Abstract
A major pathogenic mechanism of chronic alcoholism involves oxidative burden to liver and other cell types. We show that adult neurogenesis within the dentate gyrus of the hippocampus is selectively impaired in a rat model of alcoholism, and that it can be completely prevented by the antioxidant ebselen. Rats fed for 6 weeks with a liquid diet containing moderate doses of ethanol had a 66.3% decrease in the number of new neurons and a 227–279% increase in cell death in the dentate gyrus as compared with paired controls. Neurogenesis within the olfactory bulb was not affected by alcohol. Our studies indicate that alcohol abuse, even for a short duration, results in the death of newly formed neurons within the adult brain and that the underlying mechanism is related to oxidative or nitrosative stress. Moreover, these findings suggest that the impaired neurogenesis may be a mechanism mediating cognitive deficits observed in alcoholism.
Alcohol dependence and abuse are among the most prevalent mental disorders in the United States, with ≈14% of the general population meeting criteria for alcohol dependence at some time in their lives and 7% having been dependent in the past year (1). Severe cognitive impairment consistently occurs in chronic alcoholism regardless of the presence of associated thiamine deficits (Korsakoff syndrome), and it includes progressive and severe anterograde learning deficits, implicating impairment in hippocampal circuits. However, no consistent pathological finding has yet been identified. The neuropathological correlate of the cognitive impairments accompanying alcoholism remains unclear (2–4). In animal models of alcoholism, a thinning of the granular layer of the dentate gyrus (DG) is attributed to neuronal loss (5). These findings, however, have been difficult to confirm in human brains (6) and have been contested in animal models as well (7). Human postmortem studies account for the duration of alcohol abuse but not the duration of abstinence before death. Interestingly, MRI studies demonstrate reduction of hippocampal volumes in alcoholics that are reversible after short periods of abstinence (8). The loss of hippocampal volume has been attributed to changes in white matter (6), but the incorporation of newly formed neurons to the DG could also be affected by alcohol. Similarly, hippocampal-dependent cognitive functions have also shown reversibility after comparable periods of abstinence.
Neurogenesis is primarily a developmental process that involves the proliferation, migration, and differentiation into neurons of primordial CNS stem cells (9, 10). Neurogenesis declines until it ceases in the young adult mammalian brain with two exceptions: the olfactory bulb (OB) and the hippocampus produce new neurons throughout adult life. In the subgranular cell layer of the DG, hippocampal progenitors proliferate and migrate a short distance into the granule cell layer, where they differentiate into hippocampal granule cells. Although multiple factors seem to regulate adult neurogenesis including hormones, neurotransmitters, and trophic factors, it has been only recently that the possible functions and effects of neurogenesis have been considered. Reduction in the number of newly generated neurons in the DG (through administration of an antimitotic agent) is associated with impairment in hippocampal-dependent tasks (11). A recent study on conditional knockout mice for the presenilin-1 gene (12) suggests that DG neurogenesis might play a role in the periodic clearance of outdated hippocampal memory traces after cortical memory consolidation. A chronic, abnormal clearance process in the hippocampus may lead to memory disorders in the mammalian brain. Therefore, it seems that DG neurogenesis does play a role in cognitive functioning.
Chronic ethanol treatment significantly impairs hippocampal long-term potentiation (13) and produces progressive learning and memory deficits across a variety of behavioral tests, including active avoidance (14) and spatial memory (15). The Lieber–DeCarli ethanol diet reliably reproduces many of the effects of alcoholism observed in humans, including liver changes, gastro-intestinal effects, and peripheral neuropathies (16). We selected and studied adult neurogenesis by using this animal model of alcohol abuse because it reliably reproduces many of the effects observed in humans.
Ethanol may mediate many of its deleterious effects through the production of oxidative stress (17). The model of chronic alcoholism that we study here has been shown to produce a significant decrease of antioxidant enzymes and altered glutathione homeostasis (18, 19). Therefore, considering the fact that oxidative damage is an important mechanism by which ethanol induces cell damage, we explored the possibility that an antioxidant could protect new neurons from the effects of alcohol consumption.
Methods
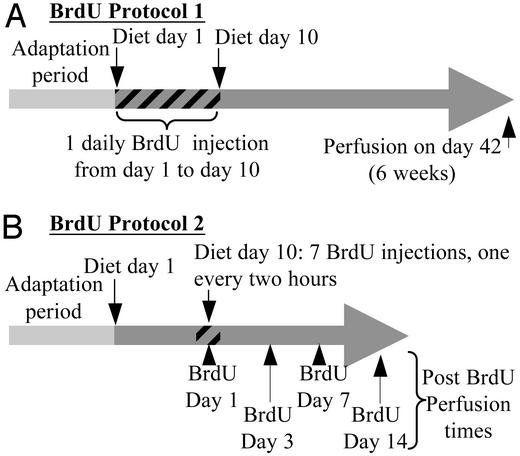
Sprague–Dawley rats (300–325 g) were divided into two groups receiving either control or alcohol liquid diet. Leber–Decarli rat ethanol or control rat diets were purchased in powder form from Bioserv (Frenchtown, NJ) or Dyets (Bethlehem, PA) and then mixed into liquid form with ethanol (final concentration, 6.4% vol/vol) and water or water alone, respectively. The antioxidant ebselen, provided by Natterman and Rhone-Poulenc-Rohrer (Cologne, Germany), was administered through the diet (0.1 mg/ml). Daily consumption of the liquid diets was recorded for each rat. The animals were allowed a 1-week adaptation period until the full diet was introduced and then they received 10 daily BrdUrd injections (40 mg/kg/i.p.; BrdUrd protocol 1, Fig. 1A). Rats were allowed to continue on their respective diets for 6 weeks and then processed for immunostaining. Animals that were used in the proliferation studies received seven BrdUrd injections (40 mg/kg/i.p.) on day 10 at 2-h intervals and were killed 1 h after the last injection (BrdUrd protocol 2, Fig. 1B). All animals were killed with a barbiturate overdose; each group had n ≥ 8. After intracardial perfusion with 4% paraformal-dehyde, brains were cut into 40-μm-thick sections (200 μm, 1 every sixth section, used for electron microscopy analysis) with a vibratome (Leica VT100M). Parasagital sections were obtained from the OB and coronal sections were obtained from the subventricular zone and the hippocampus.
