Abstract
Mifepristone (RU486), which binds with high affinity to both progesterone and glucocorticosteroid receptors (PR and GR), is well known for its use in the termination of unwanted pregnancy, but other activities including neuroprotection have been suggested. Cerebellar organotypic cultures from 3 to 7 postnatal day rat (P3—P7) were studied to examine the neuroprotective potential of RU486. In such cultures, Purkinje cells enter a process of apoptosis with a maximum at P3. This study shows that RU486 (20 μM) can protect Purkinje cells from this apoptotic process. The neuroprotective effect did involve neither PR nor GR, because it could not be mimicked or inhibited by other ligands of these receptors, and because it still took place in PR mutant (PR-KO) mice and in brain-specific GR mutant mice (GRNes/Cre). Potent antioxidant agents did not prevent Purkinje cells from this developmental cell death. The neuroprotective effect of RU486 could also be observed in pathological Purkinje cell death. Indeed, this steroid is able to prevent Purkinje cells from death in organotypic cultures of cerebellar slices from Purkinje cell degeneration (pcd) mutant mice, a murine model of hereditary neurodegenerative ataxia. In P0 cerebellar slices treated with RU486 for 6 days and further kept in culture up to 21 days, the synthetic steroid increased by 16.2-fold the survival of pcd/pcd Purkinje cells. Our results show that RU486 may act through a new mechanism, not yet elucidated, to protect Purkinje cells from death.
Keywords: neurosteroids, neuroprotection, pcd mutant mice
The synthetic steroid mifepristone (RU486) has high affinity for both progesterone and glucocorticosteroid receptors (PR and GR) and exhibits potent antagonistic activity when bound to either receptor (1). RU486 has been used for the termination of unwanted pregnancy (1, 2), and as a tool to study mechanisms of steroid receptor function (3). However, several possible uses of RU486 may not involve the classical binding to nuclear receptors. Recent studies reported that RU486 could act as a neuroprotective agent against excitotoxicity and traumatic brain injury (4, 5). When primary cultures of hippocampal neurons or organotypic hippocampal slice cultures were subjected to oxidative stress, RU486 exerted a significant protective effect. McCullers et al. (5) showed a protective effect of RU486 on hippocampal neurons, after traumatic brain injury in rats. These authors showed a protective effect on CA1 neurons but not on dentate gyrus ones, although they express GR, suggesting that GR antagonism is not the only protective mechanism involved.
The present study was designed to test whether RU486 could prevent the death of Purkinje cells observed in organotypic slice cultures of postnatal rat and mice cerebella and in a mutant strain mouse displaying a cerebellar neurodegenerative phenotype. We have indeed previously shown that Purkinje cell death by apoptosis in organotypic culture is an age-dependent process. Purkinje cells survive when the explants are taken from postnatal day 0 (P0) or P10 animals. In contrast, they undergo a massive death if the explants are taken from P1 to P7 animals, with a peak at P3 (6–8). Here, we demonstrate that this Purkinje cell death in cerebellar slices is markedly prevented by RU486. This neuroprotective activity could reflect anti-PR, anti-GR (1), as well as antioxidant actions of RU486 (4). We have therefore combined both pharmacological and genetic approaches to analyze the pathways involved in this neuroprotective ability of RU486. Using both agonists and antagonists of GR and PR, PR mutant mice (PR-KO) and brain specific GR mutant mice (9), as well as potent antioxidant agents we show that the neuroprotective activity of RU486 is independent of its antagonistic properties at PR or GR, and that it is not related to an antioxidant activity. A model of neurodegenerative cell death, i.e., Purkinje cell degeneration (pcd), an autosomal recessive mutation in the mouse, causing the death of all cerebellar Purkinje cells between the second and fourth postnatal weeks (10, 11), was also used in this study. We demonstrate that the steroid RU486 highly promotes survival of Purkinje cells in organotypic cultures of cerebellar slices from newborn (P0) pcd mutants mice.
