Abstract
New perspectives in neurorehabilitation suggest that behavioral treatments of movement disorders may modify the functional organization of central somatosensory neural networks. On the basis of the assumption that use-dependent reorganization in these networks contributes to the fundamental abnormalities seen in focal dystonia, we treated 10 affected musicians and measured the concomitant somatosensory changes by using whole-head magnetoencephalography. We found that effective treatment, using the method of sensory motor retuning, leads to alterations in the functional organization of the somatosensory cortex. Specifically, before treatment, somatosensory relationships of the individual fingers differ between the affected and unaffected hands, whereas after treatment, finger representations contralateral to the dystonic side become more similar to the less-affected side. Further, somatosensory finger representations are ordered more according to homuncular principles after treatment. In addition, the observed physiologic changes correlated with behavioral data. These results confirm that plastic changes in parallel with emergent neurological dysfunction may be reversed by context-specific, intensive training-based remediation.
Recent discoveries concerning the CNS's response to injury, as well as new insights into how patients recover lost behavioral capabilities through training, have created a new perspective in neurorehabilitation (1). On the basis of phenomena such as cortical reorganization after a lesion and CNS repair, new therapies have been developed that demonstrate substantial enhancement of extremity use and linguistic function through behavioral treatments. We extend this work to musicians with focal hand dystonia and are able to report evidence for use-dependent CNS plasticity. Previously, using neuromagnetic source imaging, we reported a smearing of the homuncular organization of the representation of the digits in primary somatosensory cortex with this population (2). Although this observation does not require causal primacy of the central mechanism, we assumed that behaviorally manipulated use-dependent plasticity could potentially be of value in changing both cortical organization and the involuntary discoordination of finger movements. On the basis of this consideration, we developed sensory motor retuning (SMR), a successful therapy for focal hand dystonia (3, 4). A disorder of the homuncular organization of the representation of the digits in primary somatosensory cortex in patients with focal hand dystonia was also presented previously by others (5). This disorder has been recently confirmed by using functional MRI to compare the brain dynamics of the sensory cortex in response to single vs. combined tactile stimulation of the index and middle fingers (6). The present study was designed to investigate whether SMR treatment would also induce observable alterations in the organization of the somatosensory cortex previously shown to be deviant in musicians with focal hand dystonia (2).
Focal hand dystonia is a motor disorder involving abnormal hand and finger positions, cramps, and noncoordinated movements of the hand and fingers (1–8). It can be so disabling that patients have to limit or give up their occupation. It develops in individuals, such as professional musicians, whose profession involves frequent repetitive movements and who try to achieve perfect stereotypical fine movements (9). The musicians particularly at risk seem to be those who perform with a high muscular force or who receive vibratory stimulation at their fingertips. Animal models using non-human primates have shown that repeated and prolonged use of the contralateral hand for the completion of motor tasks results in changes in the somatotopy in SI area 3b, which are associated with focal hand dystonia (10–12). Although the validity of this animal model has been questioned (7), practice-mediated plastic capacities can be demonstrated in the human cortex (13–16). In musicians, use-dependent plasticity in the representational cortex of string players (13) and in the motor cortex of piano learners (16) has been shown and is generally considered fundamental to the skillful playing of music (17). In addition, it has been suggested that the modification can contribute to the development of focal hand dystonia. This view suggests that synchronous activation of several digits by vigorous and frequent musical practice leads to a disordered and smeared representation of the fingers in somatosensory and probably also in motor cortex, with the inability to move the most affected fingers separately (2, 18). Also, sufficient evidence now exists to demonstrate a corresponding defective perception and abnormal sensory processing in focal hand dystonia (5, 19–22). Given these abnormalities as well as the difficulties in currently available symptomatic treatments, new sensory and motor training programs have been developed and tested (3, 4, 23). For example, Byl and McKenzie (23) combined sensory discriminative training with fitness exercises to improve sensory processing and motor control of the dystonia-affected hand and reported gains in motor control, sensory discrimination, and physical performance. In our own laboratory, treating patients with musician's cramp, we have used splints to immobilize digits other than the dystonic fingers, while the dystonic finger performed systematic training with the respective musical instrument (3, 4). In the present study, we mapped brain organization to show that, after successful treatment of focal hand dystonia in musicians, the abnormal organization in the hemisphere contralateral to the dystonic hand was significantly altered toward a normal representation. In the ipsilateral hemisphere, which can be considered as a control, we found no evidence for training-mediated changes.
