Abstract
Mice lacking the chemokine receptor chemotactic cytokine receptor 2 (CCR2) have a marked attenuation of monocyte recruitment in response to various inflammatory stimuli and a reduction of inflammatory lesions in models of demyelinating disease. In the present study, we compared nociceptive responses in inflammatory and neuropathic models of pain in CCR2 knockout and wild-type mice. In acute pain tests, responses were equivalent in CCR2 knockout and wild-type mice. In models of inflammatory pain, CCR2 knockout mice showed a 70% reduction in phase 2 of the intraplantar formalin-evoked pain response but only a modest (20–30%) and nonsignificant reduction of mechanical allodynia after intraplantar Freund's adjuvant (CFA). In a model of neuropathic pain, the development of mechanical allodynia was totally abrogated in CCR2 knockout mice. CFA administration induced marked up-regulation of CCR2 mRNA in the skin and a moderate increase in the sciatic nerve and dorsal root ganglia (DRG). In response to nerve ligation, persistent and marked up-regulation of CCR2 mRNA was evident in the nerve and DRG. Disruption of Schwann cells in response to nerve lesion resulted in infiltration of CCR2-positive monocytes/macrophages not only to the neuroma but also to the DRG. Chronic pain also resulted in the appearance of activated CCR2-positive microglia in the spinal cord. Collectively, these data suggest that the recruitment and activation of macrophages and microglia peripherally and in neural tissue may contribute to both inflammatory and neuropathic pain states. Accordingly, blockade of the CCR2 receptor may provide a novel therapeutic modality for the treatment of chronic pain.
The chemotactic cytokine or chemokine receptor family is the largest family of G protein-coupled receptors. Accordingly, the number of chemokines that binds to these receptors is large, with >50 chemokine peptides having been identified to date (for review, see ref. 1). Chemokine biology is further complicated by individual chemokines interacting with more than one receptor and chemokine receptors potentially binding more than one chemokine. Predominantly, chemokine receptors are expressed by leukocytes, and the specific interactions of chemokines with their cognate receptors are major determinants of the trafficking and localization of leukocyte subsets within tissue compartments. A subset of chemokines exhibit potent chemoattractant activity for monocytes; one of them, monocyte chemoattractant protein 1 (MCP-1), stimulates monocyte transendothelial migration (extravasation) and preferentially binds to the chemotactic cytokine receptor (CCR), CCR2. Mice lacking either MCP-1 or CCR2 show a marked attenuation of monocyte recruitment in response to various inflammatory stimuli, as well as a reduction in the development of inflammatory lesions in models of CNS demyelinating disease (2, 3). Moreover, in CCR2-deficient mice, macrophage recruitment to sites of neuronal damage is reduced, with a consequent decrease in demyelination (4, 5).
Although inflammatory and neuropathic pain syndromes are often considered distinct entities, emerging evidence belies this strict dichotomy. For example, nerve damage can stimulate macrophage infiltration, increase the number of activated T cells, and up-regulate proinflammatory cytokines (6). Under these conditions, neuroinflammatory and immune responses after nerve damage may contribute as much to the development and maintenance of neuropathic pain as the initial nerve damage itself. Recent reports suggest that chemokines may have additional roles to play in pain. Glycoprotein 120 (gp120), a component of the HIV envelope that binds to CCR5, when administered centrally or peripherally induces pain via release of IL-1 and tumor necrosis factor (TNF) from activated glia (7). gp120, RANTES (ligands for CCR1, 3, 5, and 9), SDF-1α (ligand for CXCR4), and MDC (ligand for CCR4) also induce pain when injected intradermally (8). Furthermore, dorsal root ganglia (DRG) neurons express the chemokine receptors CX3CR1, CXCR4, CCR4, and CCR5, and CXCR4- and CCR4-positive neurons also express substance P and the transient receptor potential ion channel (TRPV1, formerly named VR1) that have been implicated in nociception (8).
Among the subsets of chemokines and their receptors, MCP-1 and CCR2 have not been directly implicated in pain but clearly play a role in both inflammatory and demyelinating diseases. The aim of this study was to assess pain behavior in CCR2-deficient mice in models of inflammatory and neuropathic pain. We found that CCR2-deficient mice did not develop mechanical allodynia associated with nerve ligation, had a significant reduction of the second phase of the flinching response evoked by intraplantar formalin, and exhibited a modest attenuation of mechanical allodynia produced by complete Freund's adjuvant (CFA)-induced inflammation. Consistent with the behavioral data, immunohistochemical analyses identified a greater number of CCR2-positive monocytes/macrophages in the sciatic nerve and DRG after neuropathy than after inflammation. In addition, activated microglia expressing CCR2 were observed in the spinal cord after nerve injury. Collectively, these data suggest that CCR2 might constitute a new target for the treatment of neuropathic pain.
