking enzyme known to be associated with the in vivo apoptosis program. Here we report that apoptosis could be induced in TGase2-/- mice; however, the clearance of apoptotic cells was defective during the involution of thymus elicited by dexamethasone, anti-CD3 antibody, or γ-irradiation, and in the liver after induced hyperplasia. The lack of TGase2 prevented the production of active transforming growth factor-β1 in macrophages exposed to apoptotic cells, which is required for the up-regulation of TGase2 in the thymus in vivo, for accelerating deletion of CD4+CD8+ cells and for efficient phagocytosis of apoptotic bodies. The deficiency is associated with the development of splenomegaly, autoantibodies, and immune complex glomerulonephritis in TGase2-/- mice. These findings have broad implications not only for diseases linked to inflammation and autoimmunity but also for understanding the interrelationship between the apoptosis and phagocytosis process.
Transglutaminases (TGases) (1) are a family of thiol- and Ca2+-dependent acyl transferases that catalyze the formation of a covalent bond between the γ-carboxamide groups of peptide-bound glutamine residues and various primary amines, including the ε-amino group of lysine in certain proteins. The reaction results in posttranslational modification of proteins by establishing ε-(γ-glutamyl)lysine crosslinkages and/or covalent incorporation of polyamines and histamine into proteins. Eight distinct enzymatically active transglutaminases have so far been described (1, 2). One of them, TGase2, has additionally G protein signaling activity (3). TGase2 is ubiquitously expressed in mammalian tissues (4) found both extracellularly at the cell surface in association with the extracellular matrix (5) and intracellularly in membrane-associated as well as cytosolic form. TGase2 has been implicated in a variety of cellular processes, including signaling (6), signal transduction (3), cell adhesion and spreading (7), wound healing (8), and tissue mineralization (9).
Additionally, it has also been demonstrated that TGase2 is one of the few genes induced during the in vivo apoptotic program (10–12). Cells expressing TGase2 antisense RNA are less sensitive to apoptosis induced by various agents, whereas cells overexpressing TGase2 have increased sensitivity (13, 14). Because intracellularly GTP, Zn2+,and nitric oxide inhibit the crosslinking activity of TGase2, the accumulation of TGase2 inside the cell is not necessarily associated with the activation of its crosslinking activity (15). However, Ca2+-dependent activation of the enzyme leads to the formation of a detergentinsoluble crosslinked protein scaffold in cells undergoing programmed cell death (16). This insoluble protein scaffold may stabilize the integrity of the dying cells before their clearance by phagocytosis, thus preventing the nonspecific release of harmful intracellular components and consequently inflammatory responses and scar formation in bystander tissues (17).
Although TGase2 seems to be strongly linked to the in vivo apoptosis program (10–12), the up-regulation of TGase2 cannot be detected during the induction of in vitro apoptosis in many cells, suggesting that factors present only in the tissue environment are required for its induction in vivo (12). To assess the role of TGase2 in programmed cell death, TGase2-/- mice have been generated (18). Because these mice did not show an overt apoptosis-related phenotype during fetal life, we decided to investigate the role of TGase2 in the in vivo apoptosis program in distinct biological contexts by using the thymus and liver as model systems. Data presented in this study indicate that the lack of TGase2 affects both the killing and the clearance of dying cells. The disturbance of these events results from a deficiency in transforming growth factor (TGF)-β activation and is associated with the development of splenomegaly, autoantibodies, and glomerulonephritis in TGase2-/- mice. These findings have implications for inflammation and autoimmune diseases such as systemic lupus erythematosus.
Materials and Methods
Induction of Thymic Apoptosis in Vivo. To induce thymic apoptosis, 4-wk-old TGase2+/+ and TGase2-/- mice were injected with 50 μg of anti-CD3 mAb (PharMingen) or 0.2 mg of dexamethasoneacetate (Sigma) or exposed to 5 Gy of γ-irradiation. In some experiments, 25 μg of neutralizing anti-TGF-β mAb (R & D Systems) was injected 12 h before the treatment and a second dose at the time of apoptosis induction. Controls received the same dose of isotype control mAb (PharMingen). Thymic apoptosis was evaluated 24 h later by measuring the change in amount of thymic weight and analyzing cellular changes. For the latter, thymocytes were isolated, washed twice, and resuspended in ice-cold PBS before staining either with PE-labeled anti-CD4 and Cy5-conjugated anti-CD8 (PharMingen) antibodies or FITC-labeled annexin V in binding buffer (Sigma). Cell-bound fluorescence was analyzed in a blinded fashion by using a FACSCalibur FACScan (Beckton Dickinson). Because the total cellularity of thymus in WT and TGase2-/- mice was different, data obtained after apoptosis induction were expressed as percentage of the nontreated littermates.
Thymocyte Apoptosis in Vitro. Isolated thymocytes (106 cells per ml) were cultured in RPMI medium 1640 supplemented with 5% FCS/2 mM glutamine/1 mM Na-pyruvate/5 × 10-5 M 2-mercaptoethanol at 37°C/5% CO2. Apoptosis was induced by addition of 0.1 μM dexamethasone-acetate/10 μg/ml anti-CD3 mAb or 5μM etoposide (Sigma). In some experiments, 5 ng/ml TGF-β1 (R & D Systems) was also added. After 6 h, the extent of cell death was determined by 7-aminoactinomycin D uptake (19).