Materials and Methods
Slice Cultures. Sprague–Dawley Rats, (Janvier, Le Genest St. Isle, France) of P3, P5, and P7 and mice lacking either the progesterone receptor (PR-KO, kindly provided by B. O'Malley, Houston, TX) or the GR, specifically in the brain, (GRNes/Cre mice; ref. 9) of P3 were used. The GRNes/Cre mutant mice were developed by using the Cre/LoxP system. The brain specificity of GR inactivation was achieved by the expression of Cre, in neuronal and glial progenitor cells, under the control of the nestin promoter/enhancer. Cerebellar parasagittal slices (350 μm thick) were cut on a McIlwain tissue chopper and transferred onto membranes of 30-mm Millipore culture inserts with 0.4 μm pore size (Millicell, Millipore). Slices were maintained in culture in six-well plates containing 1 ml of medium at 35°C in an atmosphere of humidified 5% CO2. The medium was composed of 50% basal medium with Earle's salts (Invitrogen), 25% Hanks' balanced salt solution (Life Technologies), 25% horse serum (Life Technologies), l-glutamine (1 mM), and 5 mg/ml glucose (6, 7, 12).
Newborn (P0) mice of three litters issued of the mating of heterozygous pcd mutant mice (10, 11) were also used in this study. Three independent pcd mutations arose spontaneously, but, although the gene Nna1 has been identified as the mutated pcd gene (13), only two pcd strains can be identified by PCR. Unfortunately, the line we have access to is the third one. Slices from each (pcd) cerebellum were separated into two groups. One group served as control and was kept in culture for 4 wk. The other was treated with 20 μM of RU486 during the first week of culture and then maintained in culture for three more weeks [28 days in vitro (DIV)]. From the three litters studied, we distinguished slices, taken from 4 pups out of 15, with very low Purkinje cell survival (LPS), and the other slices with medium Purkinje cell survival (MPS). The Mendelian distribution of the two subgroups (25% with lower survival and 75% with median survival) suggests that the first subgroup was homozygous (Hz) and the second one comprised both heterozygous (Htz) and WT mice.
For each experiment point, at least 18 cerebellar slices from three independent animals were used in three independent experiments.
Chemicals. The principal steroids and chemical compounds used were: RU486 (mifepristone: 17β-hydroxy-11β-(4-dimethyl-amino-phenyl)-17α-(prop-1-ynyl)-estra-4,9-dien-3-one), progesterone (PROG, Sigma), 5α-dihydroprogestrone (5α-DHP, Sigma), 3α,5α-tetrahydroprogestrone (3α,5α-THP, Sigma), promegestone (R5020, a synthetic progesterone-receptor agonist, Perkin–Elmer (NEN)), estradiol (Sigma), corticosterone (Roussel-UCLAF), dexamethasone and dexamethasone 21-mesylate (Steraloids, Wilton, NH). We also used several antioxidant compounds such as: N-acetyl-L-cysteine (NAC, Sigma), reduced glutathione (GSH, Sigma), vitamin E (VitE, Sigma), and MnTBAP [Mn(III)tetrakis(4-benzoic acid) porphyrin chloride, a cell-permeable superoxide dismutase (SOD) mimetic and peroxynitrite scavenger; Calbiochem].
Doses with maximal efficiency were 1–50 μM for all compounds, except for NAC (5 mM) and MnTBAP (200 μM). Cerebellar slices were exposed to these compounds the first day of culture, and maintained for 5DIV. Media with the different steroids or drugs were replaced every 2 or 3 days.
Antibodies and Staining Procedures. Rabbit polyclonal antibody against calbindin (CaBP, diluted 1/10,000, Swant, Bellinzona, Switzerland) was used to visualize Purkinje cells. The first antibody was revealed with a secondary goat anti-rabbit CY3-labeled antibody (1/200 dilution; Jackson ImmunoResearch). Staining procedures were performed as described (6, 7).
