Abstract
New neurons are generated from stem cells in a few regions of the adult mammalian brain. Here we provide evidence for the generation of dopaminergic projection neurons of the type that are lost in Parkinson's disease from stem cells in the adult rodent brain and show that the rate of neurogenesis is increased after a lesion. The number of new neurons generated under physiological conditions in substantia nigra pars compacta was found to be several orders of magnitude smaller than in the granular cell layer of the dentate gyrus of the hippocampus. However, if the rate of neuronal turnover is constant, the entire population of dopaminergic neurons in substantia nigra could be replaced during the lifespan of a mouse. These data indicate that neurogenesis in the adult brain is more widespread than previously thought and may have implications for our understanding of the pathogenesis and treatment of neurodegenerative disorders such as Parkinson's disease.
The majority of neurons are born before or around birth. The first indications of neurogenesis in the adult mammalian brain were presented four decades ago, but it is only during the last years that it has been firmly established that new neurons are generated continuously from stem cells in certain regions of the adult brain in all studied mammals, including man (1–3). The most active neurogenic regions are the dentate gyrus (DG) of the hippocampus and the olfactory bulb. It has been estimated that ≈10,000 new neurons are added each day to the adult rat DG (4), and the rate of neurogenesis in the olfactory bulb is likely to be severalfold higher. In retrospect, it is quite remarkable that such pronounced processes went unnoticed for so long time, and it raises the question whether there may be a low level of neurogenesis in other brain regions, which has not yet been detected.
In addition to the neurogenesis in the olfactory bulb and DG, low numbers of new neurons have been suggested to be generated in other parts of the hippocampus as well as in the cortex (5–6), although the latter remains controversial (7). Moreover, neurogenesis has been demonstrated in several additional regions in response to injury (8–11).
We asked whether new neurons are generated also in the substantia nigra pars compacta (SNpc) of the midbrain, the region where dopamine-producing neurons lost in Parkinson's disease reside. We here report evidence for a slow turnover of dopaminergic projection neurons in the adult rodent brain, and that neurogenesis is increased after a partial injury.
Materials and Methods
In Vivo Labeling Techniques. Adult male C57 Bl/6 mice (2–20 months old, B & K Universal, Sollentuna, Sweden) were used, and kept 3–6 per cage. The thymidine analogue BrdUrd (Sigma) was given as single or repeated (every second hour) i.p. injections (100 mg/kg in 0.9% saline) or through drinking water (1 mg/ml for 2–6 weeks) or by continuous infusion into the right lateral ventricle using osmotic pumps (0.9–1.2 mg per day; Alzet pump model/delivery time/flow per h/BrdUrd in 0.9% saline concentration: 1003D/2 days/1 μl/50 mg/ml; 1002/10 days/0.25 μl/150 mg/ml; 2004/21 days/0.25 μl/150 mg/ml). [3H]Thymidine (4 mCi/kg, 6.7 Ci/mmol, NEN; 1 Ci = 37 GBq) was delivered over 3 days via osmotic pumps (i.p., Alzet 1003D, flow rate 1 μl/h), and animals were analyzed 6 weeks later.
Cells lining the ventricular system were labeled by a single intracerebroventricular (i.c.v.) stereotaxic injection (right lateral ventricle) of 2.5 μl of 0.2% wt/vol 1,1′-dioctadecyl-6,6′-di-(4-sulfophenyl)-3,3,3′,3′-tetramethylindocarbocyanine (DiI, Molecular Probes) in dimethyl sulfoxide or rhodamine-conjugated latex beads (3 μl, Lumafluor Inc.) diluted 1:50–100 in PBS. DiI-labeled cells were counted in the contralateral SNpc. One group of DiI-injected mice (n = 4) received cytosine-D-arabinofuranoside (Ara-Cyt, 4%, Sigma) in 0.9% saline infused on the brain surface for 14 days with an osmotic pump (Alzet 1002, flow rate 0.25 μl/h) (12).
Fluorogold [0.3 μl of 5% (wt/vol) in 0.9% saline, Fluorochrome] was injected in the left striatum (0.5 mm posterior and 2.0 mm lateral to Bregma, 2.4 mm below the dura mater) 48–72 h before death. Other animals were injected stereotaxically with 7.5·104 particles of the enhanced GFP expressing pseudorabies virus (eGFP PRV) GS518 (13) and Alexa594-conjugated cholera toxin B (CTB, Molecular Probes, one small crystal dissolved in 100 μl of virus solution, i.e., in a concentration exceeding by far the diffusion of the virus) into the right somatosensory cortex 72 h before death (14).
Tissue Preparation. Anesthetized mice were transcardially perfused with 4% paraformaldehyde in 0.1 M PBS (adding 0.25–0.5% glutaraldehyde for ultrastructural analysis) and serial coronal sections through the midbrain were cut on a cryostat (5–40 μm) or vibratome (100 μm) (15–17). Sections used for autoradiography were dipped in NTB2 emulsion (Kodak) and exposed for 4 weeks in the dark at +4°C, developed in D-19 (Kodak), fixed, and counterstained with cresyl violet. Brains used for analysis of apoptosis were dissected and frozen in -70°C isopentane, and serial cryosections were fixed in 10% Formalin (Sigma). Ultrathin diamond-knife cut sections of Durcupan-embedded (Fluka) specimens were analyzed in a Philips CM12 electron microscope (EM).
