Abstract
Two major glycolipids, which comprise ≈36% of the total lipid mass from Borrelia burgdorferi, the etiological agent of Lyme disease, were investigated. We determined the fatty acid type, sugar identity, anomeric configuration, and substituent type and position. The structures were identified as cholesteryl 6-O-acyl-β-d-galactopyranoside (B. burgdorferi glycolipid 1, BbGL-I), and 1,2-di-O-acyl-3-O-α-d-galactopyranosyl-sn-glycerol (BbGL-II). The major fatty acids were palmitate and oleate. The structures were corroborated by gas–liquid chromatography MS, matrix-assisted laser desorption/ionization time-of-flight spectroscopy, fast atom bombardment MS, detailed NMR spectrometry, and metabolic labeling. This is a previously undescribed demonstration of a cholesteryl galactoside in bacteria. Lipopolysaccharide was not detected in B. burgdorferi. The two glycolipids have several properties suggesting they may function as lipopolysaccharide: both are main components of the bacterial membrane, surface exposed, and have a three-domain structure. BbGL-I elicited specific antibodies in mice and rabbits, and BbGL-II elicited antibodies that reacted with both glycolipids.
The etiological agent of Lyme disease, Borrelia burgdorferi, is transmitted to humans through the bite of Ixodes ticks. Lyme disease is a multisystem infection, which affects the skin, joints, nervous system, and heart (1). The licensed vaccine (LYMErix, SmithKline Beecham) contains recombinant lipidated OspA. Although lipopolysaccharide (LPS) has been identified in several spirochaetales, such as Leptospira (2) and Treponema (3), there is no evidence for the presence of LPS in Borrelia species (4).
Two surface-exposed glycolipids identified in B. burgdorferi react with sera from Lyme disease patients (5–7). These immunoreactive glycolipids have been characterized as monogalactosyl diacylglycerolipids (8). It has been proposed that these glycolipids differ only in their fatty acid composition. We designated these glycolipids as B. burgdorferi glycolipid I (BbGL-I) and B. burgdorferi glycolipid II (BbGL-II). We show that although BbGL-II is a monogalactosyl diacylglycerol as reported (8), BbGL-I has the unique structure cholesteryl 6-O-acyl-β-d-galactopyranoside.
Experimental Procedures
Organism and Growth Conditions. B. burgdorferi strains B31 (ATCC 35210), BL303 (courtesy of G. Wormser, Division of Infectious Diseases, New York Medical College, Valhalla) and N40 (courtesy of L. Bockenstedt, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT) were cultivated in BSK-H medium (Sigma). Media were inoculated with 2% (vol/vol) of a frozen culture and incubated statically at 37°C for 72 h to the mid-exponential growth phase. Cells were harvested by centrifugation at 12,000 × g for 30 min, washed three times with cold PBS, and stored at -20°C.
Lipid Extraction and Purification. Lipids were extracted from washed cells as described (9). The chloroform was removed, and the dried lipids (0.1–0.2 mg/mg cell) dissolved in 1–2 ml of chloroform. Lipid extract (10 mg) in 5 ml of chloroform was then loaded onto a silica gel column (20 × 3 cm, Kieselgel 60, Merck, 230–400 mesh) and eluted sequentially with 10 bed volumes of chloroform (fraction 1); 2.5% methanol/chloroform (vol/vol; fraction 2); 5% methanol/ chloroform (vol/vol; fraction 3), 10% methanol/chloroform (vol/ vol; fraction 4) and methanol (fraction 5). The fractions were concentrated, dissolved in 1 ml of chloroform, and stored at -20°C. Fraction 2 contained BbGL-I, whereas fraction 3 contained mostly BbGL-II. Lipid extract, 200 μg per 20 μl, was chromatographed on silica gel coated aluminum plates (Merck) and developed by using 10% methanol/chloroform (vol/vol). Lipid spots were detected by iodine vapor, and glycolipids were detected by anthrone spray.
Sugar and Fatty Acid Analysis. Sugars were analyzed after hydrolysis (1 M HCl for 4 h at 100°C), reduction, and peracetylation, by Hewlett-Packard gas–liquid chromatography (GLC-MS), with HP-5 glass capillary column, programmed at 8°C per min, from 125–250°C (10). Free fatty acid methyl esters, obtained after methanolysis of dried glycolipids with 1 M HCl/MeOH for 5 h at 80°C, were analyzed under the same conditions.
Double Bond Localization. The double bond in unsaturated fatty acids was localized by GLC-MS after 4,4-dimethyloxazoline derivatization (11), and its configuration was determined by using methyl esters of oleic and elaidic acids as references.
Methylation Analysis. Native and O-deacylated glycolipids were methylated as described (12), hydrolyzed, converted to alditol acetates, and analyzed by GLC-MS (10).
O-Deacylation. Glycolipids (2 mg) were O-deacylated with 0.33 ml of 0.25 M NaOCH3 in methanol at 37°C. On completion (monitored by TLC) solvents were evaporated and the products extracted with chloroform/water (1:1 vol/vol). Both phases were analyzed for sugars and fatty acids by GLC-MS.
Absolute Configuration. d-Galactose was identified by the enzymatic method using galactose oxidase (13). The absolute configuration of glycerol was determined after O-deacylation of the glycolipid (14). In this method, the primary hydroxyl group of glycerol, released after saponification of the glycolipid, was oxidized by TEMPO and transformed into a glyceric acid residue. After acid hydrolysis, the glyceric acid was esterified with (R)-(-)-2-butanol, acetylated and analyzed by GLC-MS. The retention time was compared with those of d- and l-glyceric acids.