Induction of Hepatocyte Apoptosis in Vivo. Hepatocyte apoptosis was induced by i.v. injection of PbNO3 [10 μmol/100 g (Carlo Erba, Milan)] dissolved in physiological saline. For histological examination and evaluation of the incidence of apoptosis, small fragments of tissue were formalin-fixed, embedded in paraffin, and stained with hematoxylin/eosin. The percentage of apoptotic cells was assessed by light microscopy, i.e., counting of 500–600 hepatocytes per sample on five different sections.
Determination of TGase2 Activity. The thymus was homogenized and TGase2 activity measured by detecting the incorporation of [3H]putrescine into N,N′-dimethylcasein, as described (11).
Western Blot Analysis of TGase2 Enzyme. Thymus tissue homogenates containing 1 mg/ml protein were mixed with an equal volume of Laemmli buffer. Electrophoresis was performed in a 10% SDS–polyacrylamide gel. Separated proteins were electroblotted and probed with monoclonal (CUB7402) or polyclonal (Upstate Biotechnology, Lake Placid, NY) antibodies to TGase2.
Determination of the Expression of TGase2 by Using Quantitative PCR. Real-time quantitative PCR was carried out on an ABI PRISM 7700 Sequence Detection System (Applied Biosystems) by using the 5′ nuclease assay. Total RNA was isolated from thymus 12 h after induction of apoptosis, and 4 μg of it was reverse transcribed into DNA by using 100 units of SuperScript II reverse transcriptase (Life Technologies, Gaithersburg, MD) and 0.025 μg/μl oligo(dT)15 primer. PCRs were carried out by using 5′-CGAATCCTCTACGAGAAGTACAGC (150 nM) as forward primer, 5′-CAGTTTGCGGTTTTGCTTGG (150 nM) as reverse primer, and 5′-AGCTACCTGCTGGCTGAGAGAGATCTC (175 nM) as TaqMan probe (Applied Biosystems). The calculation of the starting concentration was based on standard curves for target DNA run in parallel. Ribosomal protein S26 was used as an internal reference of housekeeping gene transcription for normalization between different cDNA samples.
Transmission Electron Microscopy. For transmission electron microscopy analysis, thymus samples and livers were fixed with 1% glutaraldehyde in 0.1 M cacodilate buffer, cut in small pieces, and postfixed with 2% OsO4 in the same buffer. Tissue samples were then dehydrated and embedded in epoxy resin (Spurr, Electron Microscopy Sciences, Ft. Washington, PA). Ultrathin sections were stained with uranyl acetate and lead citrate and observed under a Zeiss EM900 electron microscope.
Macrophage Phagocytosis Assays. Macrophages were harvested from adult mice by peritoneal lavage with RPMI medium 1640 and seeded on 2% gelatin-treated 24-well plates in RPMI medium 1640 containing 10% FCS (2 × 105 cells). Thymocytes were isolated from 40-wk-old mice. Opsonized thymocytes were prepared by incubation with anti-CD3 or isotype-matched antibody (rat IgG2b, PharMingen, 1 μg per 106 cells). For generation of apoptotic cells, thymocytes were induced to die by addition of 0.5 μM calcium ionophore A23187 for 6 h. After rinsing, each type of cell preparation was placed on top of the macrophages at a concentration of 107 cells per ml. After 1 h of coculture, thymocytes that had not been taken up were washed away, whereas the adherent cells were prefixed with 0.5% paraformaldehyde in 0.1 M Sörensen phosphate (SP) buffer (pH 7.4) for 30 min and fixed with 2.5% glutaraldehyde in SP buffer for 3 h at room temperature. After extensive rinsing, cells were “stained” with 2% osmium tetraoxide for contrast enhancement and studied by phase-contrast microscopy. For visualizing Listeria monocytogenes uptake, bacteria were fluorescein-labeled with 2 μM CellTracker green (Molecular Probes) in Luria broth media (Difco) for 30 min and then washed in RPMI medium 1640 before being added to macrophage cultures. Cells were fixed with 1% paraformaldehyde and analyzed by using epifluorescence microscopy. For detecting the uptake of Saccharomyces cerevisiae, the technique described by Simpson et al. (20) was applied.
Autoantibody Detection. Serum samples diluted 1:40 with PBS were applied for 30 min on rat liver–kidney–stomach sections purchased from BioSystems (Barcelona). Bound antibodies were detected with FITC-labeled goat anti-mouse IgG (Fc specific) (Sigma).
Detection of Rheumatoid Factor Type Autoantibodies. Autoantibodies specific for mouse IgG isotypes (IgG1, IgG3, IgG2a, and IgG2b standard reagents, Southern Biotechnology Associates) were detected by solid-phase ELISA, as described (21). Results are given as the mean absorbance at 492 nm ± SD of the 1:100 serum dilution.