Cell Cultures. Tissue dissociation and culture conditions were as described (16) except that both epidermal growth factor and fibroblast growth factor-2 were used at 20 ng/ml. Differentiation of the neurospheres was induced by plating on fibronectin/ poly(L)-ornithin-coated slides. After 1 week, neurons and glia were detected in the cultures by using primary antibodies against βIII-tubulin (Tuj-1, mouse monoclonal, BAbCO) and glial fibrillary acidic protein (GFAP, rabbit polyclonal, DAKO).
Labeling Procedures for Tissue Sections. Primary antibodies against the following antigens were used on tissue sections for immunohistochemistry: tyrosine hydroxylase (TH, rabbit polyclonal, Pel-Freeze; mouse monoclonal, Incstar), BrdUrd (mouse monoclonal, DAKO and Boehringer Mannheim; rat monoclonal, Accurate), Hu (mouse monoclonal, clone 16A11, Monoclonal Antibody Facility, University of Oregon, Eugene), NeuN (mouse monoclonal, Chemicon), nestin (rabbit polyclonal, gift from R. D. McKay, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda), CRMP-4 (rabbit polyclonal, gift from S. Hockfield, ref. 18), green fluorescent protein (chicken, Chemicon) and fluorogold (rabbit polyclonal, Fluorochrome). Before incubation with BrdUrd antibodies, denaturation of DNA was performed by using 50% formamide and HCl or HCl and 0.25% pepsin. To study apoptosis in the midbrain, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) was used (19) with reagents from Intergen. The secondary antibodies used were linked with the following markers: fluorescein isothiocyanate, rhodamine, Cy-3, Cy-5, 7-amino-4-methylcoumarine-3-acetic acid or biotin, the latter used as substrate for the avidin-biotin peroxidase reaction (Vector) with 0.03% 3.3′-diaminobenzidine and 0.003% H2O2 as chromogens. Some sections were in addition stained with the nuclear marker 4′,6-diamidino-2-phenylindole (Sigma) or the cellular stain cresyl violet (Sigma), the latter labeling Nissl substance in neurons. Photoconversion of DiI was performed as described elsewhere (16).
Cell Counts. Bilateral cell counts were estimated by using unbiased, stereological techniques in uniform, systematic random samples of sections throughout the SNpc and DG at high magnification (15, 17). The chosen sampling fraction for optical fractionator counts (Cast-Grid, Olympus, Albertslund, Denmark) was kept low in the nigral TH+ cell counts (1–2%, yielding a coefficient of error <0.08) whereas nigral counts of DiI+, BrdUrd+ or TUNEL+ cells were performed in every sampled serial section with a sampling fraction of 50% to yield high precision, though cell numbers were low (20). Double-labeling was confirmed with a confocal laser scanning microscope (Bio-Radiance Plus, Zeiss, or Leica).
1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) Lesion. A single dose of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP·HCl, Sigma) was administered s.c. as described (17) to 10-week-old mice (40 mg·kg-1) 10 days after DiI i.c.v. or immediately after BrdUrd administration.
Results
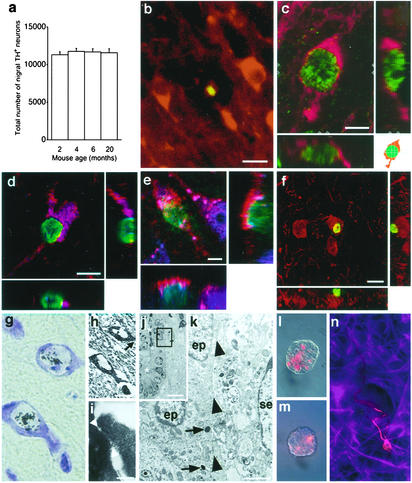
Constant Total Number Despite Apoptotic Death of Dopaminergic Neurons. We first analyzed the total number of neurons in the SNpc containing the rate-limiting enzyme for dopamine synthesis, TH, during a large part of the life span of a mouse. Unbiased, stereological cell counting did not reveal any statistically significant difference in the total number of nigral TH+ neurons in mice between 2 and 20 months of age (Fig. 1a). However, we found shrunken TH+ neurons with condensed, TUNEL+ nuclei (Fig. 1b). The number of TUNEL-labeled apoptotic neurons in the bilateral substantia nigra of 12-week-old mice was 17 ± 3 (mean ± SEM, n = 5). The finding that dopaminergic neurons are dying, whereas total nigral neuronal numbers remain constant over a large part of the life span of an adult mouse, suggested that new neurons might be added.
Fig. 1.