NMR Spectroscopy. Monogalactosyl diglyceride (MGDG, mainly 1,2-di-O-stearoyl-3-O-β-d-galactopyranosyl glycerol) was from Matreya (State College, PA); 1,2-Di-O-palmitoyl glycerol (1,2-dipalmitin) was from NuChek (New Elysian, MN); methyl α-d-galactopyranoside (Me-α-d-Galp) was prepared in-house; and methyl β-d-galactopyranoside (Me-β-d-Galp) was obtained from Aldrich (Milwaukee, WI). Deuterated solvents were from Cambridge Isotope Laboratories (Andover, MA).
NMR spectra were acquired at 300 K, by use of a Bruker DRX-500 spectrometer. Solutions of 5–10 mg of compound in CDCl3 (0.5 ml, 99.96 atom % D) or its admixtures with (CD3)2CO (99.9 atom % D) or CD3OD (99.8 atom % D) were used for the lipids, with Me4Si as a reference for 1H and 13C NMR. The anomeric methyl d-galactopyranosides (11 mg) were examined as their solutions in D2O (0.4 ml, 99.96 atom % D), with sodium 4,4-dimethyl-4-silapentanoate-2,2,3,3-d4 as a reference for 1H and 13C NMR. The data were acquired and processed by using the Bruker XWINNMR V 3.0, running on SGI O2 or Octane 2 processors. Point data sets (32,768) were used for 1D spectra, in some instances with zero-filling to 32,768 or 65,536 points. 1D 1H NMR spectra were recorded at 500 MHz with a spectral width of 4.25 kHz, a 30° pulse (3.2 μs), and a recycle time of 6 s. 1D 13C NMR spectra were acquired at 126 MHz by using a spectral width of 25.1 kHz, a 45° pulse (3 μs), and a recycle time of 1 s, except that for studies of closely spaced 13C resonances of cholesterol, 65,536 point data sets were used, with zero-filling to 131,072 points. 1D 13C NMR spectrum editing was conducted according to the distortionless enhancement by polarization transfer (DEPT) method, using combinations of spectra acquired with 30°,90°, 135°,or150° read pulses at the 1H frequency. 1H-coupled 13C NMR spectra were acquired with the NOE by using gated irradiation at the 1H frequency.
2D COSY-30 or -45 1H NMR spectra were collected in 2,048 × 512 point data sets, zero-filled to 2,048 × 2,048 points. Unshifted sine-bell squared window functions were applied in both dimensions before Fourier transformation, after which the frequency data were displayed in magnitude mode. 2D total correlation spectroscopy (TOCSY) 1H NMR spectra were acquired by using 16,384 × 256 point data sets, zero-filled to 16,384 × 2,048 points, by use of the gradient-selected, phase sensitive, echo/anti-echo protocol. 1D 1H NMR subspectra of individual residues were produced by extraction of F2 slices from the 2D TOCSY spectra. For some 1H NMR spectra, the assignments were also confirmed by homonuclear 1H decoupling.
Two-dimensional heteronuclear single quantum correlation (HSQC) and heteronuclear multiple bond correlation (HMBC) 1H/13C NMR spectra were recorded as 2,048 × 512 point data sets, zero-filled to 2,048 × 2,048 points, by using gradient-selected, sensitivity-enhanced, phase-sensitive echo/anti-echo mode for HSQC, and a gradient-selected, low-pass filtered, long-range, non-decoupled pulse sequence for HMBC, the data from which were displayed in magnitude mode. 2D HMBC NMR spectra were acquired with an evolution delay of 83 ms, i.e., optimized for 2,3JCH 6.0 Hz.
Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) MS. MALDI-TOF MS were obtained by using a PerSeptive BioSystems Voyager Elite DE-STR (Applied Biosystems). Spectra were accumulated for 100 laser pulses at an attenuation of 2600. The instrument was operated in the linear mode with a 20-kV accelerating voltage and a 150-ns ion extraction delay time. Sample and matrix were prepared as described (15).
Fast Atom Bombardment (FAB) MS. Native or peracetylated glycolipids were dissolved in chloroform/methanol 2:1 (vol/vol) or methanol respectively, mixed with Magic Bullet (DTT and dithioerythritol), 3-nitrobenzyl alcohol, or glycerol as a matrix, and analyzed by JEOL SX102a magnetic sector instrument with xenon. Peracetylation of the glycolipids was done as described (16).
Radiolabeling. [4-14C]cholesterol (specific activity 58.0 mCi/mmol, Amersham Pharmacia, courtesy of W. Prinz, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 1 Ci = 37 GBq) was added at 0.1 μCi/ml in a mixture of Tween 80/ethanol (1:1 vol/vol) to the inoculum of B. burgdorferi. For assessing [14C]cholesterol incorporation into BbGL-I, total lipids were extracted, separated on TLC plates, and exposed to iodine vapor. The visualized spots were scraped off into scintillation chambers containing 1 ml of scintillation liquid. Radioactivity was measured in a Perkin–Elmer scintillation counter (model I450 microbeta) and expressed as dpm.
Affinity Chromatography. Borrelia cells were washed five times, suspended in PBS, and disrupted by ultrasonic irradiation for 30 s, in a Branson sonifier operated in 50% duty cycles at 100 W. Membranes were separated from the soluble fraction by centrifugation at 12,000 × g for 30 min, and the supernatant (1 ml containing 1 mg of protein) was loaded onto a Detoxi-Gel Endotoxin Removing Gel (immobilized-polymixin B, Pierce) column (0.9 × 10 cm). The column was washed with 10 bed volumes of PBS, and eluted by 10 bed volumes of deoxycholic acid in PBS (1% wt/vol). The dissociated fraction was dialyzed against water and freeze-dried, and the lipids were extracted as above.