Fig. 1.
BrdUrd administration protocols. (A) In protocol 1, rats received one daily BrdUrd injection over 10 consecutive days starting on the first day of full liquid diet (diet day 1) and then were allowed to survive for 6 weeks after the first BrdUrd injection. (B) Protocol 2 rats received seven BrdUrd injections spaced at 2-h intervals on 1 day only (day 10 of the full liquid diet) and then were allowed to survive for different time periods.
Immunostaining. Sections were immersed free-floating in 0.1 M phosphate buffer plus 0.05% sodium azide. Twenty-four-well plates were used to keep the sections separate and preserve the order of the series. Sections that were 40 μm thick were used for cell counting, double labeling, analysis of phenotypes, Nissl staining, or terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling method (TUNEL); 200-μm-thick sections were used for analysis at the electron microscope level. Cell-specific markers used were NeuN and glial fibrillary acidic protein. Incubations were performed in 0.1 M phosphate buffer with 0.2% Triton X-100 and 10% normal goat serum. Sections were blocked by incubating for 30 min in a solution of 3% hydrogen peroxide for diaminobenzidine (Sigma) staining. BrdUrd immunohistochemistry was preceded with DNA denaturation of 25 min of incubation in 2 M HCl at 37°C. Primary antibodies used were 1:200 mouse anti-BrdUrd (Dako), 1:250 rabbit anti-glial fibrillary acidic protein (Dako), 1:300 rabbit anti-NeuN (Chemicon), and 1:200 mouse anti-proliferating cell nuclear antigen (PCNA) (Sigma).
Single-label BrdUrd immunohistochemistry was performed for stereology counts, and the secondary antibody was a 1:300 biotinylated horse anti-mouse IgG (Vector Laboratories) followed by the peroxidase-based ABC system (Pierce) using diaminobenzidine as the chromogen. For double immunohistochemistry, we used 1:200 anti-rabbit Cy2 and 1:1,000 anti-mouse Cy3 (Jackson ImmunoResearch).
Cell Counting. BrdUrd-positive cells were counted in sections spaced 400 μm apart by using a Nikon Eclipse E200 with a Nikon 40X Plan objective (0.65 numerical aperture) throughout the granule cell layer. At least 12 sections in the hippocampi and 4 in the OBs were studied in each animal. The same areas and number of sections were studied for all of the animals and all of the experimental groups. We considered as BrdUrd+ those nuclei completely filled with diaminobenzidine product or fluorescent marker or showing patches of variable intensity. We used the optical dissector technique to estimate the cell density of BrdUrd+ cells in the granule cell layer. The granule cell area was traced by using a camera lucida (Olympus), and drawings were scanned with an Hewlett–Packard ScanJet 5300C and processed with Scion (Frederick, MD) IMAGE BETA 4.0.2 software to determine the area of the granule cell layer at each level examined. Double-labeled sections were counted with a Leica TCS SP confocal with a Leica HCXPL ×63 Apo objective (1.4 numerical aperture) throughout the granule cell layer. The confocal images were processed with Leica confocal software. Data were expressed as total, for the whole structure, and also subanalyzed in three regions: anterior, middle, and posterior. Areas of the granular cell layer of the DG were calculated as described above and ranged (according to the level analyzed) from 0.22 ± 0.02 mm2 to 0.52 ± 0.025 mm2 in control animals and from 0.22 ± 0.02 mm2 to 0.52 ± 0.06 mm2 in ethanol-fed rats; these areas were not statistically different. Cells were counted in parasagital sections of the OB in selected sample areas (0.33 mm2) of the rostral periglomerular layer and in the granular layer (immediately above the accessory OB).
Electron Microscopy Analysis. Brain sections were processed as described (20). Briefly, perfusion and sectioning were done as for light microscopy. Selected sections were postfixed in 4% osmium for 2 h each time and placed in 2% uranyl acetate for 2 h at 4°C. Sections were then dehydrated in ethanol, placed in propylene oxide, and embedded in Araldite (Durcupan; Fluka Biochemika). Ultrathin sections (50 nm) were cut with a diamond knife, stained with lead citrate, and examined under a Phillips CM-10 electron microscope.