Turnover of dopaminergic neurons in the substantia nigra. The total number of TH+ substantia nigra pars compacta neurons (bilaterally, identified with immunoperoxidase and cresyl violet counterstain) did not change with increasing age (mean ± SEM) (a), although the same region contained apoptotic TUNEL+ condensed nuclei (green) in TH+ (red) neurons dispersed among TH+ neuropil and larger, nonapoptotic TH+ perikarya (b). Confocal three-dimensional image of a newborn (BrdUrd+, green nucleus) TH+ (red) neuron in the substantia nigra of a 16-week-old mouse after 2 weeks of BrdUrd through drinking water followed by 4 weeks without BrdUrd (c). Two cross sections analyzing the depth of the image (z axis) in c along the y and x axis are shown in the schematic drawing (Lower Right). TH+ (red), BrdUrd+ (green) cells express the neuronal markers (violet-blue) Hu (d) and NeuN (e). In f, a BrdUrd+ (green) cell is shown adjacent to a TH+ (red) cell, which could be mistaken for a newborn neuron if not studied in three dimensions. Newborn [3H]thymidine labeled cresyl violet stained neurons in the medial substantia nigra (g) of a 16-week-old mouse, 6 weeks after a 3-day i.p. infusion of [3H]thymidine. (h) EM image of the same neurons as in g with an enlargement showing a synapse in i, with the position indicated by an arrow in h. The synaptic cleft is shown between two arrowheads (i). Semithin section of the midbrain aqueduct (the lumen indicated by asterisks) after photoconversion of DiI, 1 day after intraventricular injection (j). The box in j delineates the area shown in an EM micrograph obtained from the adjacent ultrathin section (k). Dense staining is visible in the lumen (j) and lysosomes (k, indicated by arrows) in ependymal cells (ep). se, a subependymal cell nucleus; arrowheads, ependymal membranes. Note the absence of labeling of membranes and in cytoplasm of subependymal cells (k). Neurospheres from the midbrain aqueduct and third ventricular recess after in vivo labeling of the ependymal layer with DiI (l) or rhodamine-conjugated latex beads (n) contained label suggesting ependymal origin. Such neurospheres were passaged and induced to differentiate into neurons (Tuj-1+, red) and astrocytes (GFAP+, violet-blue) (m). [Scale bars = 10 μm (b, e, f, and h), 5 μm (c and d), 0.1 μm (i), 10 μm (j), 1 μm (k).]
Neurogenesis in the Substantia Nigra. To test whether neurons are generated in the adult substantia nigra, BrdUrd was delivered as i.p. injections (either as a single or repeated injections for 2 days), by i.c.v. infusion into the lateral ventricle or via drinking water. A few scattered TH+ neurons were found to have BrdUrd+ nuclei when analyzed three-dimensionally in the confocal laser scanning microscope (Fig. 1c) and were most frequent 21 days after initiation of BrdUrd i.c.v. infusion (Table 1). In contrast, no BrdUrd+ nuclei were found in nigral neurons in animals analyzed immediately after i.p. or after 2 days i.c.v. administration, arguing against DNA repair-associated incorporation. In agreement with earlier studies reporting an increased neuronal labeling when [3H]thymidine was given directly into the cerebroventricular system (21), i.c.v. administration over 2 days resulted in labeling of a 5-fold higher number of BrdUrd+ nerve cells in the DG and the SNpc compared with repeated i.p. injections (Table 1). Most BrdUrd+ neurons were found in the medial-rostral part of the substantia nigra, where the neuronal density is highest, whereas they were never observed in the most caudal SNpc. BrdUrd+ nerve cells were individually dispersed, rarely occurred in clusters, and never displayed an apoptotic, condensed morphology, nor were found to be TUNEL+. As expected from the low number of newly generated cells detected after prolonged administration of BrdUrd, a single BrdUrd injection rendered only one labeled TH+ neuron 5 weeks later in five analyzed mice. The neuronal phenotype of TH+, BrdUrd+ cells was confirmed by expression of the neuronal markers Hu (Fig. 1d) and NeuN (Fig. 1e). Similar to what has been described in the cerebral cortex (7), cells residing adjacent to TH+ neurons were frequently found to be BrdUrd+ (Fig. 1f), and therefore three-dimensional confocal analysis was required for establishing colocalization of the markers. The neuronal identity of the newborn cells in substantia nigra was further supported by EM analysis, which revealed synaptic contacts on the soma of [3H]thymidine-labeled cells containing Nissl substance (Fig. 1 g–i).
Table 1. BrdUrd labeled neurons in hippocampus and substantia nigra.
| BrdUrd 2 days, analyzed after 3 weeks
|
Continuous i.c.v. BrdUrd
|
||||
|---|---|---|---|---|---|
| i.p. injection | Continuous i.c.v. | Day 2 | Day 10 | Day 21 | |
| Hippocampus dentate gyrus | |||||
| BrdUrd+ granule neurons | 675±49 | 3,840±260 | 3,700±110 | 6,090±580 | 10,300±450 |
| Substantia nigra pars compacta | |||||
| BrdUrd+/TH+ neurons | 0.8±0.5 | 5.3±1.3 | 0±0 | 0.2±0.2 | 22±2 |
| BrdUrd+/TH- cells | 236±112 | 351±84 | 114±34 | 2,510±340 | 2,110±320 |
See Materials and Methods for a detailed description. More than 90% of BrdUrd+ cells in the granule cell layer were Tuj-1+ (4). Double labeling of BrdUrd and TH was verified confocally for each cell. n = 3-5 in each group, except i.c.v. BrdUrd day 21, where n = 2
Neural Stem Cells in the Midbrain of the Adult Mouse. Neural stem cells lining the ventricular system of the adult rodent give rise to olfactory bulb interneurons and have been found to reside both in the ependymal and subependymal layer of the lateral ventricle wall (12, 16). To test whether cells lining the ventricular extension in the midbrain have stem cell properties, we established cultures from the third ventricular recess and the cerebral aqueduct after an i.c.v. injection of DiI (16) or rhodamine-conjugated latex beads (12) in 12-week-old mice (n = 6). That the DiI uptake was restricted to the ependymal layer was confirmed by EM after photoconversion of DiI in the injected specimens (n = 2) (Fig. 1 j and k). Labeled cells proliferated in vitro to form clonal aggregates, i.e., neurospheres (Fig. 1 l and m). When passaged, single cells generated new neurospheres, which when induced to differentiate, generated both neurons and glia, indicating that they were neural stem cells (Fig. 1n). However, the cells did not spontaneously acquire a dopaminergic phenotype, which indicates the presence of signals for phenotypic differentiation in vivo.