Immunologic. Groups of 10 female NIH Swiss albino mice (5–6 weeks old) were immunized twice i.p. with BbGL-I or BbGL-II (10 μg per 0.1 ml) emulsified in complete Freund's adjuvant (first dose) or incomplete Freund's adjuvant (second dose), 4 weeks apart. Alternatively, mice were immunized three times, 2 weeks apart, with 10 μg of BbGL-I emulsified in 0.1 ml of DMSO, squalene, or PBS. Mice were bled 2 weeks after the last dose, and the sera were stored at -20°C. Rabbits (females, New Zealand White, 8 weeks old) were immunized with two doses of BbGL-I, 75 μg each, in Freund's adjuvant, administered i.m. in 0.1 ml and i.c. in 20 × 0.05 ml. Immunostaining of membrane lipids and ELISA assay for determination of anti-BbGL-I and anti-BbGL-II titers were determined as described (17).
Results
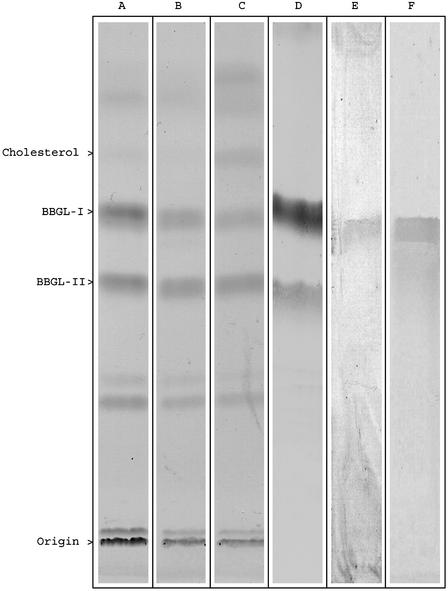
Isolation of BbGL-I and BbGL-II. Strain B31 yielded 0.7 g of total lipids from 2.17 g of dry cells, of which 163 mg (23.2%) of BbGL-I and 87 mg (12.4%) of BBGL-II were obtained, indicating that these are major glycolipids of B. burgdorferi. Similar lipid patterns (TLC) were observed from the strains BL303 and N40 (Fig. 1). Bacterial yields from B31 were the highest of all strains tested, and subsequent experiments were performed with this strain.
Fig. 1.
TLC analysis of lipid extracts from B. burgdorferi. Lipid extracts, visualized by iodine vapor, from strain B31 (A), strain N40 (B), strain BL303 (C), sprayed with anthrone reagent (strain B31) (D), immunostained with mouse anti BbGL-I (strain B31) (E), or lipid extract of Detoxy-Gel eluted material, exposed to iodine vapor (strain B31) (F).
Compositional Analysis. Galactose (Gal) was the only saccharide detected in BbGL-I, and glycerol and Gal were detected in BbGL-II. The major fatty acids detected were C16:0 and C18:1. Minor fatty acids were C14:0, C18:0, and C18:2 (Table 1). The major unsaturated fatty acid was oleate, as determined by the localization of the double bond at position Δ9, and the cis configuration.
Table 1. The chemical composition of B. burgdorferi glycolipids.
| BbGL-I
|
BbGL-II
|
|||
|---|---|---|---|---|
| Component | Native | O-deacylated | Native | O-deacylated |
| Molar ratio of sugars | ||||
| Galactose | 1.0 | 1.0 | 1.0 | 1.0 |
| Glycerol | 0 | 0 | 0.93 | 1.05 |
| Molar ratio of fatty acids | ||||
| C 14:0 | 0.07 | 0.15 | ||
| C 16:0 | 1.15 | 1.00 | ||
| C 18:2 (Δ9, 12) | 0.23 | 0.12 | ||
| C 18:1 (Δ9) | 1.0 | 0.65 | ||
| C 18:0 | 0.16 | 0.25 | ||
O-Deacylation. O-deacylation was completed in 15 h for BbGL-I, and3hfor BbGL-II. GLC-MS analysis of the hydrolyzed glycolipids indicated that Gal was in the chloroform phase of BbGL-I, and in the water phase of BbGL-II. This finding suggested that BbGL-I contained a Gal attached to a lipid by an alkali stable linkage.
Absolute Configuration. Gal was determined to be in the D configuration. The absolute configuration of C-2 in the glycerol of BbGL-II was determined to be L, with the designation of the sugar residue on C-3, and the fatty acids on C-1 and C-2. This is consistent with the sn-configuration when carbons are stereospecifically numbered.
Methylation Analysis. 1,5-di-O-acetyl-2,3,4,6-tetra–O-methyl-galactitol and 1,5,6-tri-O-acetyl-2,3,4-tetra-O-methyl-galactitol in the molar ratio of 1.0 to 0.9, demonstrated terminal and 6-substituted Gal residues in BbGL-I. Prior O-deacylation resulted in the disappearance of the 6-substituted Gal, leaving a terminal Gal as the only component. This suggested that the fatty acid chain, located at C-6 of the Gal, partially survived methylation performed as described (12).
NMR Spectroscopy. The structures of the BbGL-I and BbGL-II and their peracetyl derivatives were investigated by 1D and 2D NMR at 500 MHz. 1H NMR assignments (Table 2) were confirmed by 2D COSY, TOCSY, or selective spin-decoupling experiments, whereas 13C NMR assignments (Table 4, which is published as supporting information on the PNAS web site, www.pnas.org) were indicated by 2D heteronuclear single quantum correlation, based on the 1H NMR assignments. Interresidue connectivities and further evidence for 13C assignments were gained from 2D HMBC.