Detection of IgM-Containing Immune Complexes. The kidney was removed and horizontally sliced and tissue blocks snap-frozen with isopentane in liquid nitrogen. Four-micrometer cryosections were cut, fixed in cold acetone, and incubated with FITC-labeled anti-mouse IgM (Sigma) diluted in PBS (1:40). After rinsing in PBS, slides were mounted in buffered glycerol.
Determination of Serum Urea Concentration. The method of Rahmatullah and Boyde (22) was used.
Statistical Analysis. Statistical analysis of the data was carried out by using the unpaired t test.
Results
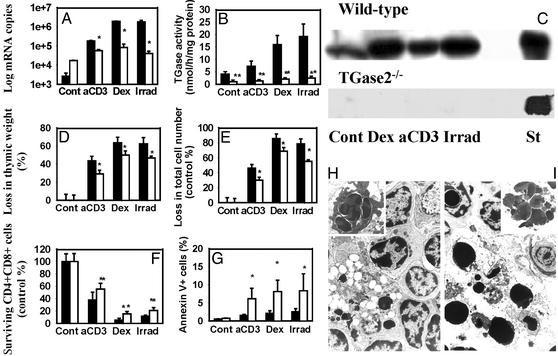
Defective Clearance of Apoptotic Cells in the Thymus of TGase2-/- Mice. Induction of thymic apoptosis by anti-CD3 mAb, dexamethasone, or γ-irradiation, signals known to initiate thymocyte death via different signaling pathways (11), also induced the in vivo expression of TGase2 mRNA (up to 700-fold) with a concomitant increase in the enzyme protein and activity in the WT animals (Fig. 1 A–C). TGase2 mRNA lacking exons 5 and 6 was expressed in TGase2-/- mice, but the respective protein was not detectable by immunoblotting with either CUB7402 monoclonal (Fig. 1 A and C) or polyclonal antibodies (data not shown), suggesting that the aberrant protein is rapidly degraded. Although the basal steady-state level of this message was higher in the knockout animals than in the WT controls, the increase observed in WT mice after the induction of apoptosis was less than that in the knockout animals, suggesting that its transcriptional activation is TGase2-dependent (Fig. 1 A).
Fig. 1.
The in vivo thymic apoptotic program of TGase2+/+ (filled bars) and TGase 2-/- (open bars) mice (n = 10) at 24 h after a single dose of anti-CD3 mAb (50 μg), dexamethasone-acetate (0.2 mg) injection, or γ-irradiation (5 Gy). (A) Changes in the expression levels of TGase2 mRNA determined by real-time quantitative PCR. (B) Changes in the transglutaminase activity expressed as nanomolar of [3H]putrescine incorporated per milligram of protein and per hour. (C) Protein levels of TGase2 detected by Western blot analysis using monoclonal antibody CUB7402. Liver extract from WT mice known to express TGase2 was used as positive control. (D) Loss in thymic weight. (E) Loss in total cell number. (F) Survival of CD4+CD8+ thymocytes expressed as percentage of the absolute CD4+CD8+ cell number of nontreated controls, and (G) percentage of annexin V-positive cells of all thymic cells determined by FACS analysis. Data represent mean ± SD. Statistically different from the WT mice (*, P < 0.05; **, P < 0.004). (H and I) Electron-microscopic sections of thymi from TGase2+/+ and TGase2-/- mice treated with dexamethasone. In WT mice (H), apoptotic cells that appear as large electron-dense bodies are found within the macrophages. (Inset) One macrophage with three engulfed apoptotic bodies. In TGase2-/- mice (I), an accumulation of free apoptotic bodies is apparent and, as shown, clusters of apoptotic bodies are frequently observed (×1,500).
The thymus in knockout mice at the age of 4 wk was smaller than in WT with a significantly (P < 0.002) reduced number of thymic cells (26.2 ± 2.3 × 107 vs. 15.9 ± 1.9 × 107, representing mean ± SD for 10 animals), but with no alteration in the percentage of the various thymocyte populations. When the tissue loss in response to apoptotic stimuli was compared between WT and knockout mice, the percentage loss in thymic weight (Fig. 1D) and cellularity (Fig. 1E) was found to be diminished in the knockout animals in each treatment group. Because all treatments affected only the CD4+CD8+ population, this difference was most evident when the percentage of CD4+CD8+ cells that survived was compared (Fig. 1F). The observed reduced loss of thymic cells in the TGase2-/- mice may be the consequence of either a reduced rate of apoptosis or phagocytosis. To discriminate between these alternatives, the changes in the percentage of the annexin V+ (free apoptotic) thymocytes after apoptosis induction was also determined. As shown in Fig. 1G, thymi of the TGase2-/- mice when compared with WT showed a significant increase in annexin V+ apoptotic cells. In accordance, electron microscopic analysis of the thymus from WT mice treated with dexamethasone showed typical atrophy of the thymus, with condensed nuclei of apoptotic cells mostly being associated with macrophages (Fig. 1H). In contrast, in the thymus of the TGase2-/- mice, free apoptotic bodies are present in abundance, often forming large clusters (Fig. 1I).