RIA of Corticosterone. Corticosterone levels were measured in the cerebellum and in the adrenal cortex of P3 rat pups as well as in RU486- or vehicle-treated P3 cerebellar slices, cultured for 12 h (when the majority of Purkinje cells are engaged in the apoptotic process; ref. 7). Tissue samples were homogenized, and steroids were extracted in 10 ml methanol. To estimate steroid recovery during extraction, 2,000 dpm of [1,2,6,7-3H]corticosterone [specific activity, 70 Ci/mmol (1 Ci = 37 GBq); NEN] were added to each sample in 100 μl of methanol. Corticosterone concentrations in aliquots of the organic extracts were measured by corticosterone RIA (14), by using an antiserum to corticosterone (1/15,000 dilution, gift of Roussel-UCLAF). The sensitivity of the assay was 8 pg.
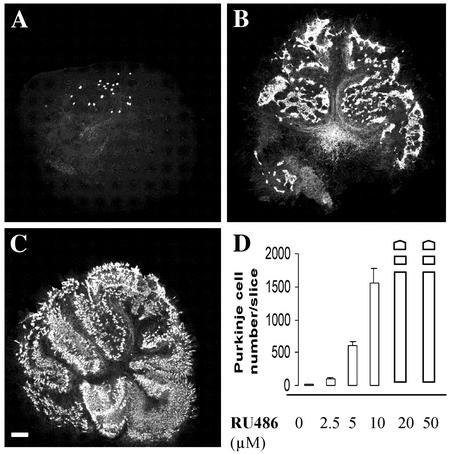
Quantification of Purkinje Cell Survival. To determine the Purkinje cell survival in the cultures, the neurons were immunostained with the anti-calbindin antibody and counted under a fluorescence microscope (Zeiss Axiovert 135M). Under these conditions, we counted the total number of surviving Purkinje cells per slice, and we calculated the means. Slices with <2,000 Purkinje cells were counted and indicated in Fig. 1 as full columns. Slices containing >2,000 Purkinje cells were represented by broken columns (see Fig. 1). Images of the immunostained Purkinje cells in organotypic slice cultures of rat and mice cerebella were acquired by using an image analyzing system, confocal Zeiss LSM 410.
Fig. 1.
RU486 prevents Purkinje cell death in organotypic slice cultures of rat cerebellum. Slices from P3 rats were cultured for 5 DIV. (A) Slices were treated with different concentrations of RU486 (0–50 μM) and were immunostained with anti-calbindin antibody to label Purkinje cells. Very few Purkinje cells were present in nontreated slices (A). However, slices treated with 10 μM RU486 (B) or with 20 μM RU486 (C) contained large numbers of Purkinje cells. (D) Quantitative analysis of Purkinje cell survival after treatment with increasing concentrations of RU486. Whole columns represent mean numbers of Purkinje cells ± SEM per slice, when slices contained no more than 2,000 Purkinje cells. When there were more than 2,000 Purkinje cells, they were represented by broken columns. (Scale bar = 250 μm.)
Results
RU486 Protects Purkinje Cells from Death in Organotypic Culture of Rat Cerebellum. In previous studies, we had shown that Purkinje cells die by apoptosis in organotypic slice cultures of rat and mouse cerebella, taken between P1 and P7. This apoptotic Purkinje cell death peaks in slices taken at P3 (6, 7) and kept in culture for 5 DIV (Fig. 1). Therefore, we chose P3 rat cerebellar cultures kept for 5 DIV to investigate whether RU486 may protect Purkinje cells from apoptosis. P3 rat cerebellar slices were treated with incremental doses of RU486 (2.5, 5, 10, 20, and 50 μM). Purkinje cell survival was assessed, after calbindin immunostaining, by counting the number of labeled Purkinje cells per slice. RU486 had a dose-dependent protective effect. In slices treated with 5 μM and 10 μM RU486, we counted, respectively, 40 times and 104 times more Purkinje cells compared with nontreated slices (Fig. 1). Purkinje cell survival was even larger when 20- to 50-μM RU486 concentrations were used (Fig. 1). We observed a similar enhancement of Purkinje cell survival when P5 and P7 cerebellar slice cultures were treated with RU486 (Fig. 2 A–B′). Thus, RU486 protects Purkinje cells from the apoptotic process observed in cultures of cerebellar slices obtained from young rats.