Table 2. 1H chemical shifts (ppm) of the galactose and glycerol residues of glycolipids and related compounds.
| Proton | BbGL-1* | BbGL-I-Ac3 | BbGL-II | BbGL-II-Ac4 | MGDG | 1,2-Dipalmitin | Me-α-D-Galp (in D2O) | Me-β-D-Galp (in D2O) |
|---|---|---|---|---|---|---|---|---|
| H-1′ | 4.327 | 4.545 | 4.943 | 5.124 | 4.232 | 4.846 | 4.323 | |
| H-2′ | ≈3.617 | 5.184 | ≈3.84 | 5.106 | 3.550 | 3.836 | 3.509 | |
| H-3′ | ≈3.617 | 5.024 | 3.770 | 5.317 | 3.502 | 3.812 | 3.653 | |
| H-4′ | 3.877 | 5.370 | 4.096 | 5.460 | 3.896 | 3.976 | 3.931 | |
| H-5′ | 3.666 | 3.886 | 3.807 | 4.203 | 3.510 | 3.905 | 3.703 | |
| H-6′a | 4.351, 4.346 | 4.191 | 3.927 | 4.107 | 3.829 | 3.768 | 3.804 | |
| H-6′b | 4.300, 4.294 | 4.101, 4.104 | ≈3.82 | 4.075 | 3.757 | 3.742 | 3.759 | |
| OMe | 3.422 | 3.581 | ||||||
| OAc | 2.142, 2.061, 1.982 | 2.138, 2.071 | ||||||
| 2.041, 1.988 | ||||||||
| H-1″″a | 4.377 | 4.340 | 4.381 | 4.319 | ||||
| H-1″″b | 4.128 | 4.147 | 4.229 | 4.238 | ||||
| H-2″″ | 5.253 | 5.198 | 5.279 | 5.083 | ||||
| H-3″″a | 3.840 | 3.816 | 3.954 | 3.751, 3.738 | ||||
| H-3″″b | 3.634 | 3.620 | 3.723 | 3.724, 3.710 |
HO-2′, HO-3′, and HO-4′ signals observed at δ 2.996 (d, J 7.5 Hz), δ 2.711 (d, J 8.6 Hz), and δ 2.771 (t, J 7.0 Hz), varied with concentration, etc
The values of certain key homo- and heteronuclear coupling constants for the glycolipids are reported in Table 3. NMR data for several reference compounds or structural components of the glycolipids are also reported in Tables 2, 3, 4. Assignment of the 13C NMR resonances was further assisted by 1D DEPT NMR spectrum editing experiments.
Table 3. Coupling constants (J, Hz) of galactopyranose and glycerol residues of glycolipid derivatives and related reference compounds.
| BbGL-I CDCl3 | BbGL-I-Ac3 CDCl3 | BbGL-II CDCl3 | BbGL-II-Ac4 CDCl3 | MGDG CDCl3:CD3OD* | 1,2-Dipalmitin CDCl3 | Me-α-d-Galp D2O | Me-β-d-Galp D2O | |
|---|---|---|---|---|---|---|---|---|
| J1′,2′ (Gal) | 7.5 | 8.0 | 3.8 | 3.7 | 7.3 | 3.4 | 8.0 | |
| J2′,3′ | ND | 10.5 | 9.8 | 10.0 | 9.7 | 10.1 | 9.9 | |
| J3′,4′ | 3.2† | 3.5 | 3.2 | 3.5 | 3.3 | 2.8 | 3.5 | |
| J4′,5′ | 1.0† | 1.0 | 1.1 | 1.1 | 1.1 | 1.6 | 0.8 | |
| J5′,6′a | 6.3 | 6.7 | 5.1 | 6.4 | 6.5 | 6.8 | 7.9 | |
| J5′,6′b | 7.2 | 6.7 | ND | 7.0 | 5.4 | 5.5 | 4.4 | |
| J6′a,6′b | 11.1 | 11.2 | 11.5 | 11.2 | 11.6 | 11.7 | 11.7 | |
| 1JC-1′,H-1′ | 158.7 | 157.4 | 170.5 | 172.4 | 160.2 | 170.2 | 160.6 | |
| J1″″a,1″″b (Gro) | 11.9 | 11.8 | 12.1 | 11.9 | ||||
| J1″″a,2″″ | 4.1 | 4.1 | 3.2 | 4.5 | ||||
| J1″″b,2″″ | 5.9 | 6.1 | 6.7 | 5.7 | ||||
| J2″″,3″″a | 4.8 | 4.3 | 5.4 | 4.8 | ||||
| J2″″,3″″b | 6.2 | 5.2 | 6.0 | 5.2 | ||||
| J3″″a,3″″b | 10.9 | 11.2 | 10.9 | 12.2 |
ND, not determined
4:1 vol/vol
In (CD3)2CO:CDCl3 (7:3 vol/vol) solution
BbGL-I. The 1D 1H NMR spectrum of BbGL-I in chloroform-d solution was incompletely dispersed at 500 MHz, the H-2′ and H-3′ signals of the sugar residue being significantly overlapped at ≈3.62 ppm. (Cholesterol, galactose, palmitic acid, oleic acid, and glycerol residues are labeled as unprimed, and single, double, triple, and quadruple primed, respectively.) Superimposition of the H-1′, H-6′a, and H-6′b multiplets was also observed (for protons labeled as a and b, the a label refers to the proton that resonates at lower field, whereas the b label refers to the higher field proton), and a 1D slice taken through these signals in the 2D TOCSY spectrum yielded a subspectrum that contained all seven of the sugar chain proton signals (H-1′ to H-6′b), and also the hydroxyl multiplets HO-2′, HO-3′, and HO-4′. Deshielding of the sugar methylene protons of the glycolipid by 0.55 ppm with respect to those of Me-β-d-Galp (Table 2) suggests that O-6′ of the sugar is acylated. This TOCSY experiment also proved that the 1:2:1 triplet of 1:2:1 triplets at δ 3.553 is not part of the sugar proton spin system, and this multiplet was assigned to H-3 of the cholesterol moiety, particularly because it was also observed in a 1D TOCSY slice taken through the olefinic proton signals of BbGL-I. By integration, the latter signals amounted to ≈2.5 protons in the 1D 1H NMR spectrum, because of coincidence of the single olefinic proton (H-6) signal of the cholesterol moiety with the olefinic proton signals of a proportion of unsaturated fatty acids in the isolated glycolipid. In support of this assignment, the TOCSY slice through the olefinic proton signals also contained a large number of multiplets in the aliphatic proton region (δ 2.8–0.9) because of connectivity to aliphatic protons of both cholesterol and unsaturated fatty acids.