Methods
This study was approved by the Ethics Committee of the University of Konstanz. Before the experiment began, written consent was obtained from all participants.
Ten professional musicians (mean age 41.7 ± 6.8 years; range, 30–52; eight males and two females) suffering from no neurological conditions other than unilateral focal hand dystonia, as confirmed by a neurologist, served as subjects. Core symptoms required included (i) painless loss of finger motor coordination exclusively when playing the musical instrument, and (ii) restriction of the motor disorder to the involuntary flexion of single digits, and (compensatory) extension of adjacent fingers while performing music. One exclusion criterion was the presence of neurological signs other than the dystonia itself. Specifically, no sensory deficits in other sensory submodalities, including the senses of touch, pain, temperature, joint position, graphesthesia, and vibration, were allowed. Furthermore, two-point discrimination had to be normal or even above average at the tips of the fingers, the palm, and the back of the hand. Nerve compression syndromes were excluded by median and ulnar nerve neurography. An additional exclusion criterion was the use of maintenance medications for dystonia in the 3 months before the beginning of the study. All patients were right-handed, as determined by the Oldfield Handedness Questionnaire (24). For 8 consecutive days, subjects underwent SMR, a behavioral therapy for focal hand dystonia recently developed in our laboratory. In SMR therapy, a hand splint tailored to the hand anatomy of each patient immobilizes one or more finger(s) while leaving the remaining digits free. The intervention involves immobilizing different finger(s) by means of the splint. The splint holds the patient's finger(s) in its characteristic rest position on the instrument, simulating those positions experienced during normal playing. In this way, the focal dystonic finger can participate in alternating individual finger movements with all possible permutations of the other fingers of the dystonic hand. During splinting, the subjects are required to make sequential movements of two or three digits in extension, including the affected digit (D), for periods of 10 min in ascending and then in descending order in continuous repetition (e.g., D3, D4, D5, D4, and so on, with D3 being the focal dystonic finger and D2 the immobilized digit) for 8 consecutive days, under a therapist's supervision, reaching a total duration of 1.5–2.5 h per day, depending on the patient's fitness (4).
The first day before therapy began and the last day after treatment, the patient's sensory-evoked magnetic fields from all fingers of both hands were recorded by means of a 148-channel whole-head magnetometer (Magnes 2500, 4-D Neuroimaging, San Diego). Measurements were done in a magnetic shielded room (Vacuumschmelze, Hanau, Germany) and were video-controlled. Nonpainful somatosensory stimulation (512 stimuli) was applied to all fingers of both hands as described elsewhere (2). Test conditions were held constant from pre- to posttreatment. Epoch data (epoch duration, 300 ms) were collected with a sampling rate of 678.17 Hz and online bandpass-filtered from 1 to 200 Hz. Responses exceeding a range of 5 pT in any of the magnetoencephalography (MEG) channels were omitted from the averaging. For source analysis, responses were filtered from 4 to 30 Hz. A first major peak in the time window of 30–80 ms (average time pretreatment = 68.7 ms, SD = 19.1 ms; average time posttreatment = 68.6 ms, SD = 16.7 ms) was identified as the evoked sensory field arising from SI. Average latencies pre- and posttreatment were 66.2 ± 16.4 ms SD and 67.9 ± 16.0 ms SD for the dystonic and 68.9 ± 27.2 ms SD and 67.9 ± 17.6 ms SD for the homologous nondystonic fingers. A single equivalent current dipole, using the best-fitting local sphere, was fitted to the distribution of the measured fields. Data fulfilling the following criteria were selected: (i) rms values in the contralateral channel group of >14 fT; (ii) a goodness of fit of the equivalent current dipole model to the measured field of >0.95 (average goodness of fit pretreatment = 0.98, SD = 0.02; average goodness of fit posttreatment = 0.98, SD = 0.01); and (iii) a