Materials and Methods
Animals. The procedures used in these studies were approved by the Institutional Animal Care and Use Committee at Merck. Mice lacking CCR2 (CCR2-/-) were generated by homologous recombination as reported previously (9). Both CCR2-/- and wild-type mice were of the genetic background C57BL/6J×129P3/J (Taconic Farms). The CCR2-/-mouse was a random intercross on the C57BL/6 × 129/Ola background, and wild-type mice were of the genetic background C57BL/6 × 129SvEvTacF1 (Taconic Farms). C57BL/6 mice were used for the following experiments: intraplantar MCP-1, real-time PCR, and immunohistochemistry analysis. Only male mice (22–32 g) were used in this study.
Behavioral Testing. Rota-rod. Initially, mice were trained on the rota-rod for 3 min at a speed of 10 rpm. For testing, the speed was set at 10 rpm for 60 s and subsequently accelerated to 600 rpm. The time taken for mice to fall after the beginning of the acceleration was recorded.
Hot plate. Mice were habituated to the hot-plate apparatus with temperature set at 45°C for 2 min. Subsequently, mice were placed on the hot-plate and the temperature was sequentially changed to 52.5°C and 55.5°C (cutoff set at 30 s) each and then to 58.5°C (cutoff set at 20 s). The time when mice either licked their paws or jumped was recorded.
Formalin test. For 4 days before testing, mice were acclimated for 2 h every day on the test platform. On the day of the study, mice were placed for 1 h on the test platform and subsequently were administered 10 μl of 2% formalin in the plantar surface of the left paw. The time mice spent either licking or lifting the injected paw was recorded over 2-min periods at 5-min intervals for 50 min. To quantify the magnitude of the inflammatory response, paw diameters were measured with calipers 90 min after formalin injection. MCP-1 intraplantar. To investigate whether MCP-1 evokes hyperalgesia, MCP-1 (150 or 500 ng in 5 μl, Research Diagnostics, Flanders, NJ) was injected s.c. and mechanical sensitivity assessed with von Frey filaments at various times after MCP-1 administration.
Thermal and mechanical stimulation. Thermal sensitivity was assessed by measuring paw withdrawal latencies to a radiant heat stimulus (10). Mechanical sensitivity was determined with calibrated von Frey filaments by using the up-and-down paradigm (11).
CFA. Mice received a unilateral 30-μl intraplantar injection of CFA (0.5 mg/ml, Sigma) into the left paw. Thermal and mechanical paw thresholds were determined before and up to 2 wk after CFA administration.
Nerve injury. Mice were anesthetized with a mixture of ketamine (50 mg/kg, i.m., Pfizer Animal Health, Exton, PA) and medetomidine (1 mg/kg, i.m., Pfizer Animal Health). An incision was made just below the hip bone, parallel to the sciatic nerve. The nerve was exposed and any adhering tissue removed from the nerve. A tight ligature with 6-0 silk suture thread around one-third to one-half of the diameter of the sciatic nerve was made (12). Muscles were closed with suture thread and the wound, with wound clips. The response of the mice to mechanical stimulation was tested before and up to 15 days after nerve injury.
Real-Time PCR Analysis. Real-time PCR was used to assess CCR2 mRNA regulation after injury. Various tissues were dissected ipsilateral to the injury (plantar paw skin, sciatic nerve, DRG: L4, L5, and L6 and lumbar spinal cord) in naïve mice, in mice 2 days after CFA administration, and in sciatic nerve-ligated mice 2, 4, and 7 days and 2, 3, and 4 wk after ligation. Tissues were homogenized by using a polytron in Ultraspec reagent (Biotecx Laboratories, Houston). RNA was isolated by using the Ultraspec RNA isolation system according to the manufacturer's protocol. mRNA was isolated by using the Qiagen oligotex kit (Valencia, CA). Reverse transcription was performed in a 100-μl reaction mixture containing 1× RT-PCR buffer, 5.5 mM MgCl2, 500 μM dNTP mix, 2.5 μM random hexamers, 0.8 units of RNase inhibitor, and 3.75 units of MultiScribe RTase (Applied Biosystems). The reaction mixture was incubated for 10 min at 25°C, then 30 min at 48 and at 95°C for 5 min, and then stored at -20°C until further PCR analysis.
Real-Time Quantitative PCR. Quantitation of mRNA for CCR2 and GAPDH was performed by using an Applied Biosystems PRISM 7700 sequence detection system. Samples of cDNA from control, inflamed, and neuropathic groups or samples from neuropathic groups at different times were analyzed simultaneously by real-time PCR, with each sample run in duplicate. The PCR mixture was prepared by using the multiplex real-time PCR protocol according to the manufacturer's instructions, and the PCR and data analysis were run with the system software. Five microliters of reverse transcription product for each sample was used as the template in a 50-μl reaction mixture. The primers and the TaqMan probe for CCR2 were as follows: 5′-AACAGTGCCCAGTTTTCTATAGG-3′, 5′-CGAGACCTCTTGCTCCCCA-3′ and 5′-6FAM-ACAGCAGATCGAGTGAGCTCTACATTCACTCC-TAMRA-3′. The primers and TaqMan probe for GAPDH were as follows: 5′-TGCACCACCAACTGCTTAG-3′, 5′-GGATGCAGGGATGATGTTC-3′ and 5′-VICCAGAAGACTGTGGATGGCCCCTC-TAMRA-3′. At the completion of the PCR (total of 40 cycles), the amount of a target message in each sample was estimated from a threshold cycle number (Ct). Average Ct values were normalized to average Ct values for GAPDH mRNA from the same cDNA preparations. Results presented are expressed as fold increases over control values.