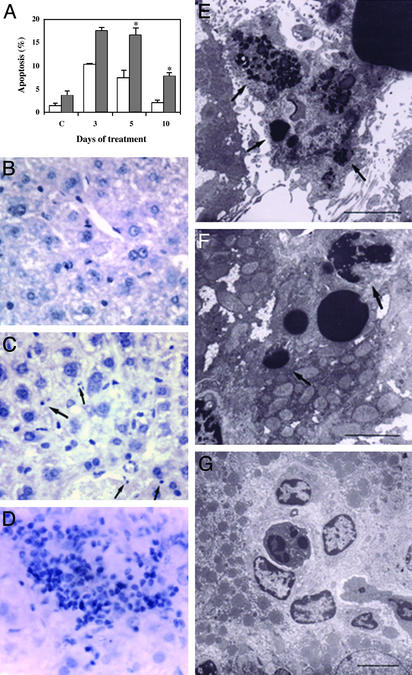
Defective Clearance of Apoptotic Cells Is Accompanied by Inflammatory Reaction in the Liver of TGase2-/- Mice. To investigate the clearance of apoptotic cells in a different organ, we injected adult TGase2-/- mice with a single dose of PbNO3. This treatment induces a proliferative response leading to doubling of the liver mass in 3 days, followed by an involution phase that involves apoptosis and induction of TGase2 protein and activity (23), then the liver recovers its weight within 2–3 days. Apoptosis was determined by counting morphologically typical apoptotic cells on tissue sections (Fig. 2 B and C). As shown in Fig. 2 A, in the liver of TGase2-/- mice, at 5 and 10 days postinjection, a significant increase in apoptotic cells was detected as compared with WT. Concomitant with the accumulation of apoptotic cells, an increase in total liver weight in TGase2-/- mice as compared with WT animals was observed (30% at day 5 and 80% at day 10 postinjection). This delayed recovery of liver weight was characterized by the accumulation of morphologically typical apoptotic cells, mostly not associated with macrophages (Fig. 2E). In contrast, WT mice treated with a similar dose of PbNO3 showed a lower number of apoptotic cells, mostly taken up by Kuppfer cells (Fig. 2F). Even in vehicle-treated animals, a lower amount of apoptotic cells was observed in WT liver as compared with TGase2-/- liver (Fig. 2 A). Taken together, these data suggest that TGase2-/- mice have a constitutive deficiency in the clearance of apoptotic hepatocytes. After apoptosis induction, this deficiency was associated with an inflammatory response, as evidenced by the presence of large infiltrates of blood cells in many parts of the liver tissue (Fig. 2 D and G). Such infiltrates were not seen in the WT mice.
Fig. 2.
Morphological changes occurring in liver of WT and TGase2 null mice after PbNO3 treatment. (A) Hepatocyte apoptosis in control and treated mice at 3, 5, and 10 days after injection of PbNO3. Filled bars, WT; open bars, TGase2-/- livers. Values are presented as mean ± SD of three independent experiments. *, Statistically different from WT mice (P < 0.05). (B–D) Light microscopy of treated livers after 5 days. Hematoxylin/eosin-stained sections (×63) demonstrate the presence of numerous apoptotic hepatocytes (arrows) and massive inflammatory infiltrate in TGase2-/- (C and D), but not in WT liver (B). (E–G) Electron microscopic ultrastructural analysis of livers shows Kupffer cells actively engulfing apoptotic cells in WT mice as demonstrated by the presence of apoptotic remnants inside the cytoplasm (E, arrows). In contrast, in TGase2 null livers (F) the apoptotic bodies (arrows) not cleared by Kupffer cells fill the lumen of hepatic sinusoids and inflammatory cells, mostly lymphocytes and polymorphonuclear leukocytes, have infiltrated the liver parenchyma (G). [Bar = 1 μm (E), 2 μm (F and G).]
The Production of Active TGF-β1 Promotes Phagocytosis of Apoptotic Cells by Macrophages and Requires TGase2. To determine whether TGase2 in apoptotic cells or macrophages is required for proper phagocytosis, peritoneal macrophages were isolated from WT and TGase2-/- mice and exposed to apoptotic thymocytes in different combinations (Table 1). Although both WT and knockout macrophages were competent in the phagocytosis of apoptotic thymocytes, the macrophages isolated from the knockout mice showed a marked reduction in the average number of dead thymocytes found inside a cell. The total number of apoptotic cells ingested within 1 h by 100 macrophages isolated from the knockout mice was only 50% of that of the WT animals. The deficiency seems to be selective for macrophages, because there was no difference in the phagocytotic efficiency when WT or knockout thymocytes were used (Table 1). Additionally, the deficit seems to be restricted to phagocytosis of apoptotic cells as TGase2-/- macrophages showed a normal ability to ingest bacteria, yeast, or opsonized nonapoptotic thymocytes, as shown in Table 1.