correlation of the recorded measurements with those obtained by plugging the dipole estimate into the forward equation of >0.95 (average correlation pretreatment = 0.99, SD = 0.01; average correlation posttreatment = 0.99, SD = 0.01) and a confidence volume of the equivalent current dipole location of <1,000 mm3 (average confidence volume pretreatment = 384.5, SD = 709.7; average confidence volume posttreatment = 149.0, SD = 224.4). If for a single finger no data fulfilling all of the criteria mentioned above were found, the next higher confidence volume, as well as the best values for the remaining parameters, were then localized. Only those dipole solutions having at least a goodness of fit and a correlation of ≥0.90 were included in the analyses (25). One finger of one patient did not meet these criteria and therefore had to be excluded from the analyses. MEG data were statistically analyzed by a repeated-measures ANOVA with the factors Treatment (pre- vs. posttreatment), Hand (dystonic vs. nondystonic), and Distance (Euclidean distances: D1–D2, D1–D3, D1–D4, D1–D5, D2–D3, D2–D4, D2–D5, D3–D4, D3–D5, D4–D5). To test for the ordered/disordered topographic arrangement of the fingers in the somatosensory cortex, another repeated-measures ANOVA comprising the factors Treatment (pre- vs. posttreatment), Hand (dystonic vs. nondystonic), and Distance (Euclidean distances D1–D2, D2–D5, and D1–D5) was also calculated. The Euclidean distance arrangement D1–D2 < D2–D5 < D1–D5 was defined as representing a normal arrangement (15). This distance profile can be considered as an indicator of a normal arrangement of the cortical finger representations in the sensory cortex because it is congruent with the peripheral finger distance profile and, consequently, with the well accepted concept of homuncular cortical representations for the sensory cortex. Subjective ratings of patient's symptom appraisal were also obtained pre- and post- treatment with the Dystonia Evaluation Scale (DES; ref. 4) and were evaluated with a one-factorial repeated-measures ANOVA with the factor Time (pre- vs. posttreatment). The ratings on the scale are as follows: 0, dystonia as bad as at its worst; 1, slightly improved; 2, moderately improved; 3, almost normal; and 4, normal. In an attempt to objectively depict the DES ratings, we developed a dexterity displacement device (Fig. 1). This device continuously recorded finger movements during metronomepaced displacements of two fingers including the dystonic digit. Movements were performed for 50 s, and off-line spectral analysis of the recorded data provided information on the smoothness of the movements before and after treatment. We evaluated the data obtained during the first and last therapy day preceding the beginning of the daily SMR session. For quantification, we divided the spectral power in the frequency of the metronome (0.9–1.2 Hz) by the power in the side bands (0.1–0.9 Hz plus 1.2–1.9 Hz). Thus, the side bands contained the record of movements that were irregular. [Values are missing for one patient because of procedural problems. See Candia et al. (4).] We then correlated the difference between post- and pretreatment (post- minus pretreatment) on both measures by means of a simple linear regression using the DES as a dependent variable. Further, the difference for the data collected with the displacement dexterity device and the difference post- minus pretreatment of the MEG-recorded dipole moment (Q values) for the dystonic fingers were then correlated by using the behavioral data as a predictor for the selected Q values. The Q values are indicators of the total neuronal activation because they, in essence, represent a measure of depolarization in the apical dendritic tree. For eight of the patients, part of the subjective ratings as well as part of the data collected with the displacement dexterity device, but not the brain measures, was included in an earlier report (4). The area of the finger triangle was calculated from the cross product of vectors a × b, where a = [finger 1(nondystonic) - finger 2(dystonic)] and b = [finger 3(nondystonic) - finger 2(dystonic)]. The area is calculated as 0.5 ∥ a × b ∥, where ∥ a × b ∥ is the norm of the vector product.
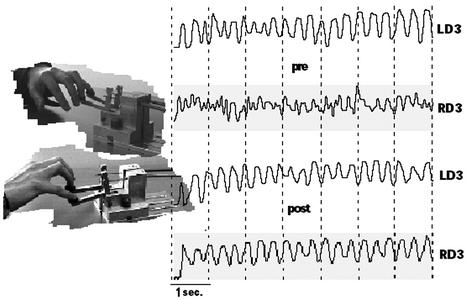
Fig. 1.