Immunohistochemistry. Mice were deeply anesthetized with sodium pentobarbital (100 mg/kg i.p.) and perfused through the ascending aorta with 4% formaldehyde [in 0.1 M phosphate buffer (PB), pH 7.4]. The spinal cords, DRG, sciatic nerves, and hind-paw skin were removed and placed in 4% formaldehyde for 4 h and then cryoprotected in 30% sucrose (in 0.1 M PB). Tissues were sectioned (20–40 μm) on a freezing microtome (Leica SM 2000R, Nussloch, Germany) and collected into 0.1 M PB. Sections were incubated for 60 min at room temperature in 3% normal goat serum in PB with 0.9% sodium chloride and 0.3% Triton-X. Sections were then incubated overnight in CCR2 antiserum at 1:400 (4.25 μg/ml). This antibody raised against the C-terminal part (365–373) was raised and tested in-house on CCR2- and CCR5-transfected Chinese hamster ovary (CHO) cells via immunocytochemistry, and Western blots. The antibody was shown to have minimal crossreactivity to murine CCR5, and no reactivity to nontransfected CHO cells was observed. Moreover, in CCR2-/-mouse tissues, no specific labeling was detected. After the primary antiserum incubation, tissue sections were washed three times in 0.1 M PB and then incubated in CY-2- or CY-3-conjugated goat anti-rabbit IgG (1:600 in 0.1 M PB; Jackson ImmunoResearch) for 2 h at room temperature. The sections were washed three times in 0.1 M PB, mounted on gelatin-coated slides, dried, and coverslipped with DPX (Aldrich).
To identify CCR2-positive cells in the skin, DRG, and sciatic nerve, F4/80 (1:100; Serotec) was used as a monocyte/macrophage marker. For cells expressing CCR2 in the spinal cord, either the neuronal markers, MAP-2 or synaptophysin (both 1:200; Sigma) or glial markers for astrocytes (glial fibrillary acidic protein; 1:20,000; Sigma), oligodendrocytes (CNPase; 1:25; Chemicon), and microglia (OX-42; 1:4,000; Cedarlane Laboratories) were used. Phospho p38 mitogen-activated protein kinase (pp38; 1:200; Santa Cruz Biotechnology) was used as a marker for glial activation. Double-labeling studies with monoclonal antibodies in mouse spinal cord presented very poor staining; therefore, rat spinal cord was used for those studies (Fig. 3 F–I). The secondary antibody was Cy-2-conjugated goat anti-mouse IgG (1:600 in 0.1 M PB; Jackson ImmunoResearch). Images were acquired by use of a Nikon microscope (Microphot-FXA) with a Spot camera (Diagnostic Instruments, Sterling Heights, MI), and montages were created in PHOTOSHOP version 6.0 (Adobe Systems, Mountain View, CA).
Fig. 3.
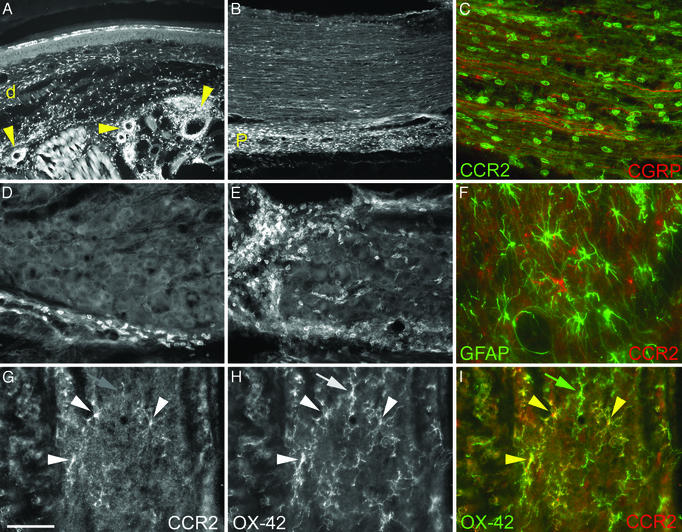
CCR2 expression after chronic injury in the skin (A), sciatic nerve (B and C), DRG (D and E), and dorsal horn of the spinal cord (F–I). Numerous monocytes/macrophages express CCR2 in the skin dermis (d) and around blood vessels (arrows in A) 2 days after CFA injection into the mouse hindpaw. Fewer CCR2 monocytes/macrophages are found in the sciatic nerve (not shown) and the DRG (D) after CFA. Conversely, abundant CCR2 cells are found both in the perineurium (P) (B) and surrounding axons (as seen with CGRP-LI; C) in the sciatic nerve and the DRG (E). Nerve injury induced the activation of both astrocytes and microglia in the spinal cord. However, CCR2-expressing cells do not colocalize with astrocyte marker (glial fibrillary acidic protein; F) but colocalize with microglia marker (OX-42; arrowheads in G–I) in the dorsal horn of the spinal cord. Not all microglia express CCR2 (green arrow in I). (Bar in G = 100 μmin A and B;25 μmin D and E; and 10 μm in C and F–I.)