Table 1. Phagocytosis of L monocytogenes, S. cerevisiae, and opsonized and nonopsonized thymocytes by WT and TGase2-/- macrophages (MΦ).
| WT MΦ with ingested particles, % | TGase2-/- MΦ with ingested particles, % | |
|---|---|---|
| WT apoptotic thymocytes | 76.7±8.2 | 51.1±4.7* |
| TGase2-/- apoptotic thymocytes | 77.1±9.1 | 51.7±3.7* |
| Nonopsonized thymocytes | 7.1±1.0 | 6.2±0.8 |
| Thymocytes opsonized with anti-CD3 mAb | 46.7±9.8 | 49.1±9.9 |
| Thymocytes with isotype control mAb | 14.1±2.3 | 11.7±1.6 |
| L. monocytogenes +50 μM | 77.6±12.1 | 82.7±13.1 |
| cytochalasin B | 17.6±5.2 | 14.7±6.9 |
| S. cerevisiae +50 μM | 67.0±20.4 | 68.9±8.4 |
| cytochalasin B | 22.8±11.7 | 24.7±14.1 |
Each experimental group included three independent experiments with macrophages isolated from separate mice. In the case of opsonized thymocytes, cells treated with isotype control mAb served as control for nonspecific binding, whereas in the case of bacterial and yeast phagocytosis, treatment with cytochalasin B, an inhibitor of phagocytosis, was used for discrimination of nonspecific interaction with macrophages. Data represent mean ± SD
Significantly different from the WT (P < 0.002)
Macrophages are known to express TGase2, which is required for the activation of latent TGF-β1 (6), a cytokine specifically released by macrophages ingesting apoptotic cells. To test the possibility that the reduced rate of clearance of apoptotic cells observed in the TGase2-/- mice is related to a deficiency in TGF-β1 activation, apoptotic thymocytes were exposed to either WT or TGase2-/- macrophages for 1 h. The conditioned media were then collected and together with a fresh aliquot of apoptotic thymocytes added to TGase2-/- macrophages. Apoptotic cells were more efficiently ingested by TGase2-/- macrophages in the presence of conditioned medium derived from WT macrophages than in conditioned medium derived from TGase2-/- macrophages (Table 2), demonstrating that TGase2 participates in the production of a soluble factor that stimulates phagocytosis. Moreover, the inclusion of a neutralizing anti-TGF-β1,2,3 antibody was sufficient to inhibit this enhancement, demonstrating that the soluble factor was TGF-β.
Table 2. Deficit in the phagocytosis of apoptotic cells by TGase2-/- macrophages can be compensated by TGF-β activated by WT macrophages.
| Phagocytosis of WT apoptotic thymocytes by
|
||
|---|---|---|
| TGase2-/- macrophages in CM derived from WT macrophages* | TGase2-/- macrophages in CM derived from TGase2-/- macrophages* | |
| 238±21† | 126±17 | |
| + a-TGF-β | 129±18‡ | 122±13 |
| + isotype specific mAb | 247±22 | 128±15 |
The amount of phagocytosis was determined after 1 h. Data represent mean ± SD from four separate experiments. CM, conditioned medium collected after 1 h of phagocytosis of apoptotic cells by WT or TGase2-/- macrophages, as indicated; a-TGF-β, anti-TGF-β 1,2,3 neutralizing monoclonal antibody (R & D Systems, 10 μg/ml); isotype-specific mAb, mouse IgG1, control for the neutralizing monoclonal antibody (Pharmingen, 10 μg/ml)
Number of apoptotic bodies/100 macrophages
Significantly different from that observed in CM derived from TGase2-/- macrophages (P < 0.002)
Significantly different from that observed in the absence of the neutralizing mAb (P < 0.002)
TGF-β1 Is Responsible for the Induction of TGase2 Expression During in Vivo Apoptosis. TGF-β1 can induce TGase2 expression via a TGF-β response element in its promoter (24). Because our previous experiments suggested that the in vivo up-regulation of TGase2 requires unknown factors present only in the tissue environment (12), and the TGase2 promoter in the TGase2-/- thymus, in contrast to WT thymus, was only marginally induced during in vivo apoptosis (Fig. 1 A), we asked whether active TGF-β could be one of the unknown tissue mediators. If this is the case, injection of neutralizing anti-TGF-β1,2,3 antibodies should reduce the up-regulation of TGase2 in WT mice. Indeed, whereas the induction of the TGase2 activity as compared with the physiological saline treated littermates was 1.7 ± 0.2-, 3.8 ± 0.3-, and 4.7 ± 0.4-fold after anti-CD3, dexamethasone treatment, or γ-irradiation, respectively, in the presence of anti-TGF-β antibodies the induction was only 1.3 ± 0.1-, 2.0 ± 0.2-, and 3.1 ± 0.2-fold. In each treatment group, the increase in TGase2 expression was significantly reduced in those mice that received the neutralizing anti-TGF-β antibodies (P < 0.05, n = 4). The same dose of isotype matched unrelated antibody had no effect.
TGF-β1 Promotes Apoptosis of Thymocytes. Because the ex vivo rate of thymocyte apoptosis of TGase2-/- mice was not altered (18), but the in vivo rate was slower (Fig. 1) and induction of the expression of TGase2 has been shown to promote apoptosis (13), we tested whether TGF-β1 would promote the rate of cell death in ex vivo thymocyte cultures. Addition of TGF-β1, which by itself induced only a 2.1 ± 0.3% increase in the 5.1 ± 1.1% rate of spontaneous death of WT thymocytes, promoted cell death induced by 0.1 μM dexamethasone, 10 μg/ml anti-CD3 mAb, and 5 μM etoposide by 26.4 ± 2.9, 13.1 ± 1.6, and 26.7 ± 2.2%, respectively (each significantly different from the control value P < 0.05, n = 4), as detected after 6 h of culture. This effect of TGF-β1, however, was not related to the up-regulation of TGase2 in apoptotic cells, because it promoted thymocyte apoptosis in TGase2-/- mice to a similar degree (data not shown).