Dexterity displacement device. Shown are pre- and posttreatment segments of the movement slopes of right D3 (RD3) and left D3 (LD3) of one patient during the performance of a trill-like task at a fast and free selected velocity. Pretreatment, the recorded movements of the dystonic finger (RD3) were uneven and uncontrolled (upper RD3 profile) compared with the movements of the homologous LD3 (upper LD3 profile), which served as a control finger. These differences are no longer present posttreatment (lower LD3 and RD3 profiles, respectively). For simplicity, only D4 and D5 are depicted in the photographs.
Results
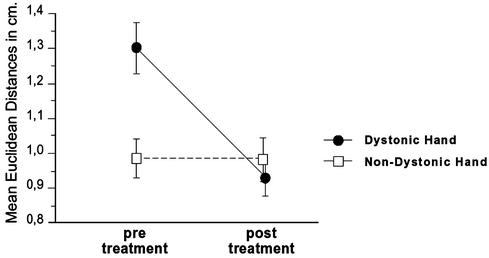
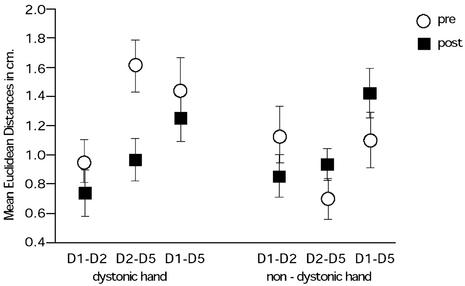
We treated 10 professional musicians having focal hand dystonia. The behavioral procedure has been detailed by Candia et al. (3, 4). MEG recordings were collected and symptom ratings were obtained with the DES before and after treatment. In addition, behavioral data were also recorded by using a finger displacement measuring device (refs. 3 and 4; Fig. 1). During the MEG recordings, we applied nonpainful stimuli individually to each finger on both hands, which allowed us to examine the evoked sensory fields. We then constructed a 3D representation of the fingers in somatosensory cortex and calculated the Euclidean distances between the various digits. A repeated-measures ANOVA examining Treatment (pre- vs. posttreatment), Hand (dystonic vs. nondystonic), and Euclidean Distance (between all digit pairs) showed a significant effect for treatment [F(1,8) = 5.1, P = 0.05] and a significant Treatment by Hand interaction [F(1,8) = 7.9, P < 0.05] whereby the indices of functional organization in both hemispheres were deviant before but similar after the treatment (Fig. 2). The Euclidean distances for the nondystonic, nontreated hand were nearly the same across both measurements (df = 9, t = 0.27, P = 0.79), whereas the average of the Euclidean distances contralateral to the dystonic hand decreased significantly (df = 9, t = 3.3, P < 0.05). A second ANOVA (Treatment, Hand, and Euclidean Distance) involving only the nondystonic fingers (D1, D2, and D5) of both the dystonic and nondystonic hands showed a significant main effect for Distance [F(2,16) = 4.4, P < 0.05] and for the Treatment by Hand by Distance interaction [F(2,16) = 4.5, P < 0.05]. Further post hoc exploration with Fisher's probable least-squares difference test located this difference between D1–D2 and D1–D5 (D1–D2 < D1–D5; significance level 5%, mean difference = -0.397, critical difference = 0.286, P < 0.05). After treatment, the ordered homuncular cortical representation of digits was found in both hands (D1–D2 < D2–D5 < D1–D5) as seen in Fig. 3. Examining the three geometric relationships between the dystonic finger and the left and right neighboring fingers revealed a significantly smaller area for the nontreated hand before treatment (mean difference = 0.273, t(8) = 2.7, P < 0.05), as well as a reduction for the dystonic hand after treatment (mean difference = 0.189 t(9) = 2.1, P < 0.05). The patients' DES ratings also reflected significant improvement after therapy [F(1,9) = 22.2, P < 0.05], which was also confirmed by the behavioral data from the displacement dexterity device. The correlation of the differences post- minus pretreatment on both measures for the dystonic fingers and DES ratings was r = 0.75 (P < 0.05). Performing a correlation on those patients who showed shorter mean Euclidean distances for the dystonic hand after treatment (eight of nine), we found that differences post- minus pretreatment of the behavioral data correlated with the dipole moment Q of the dystonic fingers (r = -0.75, P < 0.05). Including all patients gave similar results (r = -0.6, P < 0.05). Homologous calculations including the analogous fingers of the nondystonic, nontreated hand did not reveal any significant relationships.
Fig. 2.