Results
CCR2-/- mice did not exhibit any impairment of motor coordination. Thus, retention times using the rota-rod test were 23.6 ± 2.4 s for CCR2-/- mice and 24.1 ± 3.8 s for CCR2+/+ mice (t test, P = 0.89, n = 18–19 per group).
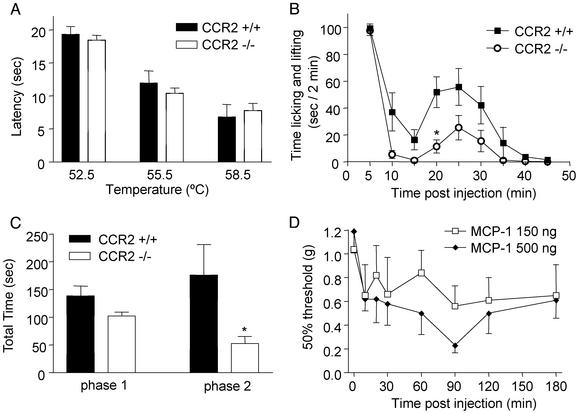
Acute Nociception. In the hot-plate test (Fig. 1A), no differences in latency period were found at the three tested temperatures (52.5, 55.5, and 58.5°C) between the two groups of mice (ANOVA P = 0.675 for “group,” n = 9 per group). CCR2-/- mice displayed a markedly attenuated behavior, compared with CCR2+/+ mice, in their responses to formalin injection (Fig. 1 B and C). Thus, phase 1 (0–10 min) responses were decreased by 24% (not significant) in the CCR2-/- mice compared with the CCR2+/+ mice, and phase 2 (15–50 min) responses were significantly (P = 0.0285; n = 9 per group) decreased by 70% in the CCR2-/- mice compared with CCR2+/+ mice. Paw edema, measured 90 min after formalin injection, was not different in the two groups. Furthermore c-Fos expression in the lumbar spinal cord after formalin injection was only modestly reduced by 20% (not significant, n = 7–8 per group) in CCR2-/- mice compared with CCR2+/+ mice.
Fig. 1.
Nociceptive responses to thermal or chemical stimulations. (A) Licking or jumping latency in the hot-plate assay; no differences between the two groups of mice. (B) Duration of licking and lifting in response to intraplantar formalin injection is significantly reduced in the homozygous mutant as compared with wild-type mice. (C) Area under the curve in B showing slight reduction in phase 1 (0–10 min) but a significant decrease in phase 2 (15–50 min) in mutant as compared with wild-type mice. (D) Mechanical allodynia after intraplantar MCP-1: maximal allodynia is detected 90 min after the injection. *, P < 0.05.
The effects of intraplantar injection of MCP-1 (150 and 500 ng) on mechanical allodynia were assessed in C57BL/6 mice (Fig. 1D). At a dose of 150 ng, moderate allodynia (20–40% decrease in mechanical threshold) was observed. However, 500 ng of MCP-1 significantly decreased mechanical threshold (Kruskal–Wallis followed by Dunn's test, P < 0.01; n = 7–9 per group; Fig. 1D).
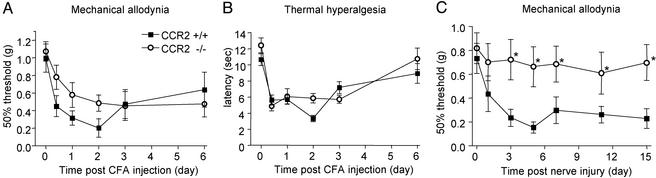
Persistent Pain States. After inflammation induced by CFA administration, CCR2 knockout mice developed attenuated mechanical allodynia as compared with the wild-type group (n = 15–16 per group). This decreased response (20–30%, not significant) was observed from 6 h to 2 days after CFA (Fig. 2A). No differences between genotypes were evident in the development of thermal hyperalgesia (Fig. 2B).
Fig. 2.
Nociceptive responses after two types of chronic pain: inflammation (A and B) and nerve injury (C). (A) Withdrawal threshold to mechanical stimulation (von Frey): mutant mice show a nonsignificant reduction in mechanical allodynia after CFA. (B) Paw withdrawal latencies to noxious thermal stimuli: no differences between the two groups. (C) After nerve injury, mutant mice did not display mechanical allodynia. *, P < 0.05.