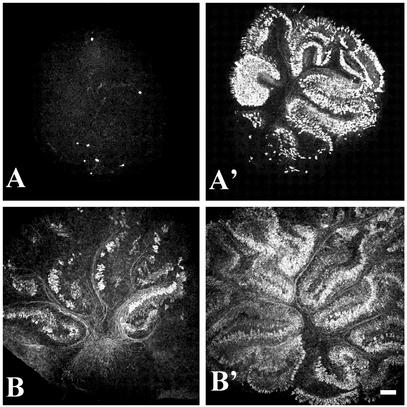
Fig. 2.
Effects of RU486 on Purkinje cell survival in P5 and P7 rat cerebella. Organotypic slices (suppress cultures) of P5 and P7 rat cerebella (A and B, respectively) were cultured for 5 DIV in the absence (A and B) or presence (A′ and B′)of20 μM RU486. At all ages, RU486 protects Purkinje neurons from cell death. (Scale bar = 250 μm.)
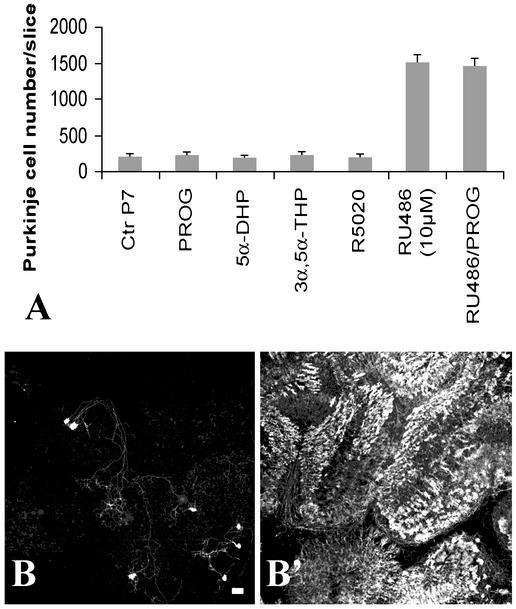
Progesterone and Progestins Are Not Protective and RU486 Action Does Not Involve Nuclear Progesterone Receptors. RU486 binds to PR with potent antagonist activity. To investigate whether PR agonists might block the RU486 effect on Purkinje cell survival, PROG and R5020 were tested. We also tested the 5α-reduced metabolites of PROG (5α-DHP and 3α,5α-THP). This study was carried out on P7 cerebellar slices, the age at which the spontaneous apoptotic process affecting Purkinje cells is much less intense than at earlier ages, P3 and P5 (Fig. 2B and ref. 7), allowing us a more complete analysis of the presumptive effects of steroids on apoptosis and differentiation of Purkinje cells. As shown in Fig. 3A, none of the tested compounds provoked either a significant increase of cell death or a neuroprotective effect on Purkinje cells. Treatment of cultures with estradiol (up to 50 μM) also had no significant effect on Purkinje cell survival (data not shown). Moreover, the protective effect of RU486 was not influenced by the presence of PROG in the culture medium (Fig. 3A). PROG at 50 μM was not able to antagonize the survival effect of 10 μM RU486, suggesting that the neuroprotective effect of RU486 was not mediated by the PR. To corroborate these observations, we studied the effect of RU486 on cerebellar cells from mice lacking the PR (PR-KO; ref. 15). Cerebellar slices were taken from PR-KO mice at P3 and were separated into two groups: one group served as control (slices treated with vehicle) and the other group was treated with 20 μM RU486. The results shown in Fig. 3 B and B′) demonstrate that, even in homozygous PR-KO mice cerebellar slices (as determined by PCR, data not shown), the intensity of RU486-neuroprotective action was not decreased. Thus, the protective effect of RU486 was PR-independent.