Mean Euclidean distances of the dystonic (treated) and nondystonic hands pre- and posttreatment. The indices of functional organization in the hemispheres were deviant before but similar after the treatment.
Fig. 3.
Euclidean distance arrangement for the distances D1–D2, D2–D5, and D1–D5 for the dystonic and nondystonic hands. Posttreatment, the normal arrangement was fitted in both hands (normal arrangement D1–D2 < D2–D5 < D1–D5).
Discussion
Our results demonstrate that after SMR therapy, individuals with focal hand dystonia report both subjective improvement and a cortical reorganization. Data from multiple levels, including self-report, behavioral quantification of the degree of dystonia, and cortical representation of sensory processes, portray a correlated picture of change associated with SMR therapy. In specific, pre- vs. posttreatment changes on the DES correlated with changes in behavioral data collected with a finger displacement dexterity device. After treatment, these changes in turn correlated negatively with changes in dipole moment of those affected fingers. After treatment we observed a significant decrease of the 3D Euclidean distances between the cortical representations of all of the fingers of the affected hand, which also resulted in a more orderly representation of the arrangement of those distances including the fingers D1, D2, and D5. As expected, there was little change of the distance dimensions in the cortical hemisphere representing the nondystonic hand. These results are consistent with a variety of studies that suggest that cortical organization may be modified through extensive use. For example, cortical organization of the somatosensory area in both Braille readers (14, 15, 26) and long-term violin players (13) has been shown to differ from matched controls. It should be noted that this previous work examined individuals whose cortical organization reflected years of practice in a particular skill. What is intriguing in our present report is that the cortical reorganization observed resulted from extensive and successful practice in a relatively brief period. In this way, it parallels results demonstrating cortical reorganization after constraintinduced movement therapy after stroke (1). Nevertheless, on the basis of the present data, a transitory modification of the cortical organization related to the treatment but unrelated to the clinical impact cannot be completely ruled out. For example, such transient changes in cortical motor organization have been demonstrated by Classen et al. (27) for thumb movements practiced for a brief period. In addition, Karni et al. (28) have discussed motor learning of skilled movements as a multistage process including rapid organizational changes at its beginning, with consolidation of specific gains after long-term practice. Because our measurements were performed within a short time period, we might have measured in part those initial stages of a much longer ongoing process, a fact preventing a final conclusion. Interestingly, and in agreement with the data presented by Karni et al. (29) for finger opposition task sequences, changes measured for the trained dystonic hand were not reflected in the Euclidean distances of the nontreated hand. Even though SMR may induce plastic changes leading to a more normal sensory representation and reverse the dystonic symptoms, it is still possible that such a mechanism is not a reversal of alterations in functional organization, but represents yet another change in cortical organization leading to behavioral gain. What we do not know at this time is whether our results reflect the establishment of a new motor program, a change in the brain dynamics that inhibits the expression of dystonia, or the reactivation of existent but not accessible functional motor programs.
A limitation of the present data is the lack of a control group in the treatment design. Several reasons preclude the recruitment of a perfect control group for these types of patients. For example, an ethical question arises in terms of training affected patients with a noneffective procedure. It would also be problematic to follow an ineffective therapy with our more effective SMR therapy in terms of patient motivation. Further, carrying out a traditional double-blind placebo procedure with the present treatment, where intensive therapist–patient interaction is fundamental and necessary, would not be possible because there is no meaningful way of blinding the therapist to the nature of the therapy. Thus, it would always seem likely that the nature of an intended placebo procedure would communicate itself to patients, either explicitly or implicitly. An additional and very important constraint against using a matched control group comprising nonaffected musicians would be the highly probable and almost nonavoidable lack of motivation for enrollment in such a training protocol. This, added to the fact that changes in cortical organization have been repeatedly reported after repetition of motor movement sequences, also imposes ethical concerns, considering the potential danger of unpredictable outcomes. Clearly, future research needs to address these issues and to consider experimental procedures to clarify the active ingredients in the therapy.