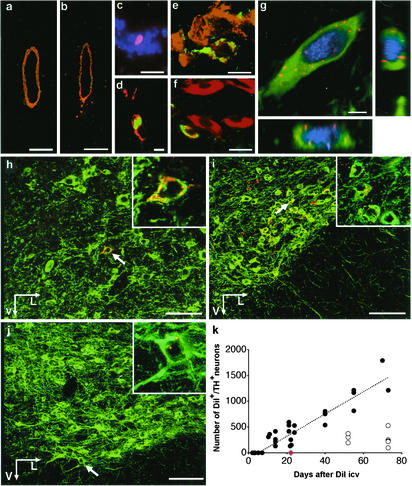
Origin of Adult-Born Neurons in the Substantia Nigra. We next asked whether neural stem cells in the midbrain give rise to the observed new neurons in the adult substantia nigra and labeled cells lining the ventricular system by an i.c.v. injection of DiI. Shortly after the injection, the label was confined to the ependymal layer (Figs. 1 j and k and 2a). A few days later, DiI-labeled cells were also seen outside the ependymal layer (Fig. 2b). Proliferation of cells lining the aqueduct was evident in animals receiving BrdUrd (Fig. 2c) and DiI-labeled BrdUrd+ cells with a morphology associated with migratory cells were seen along the ventral midline (Fig. 2d). A few DiI-labeled cells outside the cerebroventricular system were CRMP-4+ (Fig. 2e), and some nerve cells in substantia nigra were both nestin+ and TH+ (Fig. 2f). Starting about 10 days after a DiI injection, DiI-labeled TH+ cells were seen in SNpc (Fig. 2 g–i), suggesting that nigral dopaminergic neurons generated in the adult mouse derive from stem cells lining the ventricular system. The neuronal identity of the DiI-labeled TH+ cells was supported by NeuN labeling (Fig. 2g). Similar results were obtained after i.c.v. injection of rhodamine-conjugated latex beads (Fig. 2j).
Fig. 2.
Newborn substantia nigra neurons derive from cells lining the ventricular system. Six hours after an injection of DiI into the lateral ventricle, the label was restricted to the ependymal layer of the midbrain aqueduct (a). Four days later, animals receiving BrdUrd through drinking water, displayed DiI+ cells (red, b and d) with BrdUrd+ nuclei (green in d). BrdUrd incorporation (red) after six i.p. injections of the nucleotide analogue 12 h before analysis was found along the surface of the aqueduct (c). Cell nuclei are visualized with 4′,6-diamidino-2-phenylindole (violet-blue). Seven days after DiI (e), a DiI-labeled cell (red) was observed to colabel with the immature neuronal marker CRMP-4 (green). A TH+ cell (red) showing the immature cell marker nestin (green) in the cytoplasm is shown in f.In g (see Movie 1, which is published as supporting information on the PNAS web site, www.pnas.org), aTH+ (green), DiI+ cell (red) in the substantia nigra expressing the neuronal marker NeuN (violet-blue) is shown. TH+ (green) neurons containing punctate DiI (red) are shown in the substantia nigra pars compacta 14 days (h) and 55 days (i) after a DiI injection into the contralateral ventricle. Arrows point at cells shown at higher magnification. Note that DiI is also present in punctate clusters outside neurons, putatively labeling precursor cells or glia in the substantia nigra. A TH+ (green) neuron containing rhodamine-conjugated latex beads (red) is shown 6 weeks after an i.c.v. bead injection in the contralateral ventricle (j). In k, the total number of DiI+/TH+ neurons in the adult mouse substantia nigra pars compacta are shown at different time intervals after DiI injection [each black dot corresponds to one animal, n = 29, r2 (correlation coefficient) = 0.87, bilateral results are based on cell counts in SNpc contralateral to injection]. The number of DiI+/TH+ neurons was reduced in animals receiving BrdUrd in the drinking water for the first 42 days after DiI (open circles, n = 7). Animals that were infused with the anti-mitotic agent Ara-Cyt (n = 4, indicated by red diamond), completely lacked DiI-labeled TH+ neurons. Images in d–j were collected in the confocal laser scanning microscope. V, ventrally; L, laterally. [Scale bars = 100 μm(a and b), 20 μm (c), 5 μm (d and e), 10 μm (f), 50 μm (h–j).]
The number of DiI-labeled TH+ neurons in the contralateral substantia nigra increased in a linear fashion over time (Fig. 2k, filled circles). We did not see any nigral DiI-label 7 days after an i.c.v. DiI injection, arguing against the possibility that the label was unspecific or that neurons had been labeled retrogradely by transport from the injection site, which is a fast process. Importantly, double-labeled neurons were observed in the rostral SNpc, but never in the most caudal part, where sparse contralateral nigrostriatal projections have been observed (22). Dense punctate DiI label was also found in nonneuronal nigral cells (red clusters in Fig. 2 h and i).