Results
Neurogenesis Is Impaired in a Model of Chronic Alcoholism. Adult rats were divided into two groups and received either control or alcohol liquid diet (16). The animals were allowed a 1-week adaptation period until full diet was introduced and then received 10 daily BrdUrd injections (40 mg/kg/i.p.). Rats were allowed to continue on their diets for 6 weeks and then processed for immunostaining. Newly formed cells were detected by BrdUrd immunostaining and counted throughout the hippocampus and the OBs.
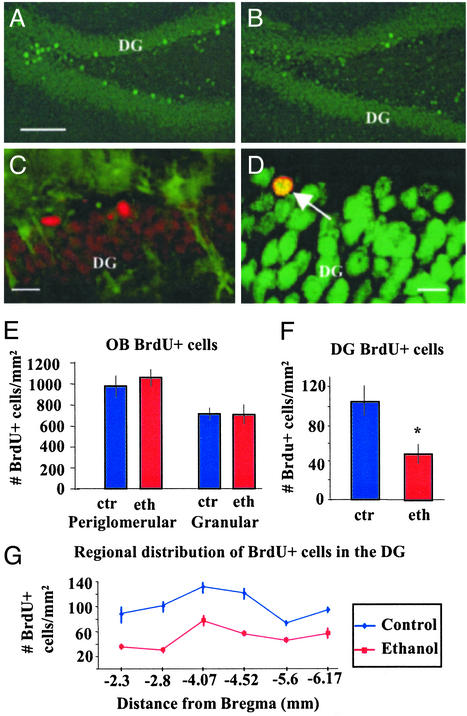
Ethanol levels of alcoholic rats ranged from 0.095% to 0.18%, which was similar to multiple previous studies using this model, and are clinically relevant (16). There was a decrease of 66.1 ± 3.1% in the number of BrdUrd-labeled cells in the DG of ethanol-receiving rats compared with controls (P < 0.01, n = 9 per group; Fig. 2 A–D, F, and G). Double-labeling studies (BrdUrd and NeuN or BrdUrd and glial fibrillary acidic protein) indicate that 93.7% of BrdUrd-labeled cells in the DG were of a neuronal phenotype (Fig. 2D), and 6.3% were astrocytes. No significant difference was found between ethanol-fed and control groups.
Fig. 2.
Ethanol selectively impairs adult neurogenesis in the hippocampus. (A and B) Immunofluorescence detection of BrdUrd-labeled cells in the DG. Photomicrograph of the DG from an animal that received control liquid diet (A) and ethanol-fed rat (B), both in BrdUrd protocol 1, 6-week survival; sgl, subgranular layer. (Bar = 150 μm.) (C) Merged confocal photomicrographs showing immunostaining for glial fibrillary acidic protein (green) and BrdUrd (red) with very few double-labeled cells in the DG. (Bar = 10 μm.) (D) Merged confocal photomicrographs of the DG showing immunostaining for BrdUrd (red) and the neuronal marker NeuN (green). Arrow points to a double-labeled cell in the DG; most BrdUrd-labeled cells in the DG were NeuN positive. (Bar = 10 μm.) (E) There was no statistical difference in the number of new cells either in the periglomerular or the granular cell layers of the OB between ethanol-fed or control rats. (F) There was an average 66.1% decrease in the number of BrdUrd-labeled cells in the DG of ethanol-fed rats as compared with controls. This effect was consistent throughout the DG. (G) Analysis at six different levels where BrdUrd-labeled cells were counted in the DG, BrdUrd protocol 1, 6-week survival. Distance of analyzed sections from bregma is expressed in mm.
The effects on neurogenesis could be observed throughout the rostral–caudal extent of the hippocampus, and the decrease of BrdUrd-positive cells in the DG of ethanol-exposed rats ranged, in different regions, between 50% and 70% (Fig. 2G). There was no difference in the extent of BrdUrd-labeled cell loss between the right and left hippocampi. However, there was a rostrocaudal gradient in the number of BrdUrd-labeled cells in the DG (higher number of cells in more anterior regions; Fig. 2G); the extent of the effect of alcohol was similar throughout this structure.
To determine whether the effect of alcohol on neurogenesis in the forebrain was restricted to the hippocampus, neurogenesis after chronic ethanol treatment was also assessed in the OB, the other neurogenic area in the adult brain. In the OB, no change in the number of BrdUrd-labeled cells was observed in ethanolfed rats (Fig. 2E). These findings suggest that the mechanism of alcohol-induced cell loss is selective for the DG and argues against a more general effect of the ethanol, such as an alteration of BrdUrd pharmacokinetics.