Development of mechanical allodynia is characteristic of the response to nerve injury. CCR2+/+ mice showed a significant (Kruskal–Wallis P < 0.001, followed by Dunn's test) decrease in mechanical threshold starting 3 days after surgery until the last time point tested, 2 wk after the nerve ligation (Fig. 2C). In contrast, CCR2-/- mice did not develop mechanical allodynia after partial sciatic nerve injury. Mechanical thresholds in CCR2-/- mice were equivalent before and after nerve injury (P = 0.96). Furthermore, mechanical thresholds were significantly (Kruskal–Wallis followed by Dunn's test, P < 0.001 at days 3, 5, 7, 11, and 15) different between CCR2-/- and CCR2+/+ mice at all time points except baseline and day 1.
CCR2 mRNA Regulation. To investigate where in the pain-processing pathway CCR2 might be implicated, real-time PCR was performed in various tissue after CFA and nerve injury of C57BL/6 mice. Basal levels of mCCR2 expression could be detected as indicated by threshold cycle number values ranging from 33.7 to 28.2. As expected, a large increase in CCR2 mRNA expression was found in the paw skin after CFA injection, whereas levels in the sciatic nerve and spinal cord increased only 2-fold (Table 1). After nerve injury, CCR2 mRNA up-regulation in the sciatic nerve and DRG was rapid, marked, and sustained; in the paw skin, there was a transient up-regulation of CCR2 mRNA after ligation, and no change was detected in the spinal cord (Table 1).
Table 1. CCR2 mRNA in various tissues during chronic pain states.
| CFA
|
Nerve injury
|
||||||
|---|---|---|---|---|---|---|---|
| 2 days | 2 days | 4 days | 1 wk | 2 wk | 3 wk | 4 wk | |
| Paw skin | 21.1±4.7 | 4.8±0.2 | 2.8±0.2 | 1.5±0.1 | 1.9±0.2 | 0.8±0.1 | 1.0±0.1 |
| Sciatic nerve | 2.4±2.4 | 6.6±0.1 | 8.3±0.5 | 3.0±0.7 | 5.0±0.8 | 1.7±0.1 | 3.4±0.4 |
| DRG | 2.8±0.4 | 5.4±0.2 | 6.0±0.6 | 4.3±0.5 | 6.3±0.0 | 3.2±0.1 | 5.6±0.5 |
| Spinal cord | 0.5±0.1 | 1.4±0.1 | 1.4±0.1 | 1.1±0.7 | 0.5±0.1 | 0.9±0.1 | 0.6±0.1 |
Results are expressed as mean ± SD fold over control
CCR2 Protein Distribution. To confirm that mRNA changes reported by the PCR experiments were accompanied by changes in CCR2 protein as well as to determine which cell types express CCR2, immunohistochemistry was used to look at CCR2 distribution in various tissues 2 days after CFA injection and 1 wk after nerve injury. In the absence of inflammation or injury, only a few or no CCR2-like immunoreactive (-LI) monocytes/macrophages were observed. Consistent with the PCR data, in the CFA-inflamed paw skin, numerous monocytes/macrophages were CCR2 positive in the dermis and around blood vessels (Fig. 3A). Macrophages were identified by immunoreactivity for F4/80; approximately two-thirds of the F4/80-positive cells were CCR2-positive. No CCR2-positive cells in the skin were detected 1 wk after nerve injury. In the sciatic nerve (Fig. 3 B and C), after CFA, a few CCR2-positive macrophages were present in the perineurium only, whereas in the neuropathic model, numerous macrophages were detected not only in the neuroma but also distant from the neuroma, in the perineurium (Fig. 3B) as well as the endoneurium (Fig. 3C). In the DRG (Fig. 3 D and E), as observed in the sciatic nerve, a few CCR2-LI cells were detected in response to CFA administration (Fig. 3D). In contrast, and consistent with PCR data, numerous CCR2-LI macrophages were present after nerve injury both in the perineurium and surrounding neuronal cells (Fig. 3E). In the spinal cord after nerve injury, cells staining positive for CCR2 were identified as microglia (double labeled with OX-42; Fig. 3 G–I). CCR2-LI cells did not double label for neuronal, astrocyte (Fig. 3F), or oligodendrocyte markers. No CCR2-LI staining was detected on neurons in either the DRGs or the spinal cord.
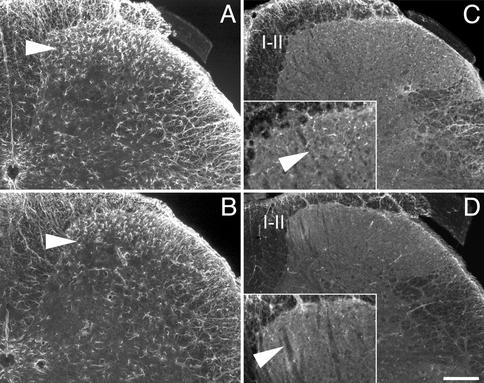
Because microglia were shown to express CCR2 in the spinal cord and because glial cells reportedly are activated during chronic pain states, astrocytes and microglia were compared in the CCR2-/- and CCR2+/+ mice 1 wk after partial nerve ligation. The number of astrocytes in the superficial laminae of the spinal cord was reduced in CCR2-/- as compared with CCR2+/+ mice (Fig. 4 A and B). Furthermore, activated p38 mitogen-activated protein kinase, as detected with a phospho-specific p38 antibody, was at lower levels in microglia of the CCR2 knockout mice as compared with wild type (Fig. 4 C and D).