To further test the specificity of DiI, four mice received intracranial infusion of the anti-mitotic agent Ara-Cyt, starting at the time of the i.c.v. injection. This completely abolished the emergence of DiI-labeled TH+ cells in the contralateral substantia nigra 3 weeks later, showing that the process depends on cell proliferation (Fig. 2k, diamond). Ara-Cyt did not affect DiI label in serotonergic neurons projecting to the cerebroventricular surface (data not shown), arguing against the involvement of inhibition of retrograde axonal transport. Based on the steady increase of DiI-labeled TH+ cells, we estimated that 20 new neurons were added each day (≈0.17% of the total nigral population, compare Fig. 1a). This estimate is substantially higher than that suggested by nucleotide analogue labeling (Table 1), and none of the used BrdUrd administration protocols labeled all DiI cells. A marked decrease in the number of DiI-labeled TH+ neurons was seen in animals which received six weeks BrdUrd administration after DiI (Fig. 2k, open circles), suggesting that BrdUrd is toxic and inhibits neurogenesis.
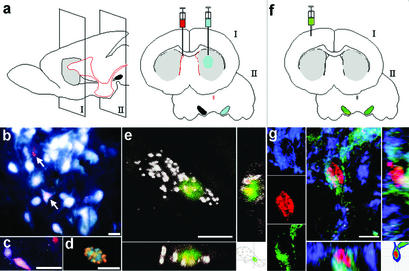
Newly Generated Neurons Project to the Striatum and Integrate into Synaptic Circuits. To test whether the nigral neurons that were generated in the adult innervate their appropriate target in the forebrain, we injected the retrogradely transported axonal tracer fluorogold into the left striatum (see schematic drawing, Fig. 3a). In animals (n = 15) that had received an i.c.v. DiI injection to label ependymal cells 4–8 weeks (but not 2 weeks) earlier, we estimated that ≈10% of the DiI-labeled nerve cells in the substantia nigra also contained fluorogold (Fig. 3 b–d). Moreover, in animals (n = 8) that had received BrdUrd 2 months (but not 3 weeks) earlier, we found a few fluorogold labeled nigral neurons that had incorporated the nucleotide analogue (Fig. 3e).
Fig. 3.
Newborn substantia nigra neurons project to the striatum. (a) Schematic drawing showing the right intraventricular injection of DiI (red) to label cells lining the ventricular system, and left intrastriatal fluorogold injection (blue) to retrogradely trace nigrostriatal projections. (b–d) Six weeks after DiI injection, a few nigral ependyma-derived cells (red) are retrogradely labeled with fluorogold (white) injected 2 days before analysis. Two DiI-labeled cells are indicated by arrows in b, and the inferior one also contains fluorogold. DiI-labeled neurons are morphologically indistinguishable from surrounding neurons (b–d). Colocalization of BrdUrd and fluorogold in the confocal microscope is shown in d. Colocalization was further analyzed three-dimensionally by confocal imaging (e) of a newborn (BrdUrd+, green nucleus) nigral neuron retrogradely labeled with fluorogold (white), injected 3 days earlier in the striatal nerve terminal field. The 10-week-old mouse received BrdUrd through drinking water for 2 weeks, and the animal was killed 2 months later. (f) Schematic drawing showing injection of eGFP PRV (green) and CTB (yellow) into the right somatosensory cortex. Both the virus and CTB were axonally transported, but the latter was not transferred over synaptic connections. Some nigral ipsilateral neurons contained both CTB and eGFP PRV, whereas contralaterally only eGFP PRV was found. (g) Three-dimensional and single channel registrations of confocal images of a transsynaptically labeled newborn neuron, TH+ (violet-blue), BrdUrd+ (red), and eGFP PRV+ (green) in an adult mouse that received BrdUrd in drinking water for 3 weeks, and was allowed to survive 1 month before eGFP PRV was administered. [Scale bars = 20 μm (b–d) and 5 μm (e and g).]
We next analyzed whether the newly generated neurons integrated into the synaptic circuitry by injecting eGFP PRV into the somatosensory cortex of adult mice 1 month after receiving BrdUrd in the drinking water for 3 weeks (Fig. 3f). Colocalization of eGFP in BrdUrd+ neurons without CTB contralateral to the injection suggested that adult-born neurons in the substantia nigra participated in defined multisynapse network connections with the cortex (Fig. 3g).
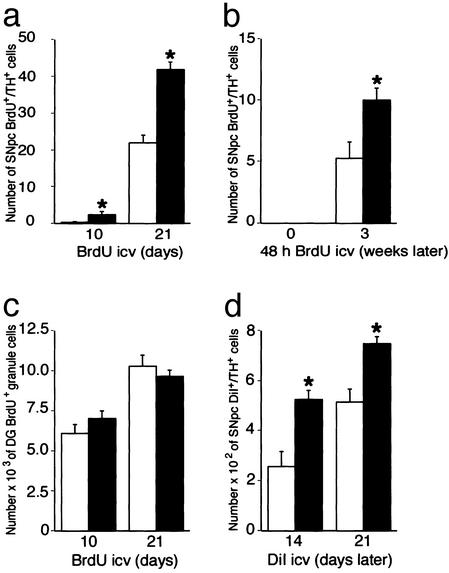
Increased Neurogenesis After a Partial Lesion. In agreement with the lesion-induced effects on neurogenesis observed in other brain regions (9–12), we found that a systemic dose of MPTP (25), known to kill approximately half of the nigral dopaminergic nerve cell population (17), led to a 2-fold increase of BrdUrd incorporation in TH+ nigral neurons 3 weeks after the lesion (Fig. 4a). Labeled neurons in SNpc did not display pyknotic nuclei or other signs of apoptosis. When MPTP-lesioned animals were killed immediately after BrdUrd administration, no BrdUrd incorporation was found in nigral neurons (Fig. 4b), arguing against BrdUrd incorporation due to DNA repair or apoptotic DNA-cleavage.