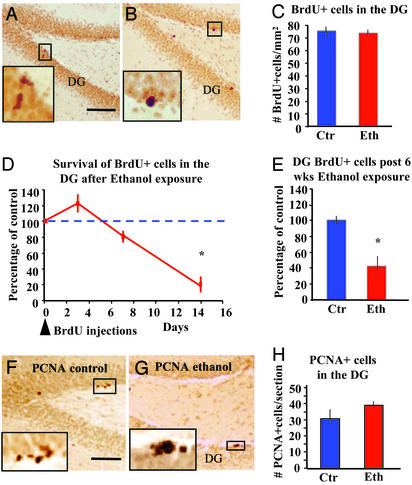
Proliferation or Survival? The ethanol-induced reductions in hippocampal neurogenesis can be attributed to two general mechanisms: an effect on cell proliferation or on cell survival. BrdUrd labeling was assessed in animals that received 10 days of ethanol (n = 4) or control diet (n = 4) and were perfused 1 h after the last of seven BrdUrd injections (one injection every 2 h, BrdUrd protocol 2; Fig. 3 A–C). The number of BrdUrd-labeled cells in both groups was nearly identical (98.6 ± 4% of control values: 74.1 ± 5 BrdUrd+ cells per mm2 in the ethanol group and 75.1 ± 6 BrdUrd+ cells per mm2 in the control group) (Fig. 3C). At increasing survival times (3, 7, and 14 days) after the seven BrdUrd injections, animals in the ethanol group showed a progressive reduction in the number of BrdUrd-positive cells in the DG. At the 7-day survival point, the decrease in the number of BrdUrd-positive cells was 16% and at 14 days was 70% as compared with respective controls (Fig. 3 D and E). These data suggest that most of the cell loss in the DG occurs within the first 2 weeks after these cells are born. Immunostaining for PCNA, a marker of cell division, allowed us to assess the rate of cell proliferation in the DG at the end of the 6-week experiments. There was no significant decrease in the number of labeled cells in ethanol-receiving rats compared with control animals (Fig. 3 F and H). These findings suggest that cell division may not be affected.
Fig. 3.
Ethanol feeding decreases the number of newly formed neurons in the DG. (A and B) Photomicrographs of BrdUrd immunostaining in the DG of control (A) or ethanol-fed (B) rats. (Bar = 100 μm.) BrdUrd was administered in seven injections at 2-h intervals, and rats were perfused 1 h after the last injection (10 days into the liquid diet treatment, BrdUrd protocol 2). Labeling is observed in the basal layers only (Insets). (C) Counting of BrdUrd-positive cells in the DG shows no impairing effect of ethanol on proliferation. (D) Rats received seven injections of BrdUrd within a 12-h period (BrdUrd protocol 2). Then, animals exposed to ethanol or control diet were allowed to survive for different time periods. Rats exposed to ethanol showed a decrease of up to 70% in the number of BrdUrd-labeled cells in the DG after 14 days. Most of the decrease seemed to occur between 7 and 14 days after the uptake of BrdUrd. (E) The number of BrdUrd-positive cells in ethanol-exposed rats at 2 weeks was similar to that observed after a 6-week exposure period, suggesting that most of the cells died within the first 14 days after BrdUrd uptake and before establishing mature contacts. (F and G) Photomicrographs of PCNA immunostaining in the DG of control (F) or ethanol-fed (G) rats. (Bar = 75 μm.) Labeling is observed in the basal layers only (Insets). (H) Counting of PCNA-positive cells in the DG shows again no impairing effect of ethanol on proliferation.
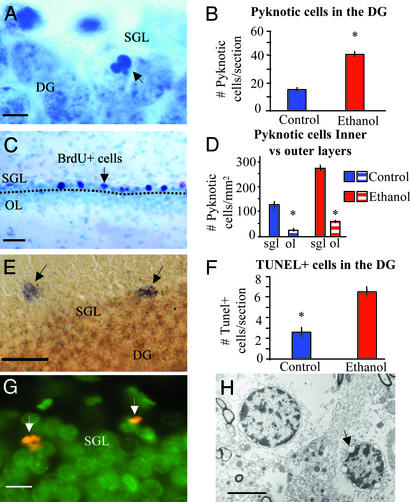
An alternate explanation for the loss of BrdUrd-labeled cells is that ethanol may hinder the survival of newly formed neurons. We used two techniques to quantify cell death within the DG and subventricular zone: TUNEL and counting of pyknotic nuclei in Nissl-stained sections (Fig. 4). No differences were noted in the subventricular zone of ethanol-fed rats as compared with controls. The number of pyknotic nuclei was 222.16 ± 41% higher in the DG of ethanol-fed rats than in controls (14.6 ± 1.3 pyknotic cells per section or 59.15 ± 3 pyknotic cells per mm2 in controls, n = 4, vs. 40.7 ± 2.9 pyknotic cells per section or 131.30 ± 5.5 pyknotic cells per mm2 in ethanol-fed rats, n = 4, P < 0.01, Mann–Whitney test; Fig. 4B). Similarly, the number of TUNEL-positive cells was 229.3 ± 40% higher (2.5 ± 0.77 TUNEL-positive cells per section in control, n = 4 vs. 6.5 ± 0.87 in ethanol-fed rats, P < 0.01, Mann–Whitney test, n = 4) in ethanol-receiving rats than in paired controls (Fig. 4 E and F). To determine whether pyknotic figures were evenly distributed throughout the DG or not, we divided the DG into its basal and outer layers. Newly born and less mature neurons are mostly located in the basal layers of the DG (Fig. 4C), and mature neurons tend to occupy the outer layers. We would expect to see an even distribution of pyknotic figures in the DG if the effect of ethanol were similar over immature or mature neurons; instead, we observed that ≈72% of pyknotic figures were located in the basal layers of the DG, which represent only 25–30% of the total area of the structure. These data indicate that there is nearly a 5-fold increase in the number of pyknotic cells in the basal layers as opposed to the outer layers of the DG, suggesting that less mature neurons are more susceptible to the effects of ethanol consumption (Fig. 4D). We examined BrdUrd uptake in the DG of ethanol-exposed rats and paired controls. These rats received BrdUrd injections in a single day (BrdUrd protocol 2, Fig. 1) and were killed between 1 h and 14 days later. Sections were counterstained with the green fluorescent marker Cyquant, allowing us to look for dense, shrunken, pyknotic figures with BrdUrd labeling. We could find pyknotic BrdUrd-labeled figures at 3- and 7-day survival times (Fig. 4G), but the low number of events (possibly due to, as we have shown, the decline in DG BrdUrd positive cells of ethanol-exposed animals appearing to occur at a steady pace for 10–14 days after cell division) did not allow for quantification. In addition, we examined the subventricular zone and the DG from brains of controls and ethanol-receiving rats at the ultrastructural level (Fig. 4H), a “gold-standard” method to assess apoptotic cell death (21). We observed the presence of apoptotic nuclei in the subgranular cell layer of the DG in ethanol-treated rats but not in the control rats examined (n = 5 per group).