The 13C NMR spectrum of BbGL-I displayed 59 major, resolved resonances, of which five were caused by quaternary 13C nuclei, i.e., not detected by 13C DEPT experiments. From low field to high field (Table 4), the five quaternary 13C resonances were assigned as two ester carbonyl resonances, and C-5 (olefinic), C-13, and C-10 resonances of the cholesterol moiety. Of the total number of 13C resonances, 15 could be identified as CH by DEPT spectrum editing (Fig. 7, which is published as supporting information on the PNAS web site), together with 27 CH2 and six CH3 groups. Five of the CH3 resonances were assigned to the cholesterol residue (Table 4), and the sixth, weaker one at δC 14.13 to a combination of the signals of the ω methyl carbons of fatty acid ester groups, which typically may not be resolved from each other. The two major olefinic CH resonances at ≈130 ppm were assigned to a predominant, unsaturated fatty acid ester group (oleic acid, 18:1). The CH resonances at 122.12 and 79.46 ppm were assigned to C-6 and C-3 of the cholesterol moiety, respectively. Good agreement was obtained between the 13C chemical shifts of BbGL-I and those of its lipid components, cholesterol, palmitic acid, and oleic acid, except for nuclei near the points of attachment of the residues (18).
The remaining five CH resonances in the range of 101.43–68.32 ppm were assigned to C-1′–C-5′ of Gal, which also exhibited the CH2 signal at 61.3 ppm (Table 4). Doubling of the C-4′, C-5′, and C-6′ of Gal and C-3 resonance of the cholesteryl moiety was observed, which may be attributed to the presence of two glycolipids bearing palmitoyl and oleoyl groups at O-6′ of the Gal (V. Pozsgay and B.C., unpublished data).
The locations of the cholesteryl group at O-1′ and acyloxy group(s) at C-6′ of the Gal residue in BbGL-I were indicated by observation of the appropriate 1H/13C cross peaks in its 2D HMBC spectrum. Analysis of the cholesterol H-3 multiplet in the 1H NMR spectrum of BbGL-I yielded the coupling constants J2eq,3 = J4eq,3 = 4.7 Hz, and J2ax,3 = J4ax,3 = 11.4 Hz. These values define the orientation of H-3 as axial, and hence the oxygen atom (O-1′) attached to C-3 is equatorial. Therefore, C-3 has the usual stereochemical configuration found in cholesterol. The NMR data for BbGL-I are consistent with a mixture of two structures, namely, cholesteryl 6-O-palmitoyl- and -6-O-oleoyl-β-d-galactopyranoside. Assignments of the sugar ring size and anomeric configuration of BbGL-I are discussed below.
BbGL-I-Ac3. The presence of Gal in BbGL-I was obtained by its peracetylation, which yielded a triacetate, the 1H NMR spectrum of which was better dispersed than that of BbGL-I, and which displayed the characteristic spin multiplets of Gal. H-2′, H-3′, and H-4′ were significantly deshielded (1.41–1.57 ppm) with respect to their positions in nonacetylated BbGL-I (Table 2), indicating that acetylation had occurred at HO-2′, HO-3′, and HO-4′. Locations of the cholesterol, and the acetyl and fatty acid ester groups were confirmed by observations of the expected 1H/13C cross peaks in 2D HMBC (Fig. 8, which is published as supporting information on the PNAS web site).
The large values of J1′,2′ observed for BbGL-I and BbGL-I-Ac3 (Table 3) indicate that H-1′ and H-2′ have the trans orientation in these glycolipids, which, therefore, have the β anomeric configuration. This was confirmed by measurement of the values 1JC-1′,H-1′ 158.7 Hz and 157.4 Hz for BbGL-I and BbGL-I-Ac3, respectively (Table 3), which fall within the appropriate range for the β anomeric configuration (19), as exemplified by the value 1JC-1′,H-1 160.6 Hz observed for Me-β-d-Galp (Table 3). The assignments of sugar ring size for BbGL-I and BbGL-I-Ac3 were made on the basis of the similarity of the J values of the sugar rings to those of Me-β-d-Galp (Table 3). The NMR data for BbGL-I-Ac3 are consistent with its characterization as a mixture of cholesteryl 2,3,4-tri-O-acetyl-6-O-palmitoyl- and -6-O-oleoyl-β-d-galactopyranosides.
BbGL-II. The 1H NMR spectrum of BbGL-II in chloroform-d solution was well dispersed; in particular, the glycerol protons are fully dispersed at 500 MHz, and both the 2D COSY and TOCSY spectra contain five-multiplet strings that represent (from low to high field) the glycerol protons H-2′″′, H-1′″′a, H-1′″′b, H-3′″′a, and H-3′″′b. The TOCSY spectrum (Fig. 9, which is published as supporting information on the PNAS web site) also exhibits an H-1′–H-4′ multiplet string that is characteristic of the Gal configuration, transmission of magnetization from H-4′ to H-5′, H-6′a, and H-6′b commonly being inhibited by the small magnitude of J4′,5′ (Table 3). A seven-multiplet string in the 2D TOCSY spectrum (Fig. 9) represents the mutual exchange of magnetization between the olefinic protons (δ 5.344) and the aliphatic protons in an unsaturated fatty acid (18:1).