Fig. 4.
MPTP increases neurogenesis in the substantia nigra. MPTP-induced effects on neuronal BrdUrd incorporation in substantia nigra (a, chronic continuous i.c.v. infusion; b, repeated systemic injections for 2 days after which mice were analyzed immediately or 3 weeks later) and hippocampus (c, chronic continuous i.c.v. infusion) and on the total number of nigral DiI+/TH+ neurons (d) are shown as bar diagrams (mean ± SEM). For details on treatment, see Materials and Methods. n = 3–5 in each group; asterisk indicates P < 0.05, Student's unpaired t test with Bonferroni′s procedure to balance for increased type I error after multiple comparisons in the same animal.
In the same animals that were used to study neurogenesis in SNpc, we quantified cell proliferation in the hippocampus after repeated systemic administration or i.c.v. infusion of BrdUrd. The number of BrdUrd+ cells in the granular cell layer of DG after MPTP did not differ from control animals at 10 days or 3 weeks after the lesion, when increases in BrdUrd+ TH+ neurons were seen (Fig. 4c). In another group of animals, an i.c.v. injection of DiI was given 10 days before MPTP, and the toxin was found to induce a similar 2-fold increase in the number of DiI-labeled TH+ nigral neurons compared with control mice (Fig. 4d). Taken together, these data suggest a significant increase in nigral neurogenesis after a partial lesion.
Discussion
Here we provide data supporting the presence of neurogenesis in the adult substantia nigra. SNpc did not lose TH+ neurons during aging, although TUNEL+ apoptotic cells were demonstrated in the same neuronal population under physiological conditions, which indirectly pointed to the possibility that new nerve cells were added during adulthood to maintain homeostasis. Indeed, markers for proliferation, i.e., the nucleotide analogues BrdUrd and [3H]thymidine, were demonstrated in nuclei of cells with nerve cell characteristics, i.e., presence of TH, Hu, NeuN, Nissl substance, and synapses on the soma. The presence of immature neurons in and around substantia nigra containing nestin, some coexpressing TH, further supported the presence of nigral neurogenesis. In agreement with functional integration of the adult-born neurons in other regions (9, 14, 24), the newly generated nigral neurons projected axons to the appropriate terminals in striatum and were integrated into multisynapse circuits to cortex. The latter was shown by identification of the neurotropic virus PRV, but not CTB, in SNpc contralateral to an injection in the somatosensory cortex, which indicated transsynaptic viral transfer (13), e.g., via crossed corticostriatal and ipsilateral striatonigral projections. Furthermore, our data suggest that the newly generated dopaminergic projection neurons derive from stem cells lining the cerebroventricular system in the midbrain, with cells putatively migrating along the ventral midline as they express the immature neuronal marker CRMP-4 (previously known as TOAD-64) (18). Although the number of neurons generated is orders of magnitude lower than in the hippocampus or the olfactory bulb, the estimated turnover rate implies, provided the rate is constant, that the entire population of dopaminergic substantia nigra neurons could be replaced within the life span of the mouse.
Considering the low number of neurons generated in the adult substantia nigra, it is not surprising that this has gone unnoticed before. Our data indicate that a high BrdUrd dose (4) is necessary to robustly demonstrate this phenomenon, because a single injection of 100 mg/kg only labeled one nigral neuron in five mice. When we infected the putative stem cells lining the ventricular surface by injecting retrovirus (data not shown), we could not observe any nigral nerve cells with the viral marker, in line with the described inefficiency of this method for labeling dividing cells (3), indicated by the fact that <20 of the estimated 10,000 newly generated nerve cells in normal rat hippocampus were found after viral injection (24). A recent study did not find evidence for neurogenesis in the adult rat substantia nigra (26). We think that a quantitative comparison with a known neurogenic region is necessary for correct interpretation of both positive and negative results on neuronal BrdUrd incorporation. Importantly, our comparison between hippocampus and substantia nigra using different BrdUrd protocols demonstrates that 5-fold more neurons can be labeled by i.c.v. administration of the thymidine analogue compared with larger, repeated peripheral doses, which is in line with earlier reports that the blood–brain barrier and/or peripheral metabolism limit the availability of BrdUrd in the brain (21). When we performed similar experiments administering DiI i.c.v. or BrdUrd via drinking water, we occasionally found labeled nigral nerve cells also in the rat (data not shown), thus it is likely that the observation by Lie et al. (26) reflects important methodological rather than species differences between mice and rats. Moreover, because their insult to the nigrostriatal system causes a near complete loss of nigral neurons (27), the different results after injury may point to an important discrepancy. Studies reporting lesion-induced up-regulation of neurogenesis used a partial lesion (9–11), in line with our results, suggesting that some instructive signals may have been lost when close to the entire nerve cell population was ablated (28). Because no nigral BrdUrd+ nerve cells were observed in animals killed immediately after 2-day administration of the nucleotide analogue, we excluded DNA repair-associated nuclear incorporation. Moreover, in animals receiving continuous BrdUrd i.c.v. infusion, we did not observe any neuronal nucleotide analogue incorporation when animals were killed 2 days after an MPTP injection, which is known to represent the postlesion interval when neuronal apoptosis is most pronounced (K.D. and A.M.J., unpublished data), thus arguing against BrdUrd incorporation reflecting transient, apoptotic processes. Interestingly, BrdUrd incorporation reached a plateau after 10 days i.c.v. infusion in nonneuronal nigral cells. In contrast, a similar upper limit for BrdUrd+ nerve cell numbers was not observed in SNpc or DG, as BrdUrd+ neurons continued to increase at 21 days. This may reflect turnover of newly generated nonneuronal cells when homeostasis was reached, whereas newly generated neurons survive for a longer time with a different turnover kinetics. In line with this interpretation, the number of dentate gyrus nerve cells was similar when BrdUrd was incorporated during a putative last cell division before neuronal differentiation and killed immediately or if the mouse survived 3 more weeks (Table 1, i.c.v. 2 days). Unspecific effects of MPTP-treatment on nucleotide incorporation are unlikely, because the toxin did not induce any changes in the total number of BrdUrd+ granule nerve cells in the dentate gyrus of hippocampus at 10 or 21 days.