Fig. 3.
The neuroprotective effect of RU486 is not mediated by the intracellular PR nor blocked by PROG. (A) Quantitative analysis of Purkinje cell survival on cerebellar slices from P7-rats treated with PROG (20 μM), 5α-dihydroprogesterone (5α-DHP, 50 μM), 3α,5α-tetrahydroprogesterone (3α,5α-THP, 50 μM), the PR agonist R5020 (20 μM), the PR antagonist RU486 (10 μM) together with PROG (50 μM), or with RU486 (10 μM) alone. Control cultures (CtrP7) were exposed to vehicle alone. Except RU486, none of these compounds modulated Purkinje cell death. (B and B′) Cerebellar slices prepared from P3 homozygous PR-KO mice were cultured for 5 DIV in the absence (B) or presence (B′) of 20 μM RU486. (Scale bar = 70 μm.)
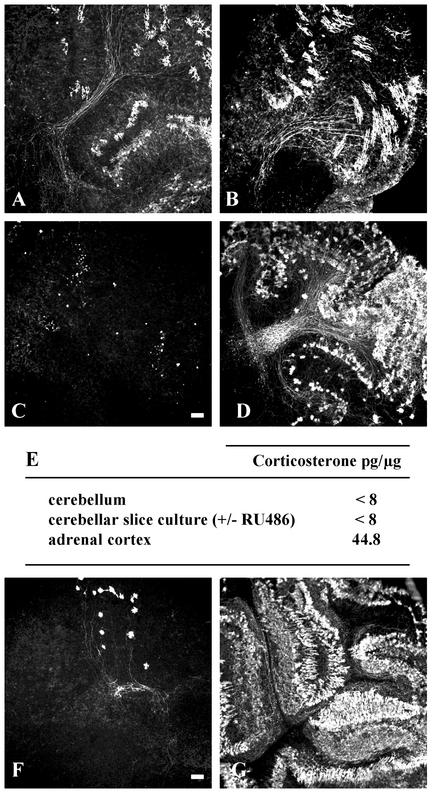
The Neuroprotective Effect of RU486 Is Not Mediated by Brain GR. RU486 is also a GR antagonist. Natural glucocorticosteroids, such as corticosterone, and their synthetic derivatives, such as dexamethasone, exert their effects through the GR. To determine whether glucocorticosteroids and GR are involved in Purkinje cell apoptosis, we treated P7 organotypic cerebellar slice cultures with different glucocorticosteroids separately or in combination with RU486. Incubating slices with dexamethasone provoked death of all of the Purkinje cells and completely abolished the neuroprotective effect of RU486 when culture media contained higher amounts of dexamethasone than of RU486 (Fig. 4C). In contrast, when its concentration was higher than that of dexamethasone, RU486 protected Purkinje neurons against both the neurotoxic effects of dexamethasone and apoptosis, suggesting a possible competitive effect between both compounds (Fig. 4D). These results evoked the possibility that the effect of RU486 could be mediated through GR. However, the natural glucocorticosteroid, corticosterone (50 μM), had no effect on Purkinje cell survival (Fig. 4B), and the presence of corticosterone did not block the neuroprotective effect of RU486. With RIA technique, corticosterone was detected neither in P3 rat cerebellum nor in cerebellar slices cultured in the presence or absence of RU486 (Fig. 4E). Thus, corticosterone (or glucocorticosteroids contained in horse serum) did not seem to be implied in this apoptotic death. In addition, in contrast to what was observed with RU486, another GR antagonist, dexamethasone 21-mesylate, did not show protective effect on Purkinje cells. When 20 μM dexamethasone 21-mesylate was used, no effect on Purkinje cell survival was observed in slices, after 5 DIV. The dexamethasone's effects may not yet be attributed to any known defined property of this synthetic steroid. Finally, we also tested the effect of RU486 on explants from GRNes/Cre animals in which the GR is inactivated specifically in neuronal and glial cells (9). Even in the absence of GR, RU486 exerted a powerful neuroprotection of Purkinje cells in slices obtained from P3 rats and mice (Fig. 4F). Taken together, these results demonstrated that programmed cell death of Purkinje neurons in organotypic cultures of P3 rat and mouse cerebella was blocked by RU486 and that this neuroprotective mechanism did not involve either PR or GR.