Fig. 4.
Ethanol exposure increases cell death in the DG. (A) Photomicrograph of Nissl-stained coronal section of rat hippocampus. Arrow shows pyknotic nucleus in the basal granular layer where precursors are located (6-week survival). (B) Counting of pyknotic nuclei shows a marked increase in the DG of such figures in ethanol-fed rats (40.75 ± 2.9 pyknotic nuclei per DG section, n = 4) as compared with controls (14.67 ± 1.38, n = 4). P ≤ 0.01, Mann–Whitney test (6-week survival). (C) BrdUrd-labeled section of the DG counterstained with Nissl, 6-week survival. Dotted line indicates chosen division between basal layers (including subgranular cell layer) and the outer layers (OL) of the DG. Arrows point to BrdUrd-positive cells in the basal layers, where most of the newly born cells can be found at this survival point. The number of pyknotic cells per mm2 (D) is clearly higher in the basal layers of the DG than in the outer layers (ol). (E) TUNEL was used to assess cell death in the DG of ethanol vs. control-fed rats. (Bar = 25 μm.) Positive labeling was observed predominantly in the basal layers (6-week survival). (F) There was a marked increase in the number of TUNEL-positive cells per section in ethanol-fed rats (6.5 ± 0.87, n = 4) as compared with controls (2.5 ± 0.87, n = 4). P ≤ 0.01, Mann–Whitney test (6-week survival). (G) BrdUrd-labeled pyknotic cells in the DG. BrdUrd was administered 3 days before perfusion (BrdUrd in red, nuclear staining in green with Cyquant 1:2,000; BrdUrd protocol 2). (Bar = 10 μm.) (H) Apoptotic nuclei were observed in the basal layers of the DG of ethanol-fed rats at the ultrastructural level (6-week survival). The arrow points to the typical fragmentation of the nuclear membranes in advanced stages of apoptosis. Also note dense chromatin within the periphery of the nucleus. (Bar = 5 μm.)
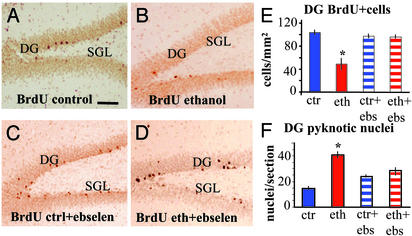
Effects of Ebselen on Neurogenesis. To examine the role of oxidative damage in the impaired neurogenesis observed here, we studied rats treated with the synthetic antioxidant ebselen. Four groups (n = 9 per group) were studied, and each received either liquid control or ethanol diet, with or without ebselen at a dose of 0.1 mg/ml. Animals received 10 daily BrdUrd injections at the beginning of liquid diets, which continued for 6 weeks (BrdUrd protocol 1, Fig. 1 A). Analysis of the number of newly formed cells and pyknotic nuclei in the DG revealed that ebselen had an important protective effect against alcohol-induced apoptosis of newly formed cells (Fig. 5). Animals that received ethanol without ebselen showed a significant decrease in the number of BrdUrd-labeled cells in the DG with respect to controls, whereas rats that received ethanol and ebselen showed no difference with rats that received either a control liquid diet alone or a control diet with ebselen. In addition, there was an increased number of pyknotic nuclei observed in the DG of ethanol-receiving rats, but no such change was observed in rats that received ethanol and the antioxidant. Ebselen, without ethanol, did not produce any significant change in the number of BrdUrd-positive cells or pyknotic nuclei in the DG (Fig. 5 E and F). The liquid diet consumption in the ethanol group was 81 ± 3.6 ml per day and 82.8 ± 2.4 ml per day in the ebselen plus ethanol group (not significant), indicating that there was no effect of ebselen on the ingestion of ethanol.
Fig. 5.
The antioxidant ebselen prevents ethanol effects on neurogenesis in the adult DG. (A–D) Photomicrographs of BrdUrd immunolabeling in coronal sections of the DG 6-week survival (BrdUrd protocol 1). (Bar = 100 μm). (A) Control rat. (B) Ethanol-fed rat. (C) Ebselen + control feedings. (D) Ebselen + ethanol feeding. (E) Ebselen prevented the deleterious effects of ethanol on newly born DG cells (ANOVA and Dunnett's post hoc analysis, P ≤ 0.01, n per group = 9) preventing cell death (F) (ANOVA and Dunnett's post hoc analysis, P ≤ 0.05, n per group = 4).