The time when BrdUrd- or DiI-labeled nigral nerve cells first appeared in the substantia nigra after administration and their preferential location in the rostral substantia nigra pars compacta support that they labeled the same stem cell progeny. However, the calculated rate of neurogenesis from cumulative DiI labeling (20 neurons per day, i.e., 0.17% of the total nigral population) is several-fold greater than estimated from repeated intermittent or continuous infusion of BrdUrd. The fact that the lower numbers found in the BrdUrd experiments would not compensate for the accounted neuronal loss by apoptosis to maintain the observed constant number of cells in the substantia nigra support the validity of the higher rate of neurogenesis estimated from the DiI experiment. We cannot rule out that the dose of BrdUrd was too low to label all dividing neurons or that the protocols used to detect neuronal BrdUrd may not have been sensitive enough to identify punctate staining of neuronal nuclei (4). However, BrdUrd has been shown to be toxic for dividing cells (23), and it is possible that the cells are killed by high doses of BrdUrd, leading to underestimates of the rate of neurogenesis. In support of this interpretation, the number of DiI-labeled TH+ neurons was lower in animals that received BrdUrd via drinking water over an extended time, suggesting that the substance inhibited neurogenesis in the substantia nigra.
It is believed that a gradual decline in the number of nigral dopamine neurons occurs with normal aging in humans, and that Parkinson's disease is caused by an abnormally rapid rate of cell death (29). Interestingly, apoptotic neurons have been found in the normal human substantia nigra (30). Neural stem cells (31) and neurogenesis (32) have been demonstrated in the adult human brain. If there is neuronal turnover also in the human substantia nigra, it is possible that Parkinson's disease could be caused, at least in some cases, by decreased neurogenesis, rather than an increased cell death. Interestingly, studies of nigral tissue from patients with neurodegenerative disorders affecting nigral neurons, including Parkinson's and Lewy-body disease, show that a surprisingly large proportion (5–11%) of the neuronal population displays signs of apoptosis (33). Based on this, and taking into account the rapid kinetics of apoptosis, one would assume that the neurons would rapidly run out. This paradox could be explained by a parallel production of neurons, though it would not keep up with the degenerative processes, leading to net loss of cells in the Parkinsonian patients. Indeed, transient processes observed during neuronal cell death, e.g., glial activation, support the presence of ongoing neuronal loss as the disease progresses over several years (29). Our data imply that disturbances in the finely tuned equilibrium of cell genesis and cell death could result in neurodegenerative disorders.
The presence of a slow physiological turnover of neurons in the adult substantia nigra points to a functional role for neural stem cells in the midbrain. Moreover, the increased neuronal replacement observed after a partial nigral MPTP-induced lesion indicates that the rate of neurogenesis can be regulated. Unveiling the molecular mechanisms controlling neurogenesis may enable the development of strategies to increase the generation of dopaminergic neurons in the adult brain, and potentially offer an attractive way to treat Parkinson's disease.
Supplementary Material
Acknowledgments
We thank Donato Di Monte, Urban Lendahl, Lars Olson, and Pasko Rakic for valuable discussions; Zhi-Qing David Xu and Tomas Hökfelt for confocal imaging; Lynn Enquist for the kind gift of PRV GS518; and Susan Hockfield and Ron McKay for generously providing antibodies. This work was supported by Swedish Research Council Grants 10816, 12183, 13473, and 14384, the Karolinska Institute, the Swedish Foundation for Strategic Research, the Göran Gustafsson Foundation, King Gustaf V and Queen Victoria's Foundation, the Ragnhild and Einar Lundström Foundation, the Hans Osterman Foundation, Swedish Association of Neurologically Disabled, Swedish Cancer Foundation, the Fredrik and Ingrid Thuring Foundation, and the Tobias Foundation.
Abbreviations: Ara-Cyt, cytosine-D-arabinofuranoside; CTB, choleratoxin subunit B; DG, dentate gyrus; DiI, 1,1′-dioctadecyl-6,6′-di-(4-sulfophenyl)-3,3,3′,3′-tetramethylindocarbocyanine; eGFP PRV, enhanced GFP expressing pseudo rabies virus; EM, electron microscope; GFAP, glial fibrillary acidic protein; i.c.v., intracerebroventricular; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SNpc, substantia nigra pars compacta; TH, tyrosine hydroxylase; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling.
See commentary on page 7430.