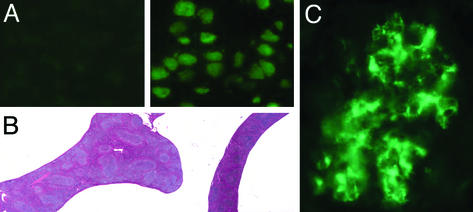
TGase2-/- Mice Develop Autoantibodies, Splenomegaly, and Immune Complex Glomerulonephritis. We then investigated the long-term effects of the TGase2 deficiency. We found that 10 of 25 1-yr-old TGase2-/- mice had autoantibodies against nuclear components (Fig. 3A) and/or smooth muscle cells, whereas none of the 20 age-matched control WT mice tested positive. Moreover, the 10 mice with autoantibodies developed splenomegaly with no change in the number CD8+ T cells, a significant decrease in the number of CD4+ T cells (13 ± 3 × 106 vs. the normal 25 ± 4 × 106), and a significant increase in the number of B cells (240 ± 60 × 106 vs. the normal 160 ± 35 × 106 P < 0.05, n = 10). These spleens showed an enlarged white pulp characteristic for hyperactive spleens (Fig. 3B). In addition, increased titers of IgG type anti-IgG2a antibodies were detected in 18 of the 25 tested knock-out animals. For these 18 animals, a mean OD492 of 0.310 ± 0.112 was measured by ELISA as compared with 0.071 ± 0.022 (n = 15) in the WT animals, (P < 0.002). (Cutoff value for nonresponders was 0.090 OD over background.) However, 5 of the 20 WT mice also had elevated titers. Elevated titers of anti-IgG1 antibodies were also detected in five of the nine antinuclear positive knockout mice and in one of the antinuclear antibody-negative mice (data not shown), whereas no anti-IgG1 antibodies were found in the WT mice. By the age of 15 mo, 5 of 10 knockout mice were terminally ill with kidney disease diagnosed as immune complex glomerulonephritis (Fig. 3C). Glomerular dysfunction was indicated by high serum concentrations of urea (31 to >100 mM vs. the normal 11 ± 3 mM). At the same age, 3 of 15 WT animals tested showed antinuclear positivity but none had signs of kidney damage.
Fig. 3.
Prevalence of autoantibodies, splenomegaly, and immune complex glomerulonephritis in TGase2-/- mice. (A) Detection of antinuclear antibodies in the sera of TGase2-/- mice using rat liver sections. (Left) A weak reaction to nuclear components; (Right) a strong antinuclear reactivity. (B) Light-microscopic images of sections of 1-yr-old TGase2-/- (Left) and WT (Right) mice spleens stained with hematoxylin/eosin. The weights of the spleens were 413 and 96 mg, respectively (×6). (C) The presence of IgM-containing immune complexes in glomeruli, revealed by staining frozen kidney sections with FITC-labeled goat anti-mouse IgM antibodies.
Discussion
The phagocytosis of apoptotic cells is very effective in the thymus, and consequently the majority of dead cells is engulfed by macrophages and normally cannot be seen by flow cytometric analysis. Thus, the apparent increase in the percentage of annexin V+ cells in the thymus of TGase2-/- mice after apoptosis induction suggests that the phagocytosis of apoptotic cells is defective in these mice. This assumption is further supported by the analysis of tissue sections, which revealed an accumulation of nonphagocyted apoptotic cells. The defect in clearance of apoptotic cells was also evident after induced liver hyperplasia, suggesting that it is not an organ-specific defect. In this case, the reduced clearance of apoptotic cells was associated with an inflammatory infiltrate. This observation is in line with previous studies showing that the efficiency of apoptotic cell clearance is a key factor in the suppression of tissue inflammation (25). However, because the increase in the percentage of all surviving thymic cells in the knockout mice as compared with WT was higher than the increase in the percentage of annexin V+ cells, the apoptosis of thymocytes is also affected in the knockout mice.
It has been shown that TGF-β1 is specifically released by macrophages ingesting apoptotic cells but not during other types of phagocytosis (26). The release of TGF-β1 is regulated by recognition of phosphatidylserine on apoptotic cells by macrophages (27), which constitutes an early step in the phagocytosis process of apoptotic cells. For TGF-β1 activation, TGase2 is also required (8). Our results demonstrate that TGase2 produced by macrophages is required for efficient clearance of apoptotic cells, and that the reduced rate of phagocytosis of TGase2-/- macrophages relates to a deficiency in activation of TGF-β1.