Many studies reported deviant indices of somatosensory and motor organization associated with dystonia in humans. Although a dysfunction in the basal ganglia is commonly assumed to be at the origin of focal hand dystonias (30), it is obvious that the whole somatomotor network is affected. Hallett (31), for instance, argued that the basal ganglia could be responsible for a weakened cortical inhibition in patients with dystonia. Thus, a lack of inhibition in somatosensory and motor cortical areas may foster a fusion and disorder of representational cortical zones. Further, current technology allows us to obtain indices of this deviant functional organization by mapping the somatosensory cortical representation. Bara-Jimenez et al. (5) examined cortical representation between D1 and D5 in six individuals who reported dystonia during a variety of tasks (including writing and instrument playing) and even during rest. These authors found an abnormality of the normal homuncular organization of the finger representations in the primary somatosensory cortex of the group overall. In our own laboratory (2), we examined the digits of professional musicians with focal hand dystonia and found a similar abnormal representation in the sensory cortex of the affected hand, which is consistent with the view that sensory dysfunction may lead to problems with fine motor control (32). Moreover, Sanger et al. (6) recently demonstrated nonlinear cortical functional activity in the primary sensory brain areas of patients with writer's cramp in response to tactile stimulation of the index and middle fingers. For the nondystonic group, adding the stimulus-related brain activity for each single finger resulted in a better prediction of the brain activity generated by means of simultaneous stimulation of the same digits. Our present report extends this earlier work and demonstrates both an abnormal mapping and a return to more normal somatosensory representation in parallel with improved motor functioning after treatment. Our work clearly supports the value of studies aimed at reordering pathological representation in the somatosensory cortex. Given the success of our treatment approach, at least two broad types of questions remain. First, the practical question remains as to the best forms of treatment. For example, future work can help to clarify differences found in dystonia treatments such as ours, which directly target the relationship between the digits, and treatments that teach alternative skills such as learning Braille as a method of modification of the disorder (32). Second, it is critical to understand both the manner in which improved motor functioning is coupled with somatosensory representation and the long-term stability of these changes. Given the difficulty of causal inference at this point, it is crucial that studies be attempted with individuals at risk for dystonia to determine at what point the cortical reorganization takes place and its relation to reported and observed pathology.
Acknowledgments
We thank Mrs. Ursula Lommen for help in data collection. This work was supported by the Deutsche Forschungsgemeinschaft.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SMR, sensory motor retuning; DES, Dystonia Evaluation Scale; D, digit; MEG, magnetoencephalography.
See commentary on page 7425.
References
- 1.Taub, E., Uswatte, G. & Elbert, T. (2002) Nat. Rev. Neurosci. 3, 228-236. [DOI] [PubMed] [Google Scholar]
- 2.Elbert, T., Candia, V., Altenmüller, E., Rau, H., Sterr, A., Rockstroh, B., Pantev, C. & Taub, E. (1998) NeuroReport 9, 3571-3575. [DOI] [PubMed] [Google Scholar]
- 3.Candia, V., Elbert, T., Altenmüller, E., Rau, H., Schäfer, T. & Taub, E. (1999) Lancet 42, 353. [DOI] [PubMed] [Google Scholar]