References
- 1.McKay, R. D. (1997) Science 276, 66-71. [DOI] [PubMed] [Google Scholar]
- 2.Gage, F. H. (2000) Science 287, 1433-1438. [DOI] [PubMed] [Google Scholar]
- 3.Momma, S., Johansson, C. B. & Frisén, J. (2000) Curr. Opin. Neurobiol. 10, 45-49. [DOI] [PubMed] [Google Scholar]
- 4.Cameron, H. A. & McKay, R. D. (2001) J. Comp. Neurol. 435, 406-417. [DOI] [PubMed] [Google Scholar]
- 5.Gould, E., Reeves, A. J., Graziano, M. S. & Gross, C. G. (1999) Science 286, 548-552. [DOI] [PubMed] [Google Scholar]
- 6.Rietze, R., Poulin, P. & Weiss, S. (2000) J. Comp. Neurol. 424, 397-408. [PubMed] [Google Scholar]
- 7.Kornack, D. R. & Rakic, P. (2001) Science 294, 127-130. [DOI] [PubMed] [Google Scholar]
- 8.Gould, E. & Tanapat, P. (1997) Neuroscience 80, 427-436. [DOI] [PubMed] [Google Scholar]
- 9.Magavi, S. S., Leavitt, B. R. & Macklis, J. D. (2000) Nature 405, 951-955. [DOI] [PubMed] [Google Scholar]
- 10.Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. (2002) Nat. Med. 8, 963-970. [DOI] [PubMed] [Google Scholar]
- 11.Nakatomi, H., Kuriu, T., Okabe, S., Yamamoto, S., Hatano, O., Kawahara, N., Tamura, A., Kirino, T. & Nakafuku, M. (2002) Cell 110, 429-441. [DOI] [PubMed] [Google Scholar]
- 12.Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. (1999) Cell 97, 703-716. [DOI] [PubMed] [Google Scholar]
- 13.Enquist, L. W., Husak, P. J., Banfield, B. W. & Smith, G. A. (1998) Adv. Virus Res. 51, 237-347. [DOI] [PubMed] [Google Scholar]
- 14.Carlén, M., Cassidy, R. M., Brismar, H., Smith, G. A., Enquist, L. W. & Frisén, J. (2002) Curr. Biol. 12, 606-608. [DOI] [PubMed] [Google Scholar]
- 15.Walters, T. L., Irwin, I., Delfani, K., Langston, J. W. & Janson, A. M. (1999) Exp. Neurol. 156, 62-70. [DOI] [PubMed] [Google Scholar]
- 16.Johansson, C. B., Momma, S., Clarke, D. L., Risling, M., Lendahl, U. & Frisén, J. (1999) Cell 96, 25-34. [DOI] [PubMed] [Google Scholar]
- 17.Chan, P., Di Monte, D. A., Langston, J. W. & Janson, A. M. (1997) J. Pharmacol. Exp. Ther. 280, 439-446. [PubMed] [Google Scholar]
- 18.Minturn, J. E., Geschwind, D. H., Fryer, H. J. & Hockfield, S. (1995) J. Comp. Neurol. 355, 369-379. [DOI] [PubMed] [Google Scholar]
- 19.Gavrieli, Y., Sherman, Y. & Ben-Sasson, S. A. (1992) J. Cell Biol. 119, 493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gundersen, H. J., Jensen, E. B., Kieu, K. & Nielsen, J. (1999) J. Microsc. 193, 199-211. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan, M. S. (1983) J. Hirnforsch. 24, 23-33. [PubMed] [Google Scholar]
- 22.Morgan, S., Steiner, H., Rosenkranz, C. & Huston, J. P. (1986) Neuroscience 17, 609-614. [DOI] [PubMed] [Google Scholar]
- 23.Kolb, B., Pedersen, B., Ballermann, M., Gibb, R. & Whishaw, I. Q. (1999) J. Neurosci. 19, 2337-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Praag, H., Schinder, A. F., Christie, B. R., Toni, N., Palmer, T. D. & Gage, F. H. (2002) Nature 415, 1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langston, J. W., Ballard, P., Tetrud, J. W. & Irwin, I. (1983) Science 219, 979-980. [DOI] [PubMed] [Google Scholar]
- 26.Lie, D. C., Dziewczapolski, G., Willhoite, A. R., Kaspar, B. K., Shults, C. W. & Gage, F. H. (2002) J. Neurosci. 22, 6639-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirik, D., Rosenblad, C. & Björklund, A. (1998) Exp. Neurol. 152, 259-277. [DOI] [PubMed] [Google Scholar]
- 28.Shihabuddin, L. S., Holets, V. R. & Whittermore, S. R. (1996) Exp. Neurol. 139, 61-72. [DOI] [PubMed] [Google Scholar]
- 29.Dunnett, S. B. & Björklund, A. (1999) Nature 399 (Suppl.), A32-A39. [DOI] [PubMed] [Google Scholar]
- 30.Anglade, P., Vyas, S., Hirsch, E. C. & Agid, Y. (1997) Histol. Histopathol. 12, 603-610. [PubMed] [Google Scholar]
- 31.Johansson, C. B., Svensson, M., Wallstedt, L., Janson, A. M. & Frisén, J. (1999) Exp. Cell Res. 253, 733-736. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson, P. S., Perfilieva, E., Björk-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A. & Gage, F. H. (1998) Nat. Med. 4, 1313-1317. [DOI] [PubMed] [Google Scholar]
- 33.Tompkins, M. M., Basgall, E. J., Zamrini, E. & Hill, W. D. (1997) Am. J. Pathol. 150, 119-131. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.