The DEPT-135 13C NMR spectrum of BbGL-II indicated six CH resonances and three CH2 signals in the sugar region, consistent with the presence of one glycerol residue and one aldose residue. Also observed were 25 incompletely resolved CH2 signals and one CH3 resonance in the aliphatic carbon region that were assigned to fatty acids. Two strong resonances at δC 130.06 and 129.70 suggested the presence of one, predominant unsaturated fatty acid residue. Three strong 13C resonances were observed in the C = O region, together with a weaker one. Substantial deshielding (0.567 to 1.470 ppm) of H-1′″′a, H-1′″′b, and H-2′″′ of the glyceryl residue of BbGL-II with respect to the corresponding protons of glycerol pointed to acylation of O-1′″′ and O-2′″′ of the glyceryl residue. Confirmation of the positions of the acyl groups and the Gal residue was provided by observation of the anticipated 1H/13C cross peaks in the 2D HMBC spectrum of BbGL-II.
BbGL-II-Ac4. Peracetylation of BbGL-II yielded a product whose 1H and 13C NMR spectra indicated formation of a tetra-O-acetate. Substantial deshielding (1.266–1.547 ppm) of the Gal H-2′, H-3′, and H-4′, and more limited deshielding (0.180 to 0.255 ppm, Table 2) of H-6′a and H-6′b pointed to acetylation of O-2′, O-3′, O-4′, and O-6′ of the Gal residue. The positions of the acetyl and fatty acid acyl groups, and Gal residue were confirmed by detection of the appropriate 1H/13C cross peaks in the 2D HMBC spectrum of BbGL-II-Ac4 (Fig. 10, which is published as supporting information on the PNAS web site).
The small values of J1′,2′ for BbGL-II and BbGL-II-Ac4 (Table 3) indicate the gauche orientation for H-1′ and H-2′ in these derivatives, indicating the α anomeric configuration of the Gal. This was confirmed by the large values JC-1′,H-1′ 170.5 Hz and 172.4 Hz, respectively, for BbGL-II and BbGL-II-Ac4 (Table 3), which fall within the range expected for α anomers (19), and observed for Me-α-d-Galp (Table 3).
The NMR data for BbGL-II are consistent with the structure 3-O-α-d-galactopyranosyl-1 (2)-O-oleoyl-2(1)-O-palmitoyl-glycerol, and those for BbGL-II-Ac4 support the structure 3-O-(2,3,4,6-tetra-O-acetyl-α-d-galactopyranosyl)-1(2)-O-oleoyl-2(1)-O-palmitoyl-glycerol. Again, the assignment of sugar ring size was based on the similarity of the coupling constants to those of Me-α-d-Galp (Table 3).
MGDG. The MGDG, a close structural analog of BbGL-II, was studied as a reference compound. Good dispersion of the 1H NMR spectrum of MGDG was obtained at 500 MHz, and the seven, characteristic spin multiplets originating from the Gal residue were readily recognized. Moreover, the ring proton coupling constants (Table 3) indicate that the Gal is present as a pyranosyl ring, in concert with the glycolipid derivatives discussed earlier. The 2D COSY and TOCSY 1H NMR spectra of MGDG contained the same highly dispersed, five-multiplet strings that are characteristic of a glycerol (Gro) residue bearing acyloxy groups at C-1′″′ and C-2″″, a substitution pattern supported by the downfield shifts of H-1″″a, H-1′″′b, and H-2′″′ (Table 2). DEPT-135 13C NMR spectra exhibited six main CH resonances and three predominant CH2 signals in the sugar region (Gal + Gro), together with 14 resolved CH2 signals and one CH3 resonance in the aliphatic region that represent two fatty acid ester residues, as confirmed by the detection of two C=O signals in the normal 13C NMR spectrum. The locations of the acyloxy groups at C-1″″ and C-2″″ and the Gal residue at O-3″″ were substantiated by observation of the expected 1H/13C cross peaks in the 2D HMBC spectrum of MGDG. The large value J1′,2′ 7.3 Hz and small value JC-1′,H-1′ 160.2 Hz (Table 3) prove that MGDG has the β anomeric configuration, i.e., the opposite configuration of BbGL-II. Together, the NMR and GLC-MS data for MGDG indicate that it consists mainly of 1,2-di-O-stearoyl-3-O-β-d-galactopyranosyl glycerol.
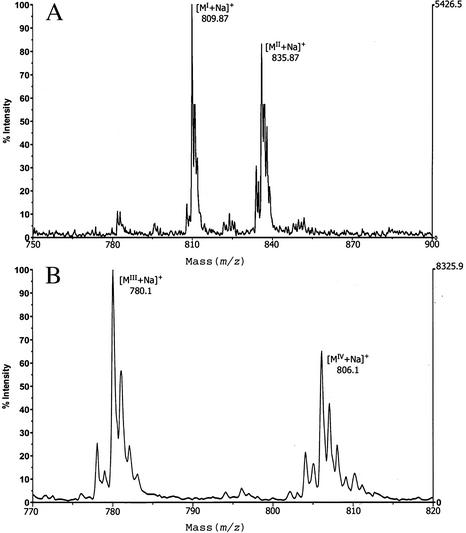
MS. MALDI-TOF spectra of BbGL-I, recorded in the positive ion mode, detected two molecular sizes of 809.87 and 835.87, representing the sodium adduct of cholesteryl galactoside substituted with either palmitate or oleate (Fig. 2). This agrees with GLC-MS data, showing the predominance of these fatty acids. FAB-MS analysis of BbGL-I confirmed molecular masses of 809.7 and 835.7 (data not shown), whereas peracetylated BbGL-I had molecular weights of 935.7 and 961.7. The increase in molecular weight of Δm/z = 126 represents the incorporation of three acetyl groups, and confirms the existence of three free hydroxyl groups in BbGL-I. MALDI-TOF spectra of BbGL-II revealed two ions with masses of 780.1 and 806.1, accounted for by sodium adducts of monogalactosyl diacyl glycerol with two palmitates, or with palmitate and oleate, respectively (Fig. 2).