Discussion
Neurogenesis Is Impaired in a Model of Chronic Alcoholism. A long-standing view that ethanol produces effects on cognition and behavior by a generalized depression of CNS activity is being reexamined (2, 8). Selective effects of alcohol on learning and memory, on the processing and use of spatial information to organize and guide behavior, and on capacities of acquisition of context conditioning (a hippocampal-dependent associative task) all stem from altered cellular activity in the hippocampus and related structures (8). This impairment seems to be reversible as evidenced by a recent neuropsychological study of former alcoholics after periods of abstinence (22). Furthermore, a recent study reported an effect of binge ethanol on hippocampal neurogenesis (23).
In the rat hippocampus, several thousand neurons are produced each day, many of which die within weeks (10, 24). Associative learning can enhance their survival, and, inversely, a substantial reduction in the number of newly generated neurons in the adult rat is correlated with impairment in hippocampal-dependent trace conditioning, a task in which an animal must associate stimuli that are separated in time (11). A similar reduction in neurogenesis does not affect learning when the same stimuli are not separated in time, a task that is independent of hippocampal integrity. The results of our studies suggest that the marked effect of ethanol on the survival of newly formed neurons in the adult hippocampus could result in impairment of hippocampal-dependent cognitive functions, or, alternatively, the changes in cognition observed in alcoholism could lead to decreased neuronal survival. This would also explain the lack of consistent results observed in pathological studies of the hippocampi in alcoholics, as this is a potentially reversible effect. Moreover, previous studies have shown that cognitive deficits occur in animal models of chronic alcoholism (14, 15).
Proliferation or Survival? The decrease in the number of newly born neurons in the DG of rats exposed to alcohol could respond to impairment in the proliferation of hippocampal stem cells, diminished survival, or both. Multiple regimes of BrdUrd administration have been reported with a wide range of dosing. There have been proponents of both low doses (25) to avoid BrdUrd-induced damage or higher doses to maximize visualization of dividing cells (26). However, BrdUrd has the potential to sensitize cells against free radical-induced damage, which is a possible mechanism through which ethanol may affect neurogenesis. Therefore, we selected a BrdUrd regime within the range in the literature that would allow us to reliably detect cells that underwent division without inducing damage (24).
To establish the time period in which newly born cells within the DG are more sensitive to ethanol, we administered seven BrdUrd injections in a 12-h period at day 10 after starting the liquid diet and allowed animals to survive for different durations. We found that, compared with control rats, animals exposed to ethanol showed the same number of BrdUrd-positive cells in the DG at 1 h after the last injection. However, after 2 weeks the number of BrdUrd-positive cells decreased to the levels observed after 6 weeks of exposure to ethanol. In addition, the DG of animals exposed to ethanol showed a marked increase in cell death, mainly in the more basal layers of the DG, where more recently born neurons are located. Quantification by Nissl staining and TUNEL yielded similar results. These facts suggest that ethanol exposure affected survival preferentially within the first 2 weeks and that proliferation was not impaired.
In a rodent model of fetal alcohol syndrome (27), it was recently shown that immature neurons seem to be highly susceptible to apoptotic cell death upon exposure to ethanol, especially if it occurred during their synaptogenesis. Cortical neurons seemed to be insensitive to the effects of ethanol after maturation but were most susceptible during the period in which they were connecting and sending processes (27). Similarly, adult DG granular cells were most affected by ethanol exposure in the first 2 weeks after mitosis and possibly before they had completed maturation.
There are several possible mechanisms that may mediate ethanol effects on neurogenesis. Pharmacologically, ethanol is both an N-methyl-D-aspartate receptor antagonist and a γ-aminobutyric acid type A agonist (27). In cerebellar granule cell cultures, ethanol leads to apoptotic death through N-methyl-D-aspartate receptor antagonism, possibly preventing the synthesis of brain-derived neurotrophic factor by the targeted cells (28). Therefore, ethanol could mediate its effects on neurogenesis through N-methyl-D-aspartate antagonism, γ-aminobutyric acid type A agonism, or effects on endogenous trophic factors (29). High levels of corticotropin (ACTH)/corticosterone are associated with impairments in neurogenesis (30). However, alcohol-fed animals showed no significant rise in plasma ACTH/ corticosterone levels (31) but did show blunted activity of the hypothalamic–pituitary–adrenal axis (32). All of these mechanisms result in the production of oxidative stress, and this shared mechanism has led us to study the possibility of preventing the effects of ethanol on this level.
Effects of Ebselen on Neurogenesis. The pharmacological mechanisms that mediate ethanol effects may result in the production of oxidative stress (17). It has been suggested that ethanol may cause tissue damage through lipid peroxidation (33, 34). Ethanol can enhance reactive oxygen species formation through induction of cytochrome P-4502E1 (CYP2E1), which is widely distributed in the brain (34). The reactive oxygen species formed from these systems are a source of oxidative stress in the brain (35). A correlation between the induction of CYP2E1 and increase in reactive oxygen species formation was shown in the brains of chronic alcohol-fed rats (34, 36). Chronic ethanol administration has been shown to decrease antioxidant enzymes, such as glutathione peroxidase, and alter glutathione homeostasis (18, 19).