Fig. 4.
Activated glial cells in the ipsilateral dorsal horn of the spinal cord 1 wk after partial sciatic nerve ligation. (A and B) The number of glial fibrillary acidic protein-positive cells was decreased (see arrows) in the superficial laminae in the CCR2-/- (B) as compared with the CCR2+/+ mice (A). Similarly, the number of microglia expressing phospho p38 mitogen-activated protein kinase (pp38, C and D) is lower in the CCR2 knockout mice (D) than in wild type (C). (C and D Insets) Higher magnification of laminae I and II. (Bar in D = 200 μm.)
Discussion
In the present report, we show that CCR2-/- mice did not develop the mechanical allodynia typically associated with neuropathy. In acute pain tests, responses of mice lacking CCR2 were not different from those of wild-type mice, whereas in models of inflammation, CCR2-/- mice showed attenuation of pain behavior in the formalin test and a slight reduction of mechanical allodynia after intraplantar CFA. Supporting the behavioral data, in wild-type mice, we found a greater number of CCR2-positive monocytes/macrophages in the sciatic nerve and DRG after neuropathy than after inflammation. We also found that activated microglia express CCR2 in the spinal cord after chronic injury. Thus, absence of pain behavior in CCR2- null mice after nerve injury might be a consequence of reduced macrophage infiltration resulting in slower Wallerian degeneration and, as a consequence, a decrease in sensitization of primary afferents and spinal cord neurons.
Neuropathic pain can be triggered by demyelination, a complex immune mediated process in which phagocytic macrophages play a key role, leading to peripheral and central hyperexcitability. Injury to the peripheral nerve initiates a complex cascade of signals involving neurons, glia, and cells of the immune system that leads to Wallerian degeneration [i.e., removal of axonal and myelin material (for review, see refs. 13 and 14)]. Wallerian degeneration is the inflammatory response of the nervous system to axonal injury, primarily attributable to the production of cytokines (e.g., TNF-α; IL-6, -1β, and -1α) that may further regulate macrophage recruitment, myelin removal, regeneration, and neuropathic pain. Inflammatory mechanisms are believed to play an important role in hyperalgesia resulting from nerve injury. Indeed, disturbance in the axon-myelin-Schwann cell unit is sufficient to induce macrophage recruitment, and it is widely accepted that this is the initial signal for the inflammatory reaction in peripheral nerve injury. Hyperalgesia after nerve injury is temporally linked with Wallerian degeneration, and macrophage recruitment is reduced in Wallerian degeneration mutant (Wld) mice, in which Wallerian degeneration is delayed (15). Expression of MCP-1 is induced by sciatic nerve axotomy in wild-type rodents but not in Wld mice (16). Our present results support the role of circulating monocytes/macrophages in Wallerian degeneration due to nerve injury (4) and as a result, development of neuropathic hyperalgesia. Macrophage depletion immediately after nerve injury could have some clinical potential in the prevention of neuropathic pain (17). More specifically, after nerve damage, Schwann cells attract macrophages by sequentially secreting cytokines: IL-6 and leukemia inhibitory factor (LIF), as well as MCP-1 (6). MCP-1 accounts for 60–70% of the chemotactic activity of Schwann cells and LIF, for the residual 30–40% (6). After nerve transection, MCP-1 mRNA levels peak 48 h after axotomy (18), consistent with the observation that after peripheral nerve damage, the major influx of macrophages begins 2–3 days after injury. The high potency and specificity of MCP-1 for monocyte recruitment, the correlation between its time course of induction and peak macrophage recruitment, and our present results further support chemokine involvement in neuropathic pain.
The contribution of CCR2 to neuropathic pain is also likely to take place at the level of the spinal cord. There, we did not detect monocytes or macrophages expressing CCR2, but we identified CCR2-positive microglial cells, which are the primary antigen-presenting cells in the CNS. Reports of neuronal CCR2 expression are not consistent, and variability between species and among investigators using antibodies from different sources is documented. However, CCR2 was found in the cerebral cortex in humans (19, 20) and rats (21). A more consistent finding, in the literature as well as with our data, is the expression of CCR2 in microglia (see references in ref. 20). Other chemokine receptors, CX3CR1, CXCR4, CCR4, and CCR5, have been identified on DRG neurons in culture (8).