Fig. 2.
Part of the MALDI-TOF mass spectra of sodium adduct of BbGL-I and BbGL-II. Two major peaks of pseudomolecular ions [M+Na]+ at m/z 809.87 and 835.87 represent palmitic (MI) and oleic (MII) derivatives of cholesteryl galactoside (BbGL-I; A), and pseudomolecular ions [M+Na]+ at m/z 780.1 and 806.1 represent monogalactosyl diacyl glycerol (BbGL-II) containing dipalmitoyl (MIII) and palmitoyl-oleyl (MIV) substituents (B).
Radioactive Labeling of BbGL-I. When B. burgdorferi was cultivated in the presence of 14C-cholesterol, 80% of the label was found in BbGL-I. No radioactivity was detected in BbGL-II, free cholesterol, or cholesterol esters.
Affinity Chromatography. Sonicated B. burgdorferi cells were loaded onto a polymixin B column, and BbGL-I was eluted with 1% deoxycholic acid, demonstrated by TLC and immunolabeling (Fig. 1). BbGL-II was detected only in the unbound fraction.
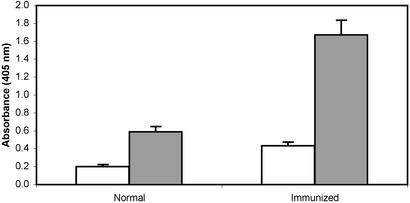
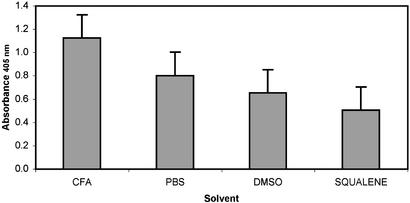
Immunologic. Specific antibodies were induced in mice and rabbits injected with various formulations of BbGL-I (Figs. 1 and 3). BbGL-II induced antibodies, mostly IgM, that reacted with both glycolipids (data not shown). BbGL-I in PBS (likely as micelles) was more immunogenic than as a solute in DMSO or squalene (Fig. 4). Highest titers were obtained with Freund's adjuvant.
Fig. 3.
Antibody titers of sera from rabbits immunized with BbGL-I. Antibody titers were measured by an ELISA, as described in Experimental Procedures, and represent an average of four different rabbit sera, diluted 1:100, each tested in three independent assays. Open bars, IgG titers; gray bars, IgM titers; brackets, standard deviation.
Fig. 4.
Antibody titers of sera from mice immunized with BbGL-I. Mice (10 mice per group) were immunized as described in Experimental Procedures, with BbGL-I in Freund's adjuvant (CFA); PBS; DMSO, or squalene. Antibody titers were determined by an ELISA in sera diluted 1:100, and are represented as an average of three independent assays. Brackets, standard deviation.
Discussion
Two major glycolipids from strains B31, N40, and BL303 of B. burgdorferi were isolated, characterized, and designated as BbGL-I and BbGL-II. These compounds were purified by silica gel chromatography, and analyzed using GLC-MS, MALDI-TOF, FAB-MS, NMR spectrometry, and metabolic labeling. The structure of BbGL-I is cholester yl 6-O-acyl-β-d-galactopyranoside (Fig. 5), and that of BbGL-II is 1,2-di-O-acyl-3-O-α-d-galactopyranosyl-sn-glycerol (Fig. 6). BbGL-II conforms to the structure of MGDG previously described (8): we confirmed the α configuration of the d-galactose moiety, the sn configuration of the glycerol, and identified the main unsaturated fatty acid as oleate. Hossain et al. (8) suggested that the other glycolipid (BbGL-I) is also a monogalactosyl diacylglycerol, and that the two differed only in their fatty acid composition. The pseudomolecular masses [M+Na]+ observed for BbGL-I by these investigators, 810 and 836, coincidently match the pseudomolecular masses [M+Na]+ of cholesteryl galactoside, substituted with either palmitic or oleic acids, respectively. Therefore, without NMR analysis, the suggested structure of BbGL-I could have been misinterpreted.
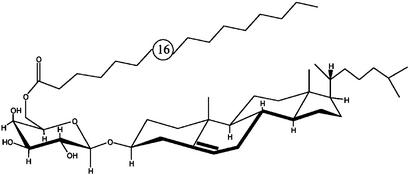
Fig. 5.
Structure of BbGL-I. Cholesteryl 6-O-palmitoyl-β-d-galactopyranoside.
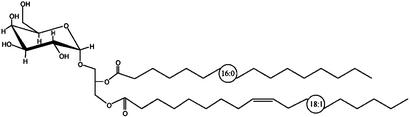
Fig. 6.
Structure of BbGL-II. 1-O-palmitoyl-2-O-oleyl-3-O-α-d-galactopyranosyl-sn-glycerol.
Free cholesterol or cholesterol esters are components of several bacterial membranes such as Mycoplasma (20), Helicobacter pylori (21), Micrococcus lysodeikticus, Bacillus megaterium, and Proteus mirabilis (22). Cholesteryl glycosides have been identified in Mycoplasmas (20, 23), H. pylori (21), and Borrelia hermsi (24), in all of which, the carbohydrate was glucose. Cholesteryl Galactoside is now identified in bacteria.
LPS has been identified in spirochaetales, but we and others (4) could not find evidence for its presence in B. burgdorferi, nor were we able to detect markers of LPS such as KDO, Lipid A or 3-hydroxy fatty acids (data not shown). Detoxy Gel, an affinity sorbent for binding (removal) of LPS, did not reveal LPS, but bound to BbGL-I. The lack of LPS, the abundance of BbGL-I and BbGL-II in the bacterial membrane, their surface exposure (as indicated by fluorescence labeling; data not shown), and their three-domain structure lead us to suggest that these glycolipids may assume LPS function.