- 4.Candia, V., Schäfer, T., Taub, E., Rau, H., Altenmüller, E., Rockstroh, B. & Elbert, T. (2002) Arch. Phys. Med. Rehab. 83, 1342-1348. [DOI] [PubMed] [Google Scholar]
- 5.Bara-Jimenez, W., Catalan, M. J., Hallett, M. & Gerloff, C. (1998) Ann. Neurol. 44, 828-831. [DOI] [PubMed] [Google Scholar]
- 6.Sanger, T. D., Pascual-Leone, A., Tarsy, D. & Schlaug, G. (2002) Movement Disorders 17, 105-111. [DOI] [PubMed] [Google Scholar]
- 7.Chen, R. & Hallett, M. (1998) Clin. Orthop. 351, 102-110. [PubMed] [Google Scholar]
- 8.Byl, N., Wilson, F., Merzenich, M., Melnick, M., Scott, P., Oakes, A. & McKenzie, A. (1996) J. Orthop. Sports Phys. Ther. 23, 234-244. [DOI] [PubMed] [Google Scholar]
- 9.Wilson, F. (1989) Semin. Neurol. 9, 146-151. [DOI] [PubMed] [Google Scholar]
- 10.Byl, N. N. & Melnick, M. (1997) J. Hand. Ther. 10, 160-174. [DOI] [PubMed] [Google Scholar]
- 11.Byl, N. N., Merzenich, M. M. & Jenkins, W. M. (1996) Neurology 47, 508-520. [DOI] [PubMed] [Google Scholar]
- 12.Byl, N. N., Merzenich, M. M., Cheung, S., Bedenbaugh, P., Nagarajan, S. S. & Jenkins, W. M. (1997) Phys. Ther. 77, 269-284. [DOI] [PubMed] [Google Scholar]
- 13.Elbert, T., Pantev, C., Wienbruch, C., Rockstroh, B. & Taub, E. (1995) Science 220, 21-23. [DOI] [PubMed] [Google Scholar]
- 14.Sterr, A., Müller, M. M., Elbert, T., Rockstroh, B., Pantev, C. & Taub, E. (1998) Nature 391, 134-135. [DOI] [PubMed] [Google Scholar]
- 15.Sterr, A., Müller, M. M., Elbert, T., Rockstroh, B., Pantev, C. & Taub, E. (1998) J. Neurosci. 18, 4417-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pascual-Leone, A., Nguyet, D., Cohen, L. G., Brasil-Neto, J. P., Cammarota, A. & Hallett, M. (1995) J. Neurophysiol. 74, 1037-1045. [DOI] [PubMed] [Google Scholar]
- 17.Pascual-Leone, A. (2001) Ann. N.Y. Acad. Sci. 930, 315-329. [DOI] [PubMed] [Google Scholar]
- 18.Münte, T. F., Altenmüller, E. & Jancke, L. (2002) Nat. Rev. Neurosci. 3, 473-478. [DOI] [PubMed] [Google Scholar]
- 19.Bara-Jimenez, W., Shelton, P. & Hallet, M. (2000) Neurology 55, 1869-1873. [DOI] [PubMed] [Google Scholar]
- 20.Bara-Jimenez, W., Shelton, P., Sanger, T. D. & Hallet, M. (2000) Ann. Neurol. 47, 377-380. [PubMed] [Google Scholar]
- 21.Grünewald, R. A., Yoneda, Y., Shipman, J. M. & Sagar, H. H. (1997) Brain 120, 2179-2185. [DOI] [PubMed] [Google Scholar]
- 22.Sanger, T. D., Tarsy, D. & Pascual-Leone, A. (2001) Movement Disorders 16, 94-99. [DOI] [PubMed] [Google Scholar]
- 23.Byl, N. N. & McKenzie, A. (2000) J. Hand. Ther. 13, 289-301. [DOI] [PubMed] [Google Scholar]
- 24.Oldfield, R. C. (1971) Neuropsychologia 9, 97-113. [DOI] [PubMed] [Google Scholar]
- 25.Mühlnickel, W., Elbert, T., Taub, E. & Flor, H. (1998) Proc. Natl. Acad. Sci. USA 95, 10340-10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual-Leone, A., Wassermann, E. M., Sadato, N. & Hallet, M. (1995) Ann. Neurol. 38, 910-915. [DOI] [PubMed] [Google Scholar]
- 27.Classen, J., Liepert, J., Wise, S. P., Hallett, M. & Cohen, L. G. (1998) J. Neurophysiol. 79, 1117-1123. [DOI] [PubMed] [Google Scholar]
- 28.Karni, A., Meyer, G., Rey-Hipolito, C., Jezzard, P., Adams, M. M., Turner, R. & Ungerleider, L. G. (1998) Proc. Natl. Acad. Sci. USA 3, 861-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karni, A., Meyer, G., Jezzard, P., Adams, M. M., Turner, R. & Ungerleider, L. G. (1995) Nature 377, 155-158. [DOI] [PubMed] [Google Scholar]
- 30.Berardelli, A., Rothwell, J., Hallet, M., Thompson, P., Manfredi, M. & Marsden, C. (1998) Brain 121, 1195-1212. [DOI] [PubMed] [Google Scholar]
- 31.Hallett, M. (1998) Arch. Neurol. (Chicago) 55, 601-603. [DOI] [PubMed] [Google Scholar]
- 32.Zeuner, K. E., Bara-Jimenez, W., Noguchi, P. S., Goldstein, S. R., Dambrosia, J. M. & Hallett, M. (2002) Ann. Neurol. 51, 593-598. [DOI] [PubMed] [Google Scholar]