Spinal cord glial cells, microglia, and astrocytes formerly were not considered to play a role in pain processing, because they were not thought to function in cell-to-cell signaling. However, microglia and astrocytes have been shown recently to be implicated in pathological pain such as s.c. inflammation, peripheral nerve injury, spinal root constriction, or bone cancer (for review, see ref. 22). Glia are functionally activated by substance P, excitatory amino acids (EAAs), and ATP, which are released by primary afferent fibers that terminate in the superficial laminae of the dorsal horn, and by NO, prostaglandins, and fractalkine, which are released by nociceptive spinal cord neurons. Once activated, glia release a variety of neuroactive substances (TNF, IL-1, IL-6, NO, EAAs, eicosanoids, and ATP) that will increase the excitability of nociceptive dorsal horn neurons. Microglia and astroglia are activated after nerve lesion, and expression of the activated phenotype correlates with the development of allodynic behavior (23, 24). Moreover, glial activation is necessary and sufficient to induce pain. Immune activation of glia by spinal administration of viral envelope proteins, such as glyco-protein 120, injected intrathecally, induces enhanced pain state via IL-1 and TNF-α release from activated microglia (7, 8).
Glial cells undergo a rapid and stereotypic pattern of activation in response to either central or peripheral nerve damage, that includes proliferation, decreased ramification, hypertrophy, and altered expression of surface molecules, growth factors, reactive oxygen species, proteases, neurotoxins, and proinflammatory cytokines (25). There is evidence that microglia express major histocompatibility complex class II (MHC II) proteins. Accordingly, they are able to function as antigen-presenting cells and form the interface between the neural parenchyma and the immune system (26). Activation of microglia to express MHC II and other phenotypic markers may be related to the presence of activated T cells arriving from the periphery. Activated microglia, especially the perivascular variety, may permit entry of and activation of memory T cells. MHC class II knockout mice exhibited attenuated allodynia after spinal nerve transection as compared with wild-type control mice (27). Persistent mechanical allodynia and enhanced spinal MHC class II and CD4 immunoreactivity are observed after peripheral nerve transection but not inflammation, suggesting that central neuroimmune activation may contribute to the maintenance of neuropathic pain after peripheral spinal nerve transection but not after a peripheral inflammatory insult (27).
We found a reduced number of activated astrocytes and microglia in the dorsal horn after chronic injury in CCR2-null mice compared with wild-type mice, consistent with the involvement of glial cell proliferation in the development and maintenance of pain states (22). The activation of glia is directly involved in inflammatory and neuropathic pain, because glial inhibitors exhibit antiallodynic properties. For instance, propentofylline, a glial modulating agent, attenuates nerve-injury-induced mechanical allodynia at doses that inhibit both spinal microglial and astrocytic activation (28). Very little information is available regarding the intracellular signal transduction mechanisms occurring in glial cells activated during chronic pain, but recent papers provide evidence that MAP kinases play a critical role (29–31). Activation of the p38 pathway plays an essential role in production of proinflammatory cytokines such as IL-1β, TNF-α, and IL-6, and induction of enzymes (cyclooxygenase-2, iNOS) (32). Our data showing a decreased number of pp38-positive microglia after nerve injury in the CCR2 knockout mice, coincident with blunted pain responses, support the involvement of MAP kinases in the cascade of mediators implicated in pain processing.
In conclusion, our results demonstrate significant roles for the chemokine receptor, CCR2, in neuropathic pain. In part and as anticipated, CCR2 is expressed at the site of injury, presumably to support macrophage recruitment and the demyelination processes. However, CCR2 is also present in the DRG and spinal cord, where macrophages and microglia release proinflammatory mediators that will sensitize primary afferents and spinal cord neurons. Therefore, chemokine receptors might constitute a new target for pain providing a nonneuronal site of action.
Acknowledgments
This work was supported by Merck Research Laboratories.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CCR2, chemotactic cytokine receptor 2; CFA, complete Freund's adjuvant; DRG, dorsal root ganglia; MCP-1, monocyte chemoattractant protein 1; -LI, -like immunoreactivity; TNF, tumor necrosis factor; PB, phosphate buffer.