To examine the role of oxidative damage in the impaired neurogenesis observed here, we studied rats treated with the organo-selenium compound antioxidant ebselen (37, 38). Ebselen has been studied as a potential agent to counteract some of the deleterious effects of ethanol. Studies demonstrate that ebselen protects the liver (39), gastric mucosa (37), CNS (40), and peripheral nerves from chronic alcohol exposure (41) by decreasing oxidative stress through inhibition of lipid peroxidation. Here, administration of ebselen results in a decrease in ethanol-induced cell death in the DG and particularly in protection of newly born neurons. Mechanistically, it has been recently demonstrated that ebselen has an efficient peroxynitrite quenching capacity (42). Preliminary results of our laboratories confirm the reduction of glutathione peroxidase activity in brain of ethanol-treated animals when compared with controls (data not shown). Ebselen could act via two different mechanisms, on one hand blocking the generation of peroxynitrite, and on the other, compensating for the loss of glutathione peroxidase activity in ethanol-treated brains. Finally, there was no effect of ebselen on the ingestion of ethanol (see Results) or on its metabolism (39).
Conclusions
Survival of newly formed neurons in the DG, but not in the OB, is impaired by ethanol. DG cells seemed to be more susceptible to ethanol effects between 1 and 14 days after their birth, suggesting that early survival more than proliferation is affected. These changes in the hippocampal structure could be part of the anatomical basis for the cognitive deficits described in alcoholism. Understanding the mechanisms that underlie alcohol-induced changes in the brain and particularly in the hippocampus may soon lead to therapeutic interventions.
Finally, the adult neurogenesis deficit was prevented by ebselen. This or similar compounds could be used in the treatment of cognitive impairment seen in alcoholic patients and possibly in other disorders where adult neurogenesis may be affected.
Acknowledgments
We thank the superb technical assistance of A. Rankin, M. Soriano, J. Martinez-Jimenez, and B. Haripal. We are grateful to Drs. S. Anderson, G. DeErausquin, D. A. Lim, and B. Seri for useful comments after reading this manuscript. D.G.H. was supported by grants from the Alcoholic Beverage Medical Research Foundation, Weill Cornell Center for Aging Research and Clinical Care, Janssen Pharmaceutica Faculty Development Award, and Reader's Digest; F.J.R. was supported by Ministry of Science and Technology Grant PM99-0177 (Madrid) and the Directorate General on Drug Abuse of the “Generalitat Valenciana.” F.B.-M. was supported by Grant UCH02/22 from the Fundación Universitaria San Pablo–Centre de Estudios Universitarios. F.J.R. and F.B.-M. also received support from the “Fundación Oftalmológica del Mediterráneo.” S.J.-S. and M.M. are research fellows of the Universidad Cardenal Herrera-Centro de Estudios Universitarios.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DG, dentate gyrus; OB, olfactory bulb; PCNA, proliferating cell nuclear antigen; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling method.
References
- 1.American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders (Am. Psychiatr. Assoc., Washington, DC), 4th Ed.
- 2.Harper, C. (1998) J. Neuropathol. Exp. Neurol. 57, 101-110. [DOI] [PubMed] [Google Scholar]
- 3.Albert, M. S., Butters, N. & Brandt, J. (1980) J. Stud. Alcohol 41, 1071-1081. [DOI] [PubMed] [Google Scholar]
- 4.Korbo, L. (1999) Alcohol Clin. Exp. Res. 23, 164-168. [PubMed] [Google Scholar]
- 5.Walker, D. W., Barnes, D. E., Zornetzer, S. F., Hunter, B. E. & Kubanis, P. (1980) Science 209, 711-713. [DOI] [PubMed] [Google Scholar]
- 6.Harding, A. J., Wong, A., Svoboda, M., Kril, J. J. & Halliday, G. M. (1997) Hippocampus 7, 78-87. [DOI] [PubMed] [Google Scholar]
- 7.Bengoechea, O. & Gonzalo, L. M. (1991) Neurosci. Lett. 123, 112-114. [DOI] [PubMed] [Google Scholar]
- 8.White, A. M., Matthews, D. B. & Best, P. J. (2000) Hippocampus 10, 88-93. [DOI] [PubMed] [Google Scholar]
- 9.Palmer, T. D., Takahashi, J. & Gage, F. H. (1997) Mol. Cell. Neurosci. 8, 389-404. [DOI] [PubMed] [Google Scholar]
- 10.Gage, F. H. (2000) Science 287, 1433-1438. [DOI] [PubMed] [Google Scholar]