The long-term consequence of the defect in TGase2 function was the development of autoimmunity. This finding is in line with the observation that systemic exposure of the immune system to apoptotic thymocytes in mice results in the transient production of autoantibodies (25, 28, 29), and that defects in clearance of dead cells are associated with autoimmunity (30, 31). This observation is partly explained by the fact that unprocessed apoptotic cells may release nuclear and cellular components, which trigger autoimmunity. Thus the lack of formation of detergent-insoluble cross-linked protein polymers in dying cells of TGase2-/- mice, which could at least partially prevent the leakage of cellular content (16), can further contribute to the development of autoimmunity. In addition, TGF-β is a well known immunoregulatory cytokine that plays a key role in the down-regulation of inflammatory and autoimmune responses (32). Because in our model the apoptosis of thymocytes is also affected, autoreactive T cells may also accumulate due to the ineffective negative selection and contribute to the development of autoimmunity. In support of this conclusion, we found IgG type anti-IgG antibodies in these mice, the production of which requires the function of autoreactive CD4+ T cells.
TGase2 up-regulation is associated with the in vivo apoptosis program in many cells (10–12). Although the presence of the enzyme promoted or, in some cells, induced apoptosis (13, 18), the apoptotic program can be induced in the absence of TGase2 activity, and in vitro many cells die without the apparent upregulation of the enzyme. In fact, the same apoptotic stimuli that up-regulate the enzyme in the thymus in vivo fail to do so in vitro, indicating that up-regulation of TGase2, at least in thymocytes, requires the tissue environment (12). Although the in vivo rate of cell death in the thymus of TGase2 null mice was found by us to be diminished, the in vitro rate of apoptosis of TGase2-/- and WT thymocytes was similar (18). Again, the difference between the in vivo and in vitro rates indicates that factors present in the tissue environment play a role in determining the in vivo rate of apoptosis of thymocytes. Two recent reports indicate that phagocytosis-related factors may play such a role in Caenorhabditis elegans (33, 34). In contrast to the conventional concept that apoptosis and phagocytosis are subsequent but independent phenomena, both the C. elegans work and our data indicate an interplay between the two processes in vivo, resulting in a complex apoptophagocytotic process. In vertebrates, this appears to play a crucial role in preventing inflammation and autoimmunity. Taken together, the data suggest that the interplay is initiated by the recognition of phosphathidylserine in the outer lipid layer of apoptotic cells by macrophages (27). This interaction induces an apoptosis-specific form of macrophage activation (26) that involves a TGase2-dependent step in the production of active TGF-β. Activated TGF-β then downregulates the inflammatory cytokine formation of macrophages (26) and, as is shown in this paper, stimulates the rate of phagocytosis. In addition, TGF-β promotes thymocyte apoptosis and the concurrent accumulation of TGase2 in thymocytes during the apoptosis program in vivo. Overall, our results provide further support for a crosstalk between cells programmed for death and recruited macrophages and demonstrate that TGase2 is an element of this apoptophagocytotic machinery, the function of which is critical to prevent inflammation and autoimmunity.
Acknowledgments
We thank S. Varga for staining macrophages, P. Aeschlimann and Z. Hartmann for technical assistance, and É. Pintye for irradiating mice. This work was supported by Országos Tudományos Kutatási Alap Grants OTKA T 034191 (to Z. Szondy) and OTKA TS 044798 (to L. Fésüs); Egészségügyi Tudományos Tanács Grant ETT 48/2000 (to Z. Szondy); the Arthritis Research Campaign, United Kingdom, Grant A0560 (to D.A.); European Union Grant QLGI-1999-00739; Ministero dell'Istruzione, dell'Universita e della Ricerca and Associazione Italiana per la Ricerca sul Cancro (to M.P. and G.M.); European Union Grant QLK3-2002-01956 (to G.M.); and European Union Grant QLK3-CT-2002-02017 (to Z. Szondy, L. Fésüs, and P.M.).
Abbreviations: TGase, transglutaminase; TGF-β, transforming growth factor β.