References
- 1.Onuffer, J. & Horuk, R. (2002) Trends Pharmacol. Sci. 23, 459-467. [DOI] [PubMed] [Google Scholar]
- 2.Fife, B. T., Huffnagle, G. B., Kuziel, W. A. & Karpus, W. J. (2000) J. Exp. Med. 192, 899-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izikson, L., Klein, R. S., Charo, I. F., Weiner, H. L. & Luster, A. D. (2000) J. Exp. Med. 192, 1075-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma, M., Wei, T., Boring, L., Charo, I. F., Ransohoff, R. M. & Jakeman, L. B. (2002) J. Neurosci. Res. 68, 691-702. [DOI] [PubMed] [Google Scholar]
- 5.Siebert, H., Sachse, A., Kuziel, W. A., Maeda, N. & Bruck, W. (2000) J. Neuroimmunol. 110, 177-185. [DOI] [PubMed] [Google Scholar]
- 6.Tofaris, G. K., Patterson, P. H., Jessen, K. R. & Mirsky, R. (2002) J. Neurosci. 22, 6696-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milligan, E. D., O'Connor, K. A., Nguyen, K. T., Armstrong, C. B., Twining, C., Gaykema, R. P., Holguin, A., Martin, D., Maier, S. F. & Watkins, L. R. (2001) J. Neurosci. 21, 2808-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh, S. B., Tran, P. B., Gillard, S. E., Hurley, R. W., Hammond, D. L. & Miller, R. J. (2001) J. Neurosci. 21, 5027-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuziel, W. A., Morgan, S. J., Dawson, T. C., Griffin, S., Smithies, O., Ley, K. & Maeda, N. (1997) Proc. Natl. Acad. Sci. USA 94, 12053-12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hargreaves, K., Dubner, R., Brown, F., Flores, C. & Joris, J. (1988) Pain 32, 77-88. [DOI] [PubMed] [Google Scholar]
- 11.Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M. & Yaksh, T. L. (1994) J. Neurosci. Methods 53, 55-63. [DOI] [PubMed] [Google Scholar]
- 12.Seltzer, Z., Dubner, R. & Shir, Y. (1990) Pain 43, 205-218. [DOI] [PubMed] [Google Scholar]
- 13.Raff, M. C., Whitmore, A. V. & Finn, J. T. (2002) Science 296, 868-871. [DOI] [PubMed] [Google Scholar]
- 14.Koltzenburg, M. & Scadding, J. (2001) Curr. Opin. Neurol. 14, 641-647. [DOI] [PubMed] [Google Scholar]
- 15.Hall, S. M. (1993) J. Neurocytol. 22, 480-490. [DOI] [PubMed] [Google Scholar]
- 16.Carroll, S. L. & Frohnert, P. W. (1998) J. Neuropathol. Exp. Neurol. 57, 915-930. [DOI] [PubMed] [Google Scholar]
- 17.Myers, R. R., Heckman, H. M. & Rodriguez, M. (1996) Exp. Neurol. 141, 94-101. [DOI] [PubMed] [Google Scholar]
- 18.Toews, A. D., Barrett, C. & Morell, P. (1998) J. Neurosci. Res. 53, 260-267. [DOI] [PubMed] [Google Scholar]
- 19.Coughlan, C. M., McManus, C. M., Sharron, M., Gao, Z., Murphy, D., Jaffer, S., Choe, W., Chen, W., Hesselgesser, J., Gaylord, H., et al. (2000) Neuroscience 97, 591-600. [DOI] [PubMed] [Google Scholar]
- 20.van der Meer, P., Ulrich, A. M., Gonzalez-Scarano, F. & Lavi, E. (2000) Exp. Mol. Pathol. 69, 192-201. [DOI] [PubMed] [Google Scholar]
- 21.Banisadr, G., Queraud-Lesaux, F., Boutterin, M. C., Pelaprat, D., Zalc, B., Rostene, W., Haour, F. & Parsadaniantz, S. M. (2002) J. Neurochem. 81, 257-269. [DOI] [PubMed] [Google Scholar]
- 22.Watkins, L. R., Milligan, E. D. & Maier, S. F. (2001) Trends Neurosci. 24, 450-455. [DOI] [PubMed] [Google Scholar]
- 23.Coyle, D. E. (1998) Glia 23, 75-83. [PubMed] [Google Scholar]
- 24.Garrison, C. J., Dougherty, P. M., Kajander, K. C. & Carlton, S. M. (1991) Brain Res. 565, 1-7. [DOI] [PubMed] [Google Scholar]
- 25.Raivich, G., Bohatschek, M., Kloss, C. U., Werner, A., Jones, L. L. & Kreutzberg, G. W. (1999) Brain Res. Brain Res. Rev. 30, 77-105. [DOI] [PubMed] [Google Scholar]
- 26.Kreutzberg, G. W. (1996) Trends Neurosci. 19, 312-318. [DOI] [PubMed] [Google Scholar]
- 27.Sweitzer, S. M., White, K. A., Dutta, C. & DeLeo, J. A. (2002) J. Neuroimmunol. 125, 82-93. [DOI] [PubMed] [Google Scholar]
- 28.Sweitzer, S. M., Schubert, P. & DeLeo, J. A. (2001) J. Pharmacol. Exp. Ther. 297, 1210-1217. [PubMed] [Google Scholar]
- 29.Ji, R. R., Samad, T. A., Jin, S. X., Schmoll, R. & Woolf, C. J. (2002) Neuron 36, 57-68. [DOI] [PubMed] [Google Scholar]
- 30.Dai, Y., Iwata, K., Fukuoka, T., Kondo, E., Tokunaga, A., Yamanaka, H., Tachibana, T., Liu, Y. & Noguchi, K. (2002) J. Neurosci. 22, 7737-7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji, R. R., Befort, K., Brenner, G. J. & Woolf, C. J. (2002) J. Neurosci. 22, 478-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono, K. & Han, J. (2000) Cell Signalling 12, 1-13. [DOI] [PubMed] [Google Scholar]