Surprisingly, despite its small size, BbGL-I elicited antibodies in mice and rabbits, mostly of the IgM isotype. These antibodies were specific to the homologous glycolipid. In contrast, BbGL-II elicited antibodies that reacted with both glycolipids. Antibodies to these glycolipids have been found in sera of Lyme disease patients (6).
First described as a tick-borne spirochetosis in 1982 (25), Lyme disease is now recognized as a cause of common and serious systemic disease worldwide. Little information exists about the protective antigen(s) or the host factor(s) that confer immunity to B. burgdorferi. The licensed vaccine, composed of a lipidated derivative of an outer membrane protein, has been withdrawn by the manufacturer. The comprehensive description of two surface antigens of B. burgdorferi provides an approach to study immunity to this pathogen.
Supplementary Material
Acknowledgments
We thank John B. Robbins, Andrej Gamian, and Ulrich Zahringer for review of the manuscript, and Noel Whittaker and Victor Livengood for FAB-MS.
Abbreviations: LPS, lipopolysaccharide; BbGL-I, Borrelia burgdorferi glycolipid I; BbGL-II, B. burgdorferi glycolipid II; MGDG, monogalactosyl diglyceride; Me-α-d-Galp, methyl α-d-galactopyranoside; Me-β-d-Galp, methyl β-d-galactopyranoside; DEPT, distortionless enhancement by polarization transfer; TOCSY, total correlation spectroscopy; HMBC, heteronuclear multiple bond correlation; Gal, galactose; MALDI-TOF, matrix-assisted laser desorption/ionization time-of-flight; GLC, gas–liquid chromatography; Gro, glycerol.
References
- 1.Steere, A. C. (2001) N. Engl. J. Med. 345, 115-125. [DOI] [PubMed] [Google Scholar]
- 2.Vinh, T. U., Shi, M. H., Adler, B. & Faine, S. (1989) J. Gen. Microbiol. 135, 2663-2673. [DOI] [PubMed] [Google Scholar]
- 3.Halter, M. R. & Joens, L. A. (1988) Infect. Immun. 56, 3152-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takayama, K., Rothenberg, R. J. & Barbour, A. G. (1987) Infect. Immun. 55, 2311-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinco, M., Banfi, E., Balanzin, D., Godeas, C. & Panfili, E. (1991) FEMS Microbiol. Immunol. 3, 33-38. [DOI] [PubMed] [Google Scholar]
- 6.Eiffert, H., Lotter, H., Jarecki-Khan, K. & Thomssen, R. (1991) Med. Microbiol. Immunol. (Berlin) 180, 229-237. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler, C. M., Garcia Monco, J. C., Benach, J. L., Golightly, M. G., Habicht, G. S. & Steere, A. C. (1993) J. Infect. Dis. 167, 665-674. [DOI] [PubMed] [Google Scholar]
- 8.Hossain, H., Wellensiek, H. J., Geyer, R. & Lochnit, G. (2001) Biochimie 83, 683-692. [DOI] [PubMed] [Google Scholar]
- 9.Bligh, E. G. & Dyer, W. J. (1969) Can. J. Biochem. Physiol. 37, 911-917. [DOI] [PubMed] [Google Scholar]
- 10.Sawardeker, J. S., Sloneker, J. H. & Jeanes, A. (1965) Biochem. J. 37, 1602-1604. [Google Scholar]
- 11.Fay, L. & Richli, U. (1991) J. Chromatogr. 541, 89-98. [Google Scholar]
- 12.Ciucanu, I. & Kerek, F. (1984) Carbohydr. Res. 131, 209-217. [Google Scholar]
- 13.Roth, h., Segal, S. & Bertoli, D. (1965) Anal. Biochem. 10, 32-52. [DOI] [PubMed] [Google Scholar]
- 14.Pasciak, M., Ekiel, I., Grzegorzewicz, A., Mordarska, H. & Gamian, A. (2002) Biochim. Biophys. Acta 1594, 199-205. [DOI] [PubMed] [Google Scholar]
- 15.Zahringer, U., Wagner, F., Rietschel, E. T., Ben-Menachem, G., Deutsch, J. & Rottem, S. (1997) J. Biol. Chem. 272, 26262-26270. [DOI] [PubMed] [Google Scholar]
- 16.Dell, A. (1987) Adv. Carbohydr. Chem. Biochem. 45, 19-72. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Menachem, G., Wagner, F., Zahringer, U., Rietschel, E. T. & Rottem, S. (1997) FEMS Microbiol. Lett. 154, 363-369. [DOI] [PubMed] [Google Scholar]
- 18.Wilson, W. K., Sumpter, R. M., Warren, J. J., Rogers, P. S., Ruan, B. & Schroepfer, G. J., Jr. (1996) J. Lipid Res. 37, 1529-1555. [PubMed] [Google Scholar]
- 19.Bock, K. & Pedersen, C. (1975) Acta Chem. Scand. B 29, 258-264. [Google Scholar]
- 20.Rottem, S. (2002) Biochem. Biophys. Res. Commun. 292, 1289-1292. [DOI] [PubMed] [Google Scholar]
- 21.Hirai, Y., Haque, M., Yoshida, T., Yokota, K., Yasuda, T. & Oguma, K. (1995) J. Bacteriol. 177, 5327-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razin, S. (1975) J. Bacteriol. 124, 570-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayberry, W. R. & Smith, P. F. (1983) Biochim. Biophys. Acta 752, 434-443. [DOI] [PubMed] [Google Scholar]
- 24.Livermore, B. P., Bey, R. F. & Johnson, R. C. (1978) Infect. Immun. 20, 215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgdorfer, W., Barbour, A. G., Hayes, S. F., Benach, J. L., Grunwaldt, E. & Davis, J. P. (1982) Science 216, 1317-1319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.