References
- 1.Greenberg, C. D., Birckbichler, P. J. & Rice, R. H. (1991) FASEB J. 5, 3071-3077. [DOI] [PubMed] [Google Scholar]
- 2.Grenard, P., Bates, M. K. & Aeschlimann, D. (2001) J. Biol. Chem. 276, 33066-33078. [DOI] [PubMed] [Google Scholar]
- 3.Murthy, S. N., Lomasney, J. W., Mak, E. C. & Lorand. L. (1999) Proc. Natl. Acad. Sci. USA 96, 11815-11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomazy, V. & Fésüs, L. (1989) Cell Tissue Res. 255, 215-224. [DOI] [PubMed] [Google Scholar]
- 5.Gaudry, C. A., Verderio, E., Aeschlimann D., Cox, A., Smith, C. & Griffin, M. (1999) J. Biol. Chem. 274, 30707-30714. [DOI] [PubMed] [Google Scholar]
- 6.Kojima S., Nara, K. & Rifkin, D. B. (1995) J. Cell Biol. 121, 439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentile, V., Thomázy, V., Piacentini, M., Fésüs, L. & Davies, P. J. A. (1992) J. Cell. Biol. 119, 464-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upchurch, H. F., Conway, E., Patterson, M. K., Jr., & Maxwell, M. P. (1991) J. Cell. Physiol. 149, 375-382. [DOI] [PubMed] [Google Scholar]
- 9.Aeschlimann, D., Kaupp, O. & Paulsson, M. (1995) J. Cell Biol. 129, 881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fésüs, L., Mádi, A., Balajthy, Z., Nemes, Z. & Szondy, Z. (1996) Experientia 52, 942-949. [DOI] [PubMed] [Google Scholar]
- 11.Szondy, Z., Molnar, P. Nemes, Z., Boyiadzis, M., Kedei, N., Tóth, R. & Fésüs, L. (1997) FEBS Lett. 404, 307-313. [DOI] [PubMed] [Google Scholar]
- 12.Szegezdi, E., Szondy Z., Nagy, L., Nemes, Z., Friis, B., Davies, P. J. A & Fésüs, L. (2000) Cell Death Differ. 7, 1225-1233. [DOI] [PubMed] [Google Scholar]
- 13.Melino, G. Annicchiarcio-Petrucelli, M., Piredda, L., Candi, E., Gentile, V., Davies, P. J. A. & Piacentini, M. (1994) Mol. Cell. Biol. 14, 6584-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliverio, S., Amendola, A., Rodolfo, C., Spinedi, A. & Piacentini, M. (1999) J. Biol. Chem. 74, 34123-341288. [DOI] [PubMed] [Google Scholar]
- 15.Melino, G. & Piacentini, M. (1998) FEBS Lett. 430, 59-63. [DOI] [PubMed] [Google Scholar]
- 16.Fésüs, L., Thomázy, V., Autuori, F., Ceru, M. P., Tarcsa, E. & Piacentini, M. (1989) FEBS Lett. 245, 150-154. [DOI] [PubMed] [Google Scholar]
- 17.Piredda, L., Amendola, A., Colizzi, V., Davies, P. J. A., Farrace, M. G., Fraziano, M., Gentile, V., Uray, I., Piacentini, M. & Fésüs, L. (1997) Cell Death Differ. 4, 463-472. [DOI] [PubMed] [Google Scholar]
- 18.De Laurenzi, V. & Melino, G. (2001) Mol. Cell. Biol. 21, 148-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecoeur, H., Ledru, H. & Gougeon, M. L. (1998) J. Immunol. Methods 217, 11-26. [DOI] [PubMed] [Google Scholar]
- 20.Simpson, D. W., Roth, R. & Loose, L. D. (1979) J. Immunol. Methods 29, 221-226. [DOI] [PubMed] [Google Scholar]
- 21.Fazekas, G., Pálfi, G., Wolff-Wisinski, B., Rosenwirth, B., Dukor, P., Gergely, J. & Rajnavölgyi, E. (1995) Int. Immunol. 7, 1125-1134. [DOI] [PubMed] [Google Scholar]
- 22.Rahmatullah, M. & Boyde, T. R. (1980) Clin. Chim. Acta 107, 3-9. [DOI] [PubMed] [Google Scholar]
- 23.Fésüs, L., Thomázy, V., Autuori, F., Ceru, M. P., Tarcsa, E. & Piacentini, M. (1989) FEBS Lett. 245, 150-154. [DOI] [PubMed] [Google Scholar]
- 24.Ritter, S. J. & Davies, P. J. A. (1998) J. Biol. Chem. 273, 12798-12806. [DOI] [PubMed] [Google Scholar]
- 25.Rosen, A. & Casciola-Rosen, L. (1999) Cell Death Differ. 6, 6-12. [DOI] [PubMed] [Google Scholar]
- 26.Fadok, V. A. Bratton, D. L., Konowal, A., Freed, P. W., Westcott, J. Y. & Henson, P. M. (1998) J. Clin. Invest. 101, 890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fadok, V. A., Voelker, D. R., Campbell, P. A., Cohen, J. J., Bratton, D. L. & Henson, P. M. (1992) J. Immunol. 148, 2207-2216. [PubMed] [Google Scholar]
- 28.Casiano, C. A. & Tan, E. M. (1996) Int. Arch. Allergy Immunol. 111, 308-313. [DOI] [PubMed] [Google Scholar]
- 29.Mervorach, D., Zhou, J. L., Song, X. & Elkon, K. B. (1999) J. Exp. Med. 223, 237-248. [Google Scholar]
- 30.Scott, R. S., McMahon, E. J., Pop, S. M., Reap, E. A., Caricchio, R., Cohen, P. L., Earp, H. S. & Matsushima, G. K. (2001) Nature 411, 207-211. [DOI] [PubMed] [Google Scholar]
- 31.Botto, M., Dell'Agnola, C., Bygrave, A. E., Thompson, E. M., Cook, H. T., Petry, F., Loos, M., Pandolfi, P. P. & Walport, M. J. (1998) Nat. Genet. 19, 56-59. [DOI] [PubMed] [Google Scholar]
- 32.Shull, M. M., Ormsby, I., Kier, A. B., Pawlowski, S., Diebold, R. J., Yin, M., Allen, R., Sidman, C., Proetzel, G., Calvin, et al. (1992) Nature 359, 693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddien, P. W., Cameron, S. & Horvitz, R. H. (2001) Nature 412, 198-202. [DOI] [PubMed] [Google Scholar]
- 34.Hoeppner, D. J., Hengartner, M. O. & Schnabel, R. (2001) Nature 412, 202-206. [DOI] [PubMed] [Google Scholar]