- 11.Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T. & Gould, E. (2001) Nature 410, 372-376. [DOI] [PubMed] [Google Scholar]
- 12.Feng, R., Rampon, C., Tang, Y. P., Shrom, D., Jin, J., Kyin, M., Sopher, B., Miller, M. W., Ware, C. B., Martin, G. M., et al. (2001) Neuron 32, 911-926. [DOI] [PubMed] [Google Scholar]
- 13.Roberto, M., Nelson, T. E., Ur, C. L. & Gruol, D. L. (2002) J. Neurophysiol. 87, 2385-2397. [DOI] [PubMed] [Google Scholar]
- 14.Walker, D. W. & Freund, G. (1971) Physiol. Behav. 7, 773-778. [DOI] [PubMed] [Google Scholar]
- 15.Santin, L. J., Rubio, S., Begega, A. & Arias, J. L. (2000) Alcohol 20, 149-159. [DOI] [PubMed] [Google Scholar]
- 16.Lieber, C. S., DeCarli, L. M. & Sorrell, M. F. (1989) Hepatology 10, 501-510. [DOI] [PubMed] [Google Scholar]
- 17.Sun, A. Y., Ingelman-Sundberg, M., Neve, E., Matsumoto, H., Nishitani, Y., Minowa, Y., Fukui, Y., Bailey, S. M., Patel, V. B., Cunningham, C. C., et al. (2001) Alcohol Clin. Exp. Res. 25, 237S-243S. [DOI] [PubMed] [Google Scholar]
- 18.Bailey, S. M., Patel, V. B., Young, T. A., Asayama, K. & Cunningham, C. C. (2001) Alcohol Clin. Exp. Res. 25, 726-733. [PubMed] [Google Scholar]
- 19.Rouach, H., Fataccioli, V., Gentil, M., French, S. W., Morimoto, M. & Nordmann, R. (1997) Hepatology 25, 351-355. [DOI] [PubMed] [Google Scholar]
- 20.Herrera, D. G., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. (1999) Ann. Neurol. 46, 867-877. [DOI] [PubMed] [Google Scholar]
- 21.Ishimaru, M. J., Ikonomidou, C., Tenkova, T. I., Der, T. C., Dikranian, K., Sesma, M. A. & Olney, J. W. (1999) J. Comp. Neurol. 408, 461-476. [PubMed] [Google Scholar]
- 22.Sullivan, E. V., Rosenbloom, M. J., Lim, K. O. & Pfefferbaum, A. (2000) Neuropsychology 14, 178-188. [PubMed] [Google Scholar]
- 23.Nixon, K. & Crews, F. T. (2002) J. Neurochem. 83, 1087-1093. [DOI] [PubMed] [Google Scholar]
- 24.Cameron, H. A. & McKay, R. D. (2001) J. Comp. Neurol. 435, 406-417. [DOI] [PubMed] [Google Scholar]
- 25.Rakic, P. (2002) J. Neurosci. 22, 614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gould, E. & Gross, C. G. (2002) J. Neurosci. 22, 619-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikonomidou, C., Bittigau, P., Ishimaru, M. J., Wozniak, D. F., Koch, C., Genz, K., Price, M. T., Stefovska, V., Horster, F., Tenkova, T., et al. (2000) Science 287, 1056-1060. [DOI] [PubMed] [Google Scholar]
- 28.Bhave, S. V., Ghoda, L. & Hoffman, P. L. (1999) J. Neurosci. 19, 3277-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tapia-Arancibia, L., Rage, F., Givalois, L., Dingeon, P., Arancibia, S. & Beauge, F. (2001) J. Neurosci. Res. 63, 200-208. [DOI] [PubMed] [Google Scholar]
- 30.McEwen, B. S. (1999) Annu. Rev. Neurosci. 22, 105-122. [DOI] [PubMed] [Google Scholar]
- 31.Ogilvie, K., Lee, S. & Rivier, C. (1997) Alcohol Clin. Exp. Res. 21, 467-476. [DOI] [PubMed] [Google Scholar]
- 32.Ogilvie, K., Lee, S., Weiss, B. & Rivier, C. (1998) Alcohol Clin. Exp. Res. 22, 243S-247S. [DOI] [PubMed] [Google Scholar]
- 33.Mi, L. J., Mak, K. M. & Lieber, C. S. (2000) Alcohol Clin. Exp. Res. 24, 207-212. [PubMed] [Google Scholar]
- 34.Montoliu, C., Sancho-Tello, M., Azorin, I., Burgal, M., Valles, S., Renau-Piqueras, J. & Guerri, C. (1995) J. Neurochem. 65, 2561-2570. [DOI] [PubMed] [Google Scholar]
- 35.Yang, M. X. & Cederbaum, A. I. (1997) Arch. Biochem. Biophys. 341, 25-33. [DOI] [PubMed] [Google Scholar]
- 36.Montoliu, C., Valles, S., Renau-Piqueras, J. & Guerri, C. (1994) J. Neurochem. 63, 1855-1862. [DOI] [PubMed] [Google Scholar]
- 37.Tabuchi, Y., Sugiyama, N., Horiuchi, T., Furusawa, M. & Furuhama, K. (1995) Eur. J. Pharmacol. 272, 195-201. [DOI] [PubMed] [Google Scholar]
- 38.Imai, H., Masayasu, H., Dewar, D., Graham, D. I. & Macrae, I. M. (2001) Stroke 32, 2149-2154. [DOI] [PubMed] [Google Scholar]
- 39.Kono, H., Arteel, G. E., Rusyn, I., Sies, H. & Thurman, R. G. (2001) Free Radical Biol. Med. 30, 403-411. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi, T., Sano, K., Takakura, K., Saito, I., Shinohara, Y., Asano, T. & Yasuhara, H. (1998) Stroke 29, 12-17. [DOI] [PubMed] [Google Scholar]
- 41.Bosch-Morell, F., Martinez-Soriano, F., Colell, A., Fernandez-Checa, J. C. & Romero, F. J. (1998) Free Radical Biol. Med. 25, 365-368. [DOI] [PubMed] [Google Scholar]
- 42.Roussyn, I., Briviba, K., Masumoto, H. & Sies, H. (1996) Arch. Biochem. Biophys. 330, 216-218. [DOI] [PubMed] [Google Scholar]