Fig. 4.
The neuroprotective action of RU486 is not mediated by glucocorticosteroid receptors. (A) Untreated cerebellar slices from P7 rats. (B–D) Cerebellar slices were treated with 20 μM corticosterone (B), with 1 μM dexamethasone or simultaneously with 20 μM dexamethasone and 20 μM RU486 (C), or with 10 μM dexamethasone and 20 μM RU486 (D). B corresponds also to the effect of another glucocorticosteroid antagonist, dexamethasone 21-mesylate, on Purkinje cell death in P7 slice cultures. (E) Corticosterone levels were measured in the cerebellum and in the adrenal cortex of P3 rat pups as well as in RU486- or vehicle-treated P3 cerebellar slices, cultured for 12 h, by using the RIA technique. Corticosterone was not detected, as its level was less than the sensitivity of the assay (8 pg). (F) Cerebellar slices from P3 GRNes/Cre mice were cultured for 5 DIV without RU486 treatment. (G) High survival of Purkinje cells in cerebellar slices from P3 GRNes/Cre mice, treated with 20 μM RU486. (Scale bar = 100 μm.)
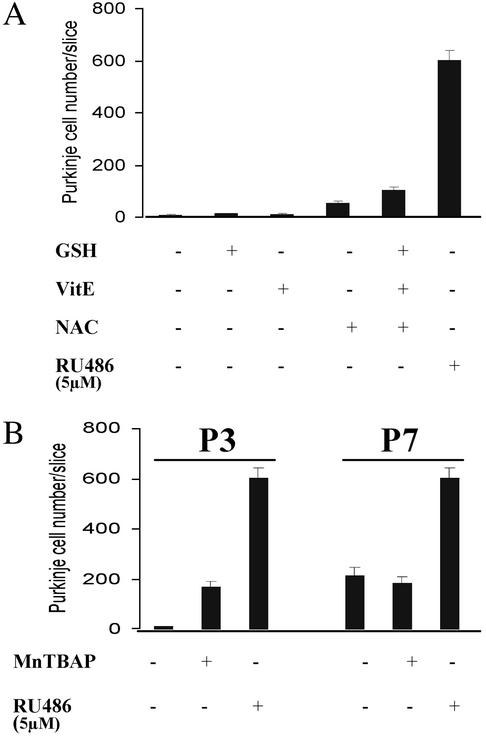
Effect of Antioxidants on Purkinje Cell Survival. To determine whether Purkinje cell apoptosis in organotypic culture results from oxidative stress and whether RU486 acts as an antioxidant to prevent this apoptosis, several other antioxidant molecules such as VitE, NAC, and GSH were used. None of these free radical scavengers could prevent apoptosis of Purkinje cells in P3–P7 cerebellar slices (Fig. 5A), even at very high concentration (i.e., 5 mM NAC). Concomitant use of these compounds was also without effect (Fig. 5A). Only when high concentrations (200 μM) of MnTBAP were used, a slight protection was observed in P3 slices (Fig. 5B). However, this compound could not protect Purkinje cells from apoptosis when P7 slices were used (Fig. 5B). We therefore conclude that oxidative stress was not responsible for the age-dependent Purkinje cell death in organotypic cultures of newborn rat cerebellum and that RU486 probably did not protect them by an antioxidant mechanism.
Fig. 5.
Purkinje cell death in organotypic slice cultures of postnatal rat and mouse cerebella does not result from oxidative stress. P3 cerebellar slices were treated with different antioxidant agents. (A) VitE (50 μM), NAC (5 mM), and GSH were used alone or in combination. (B) MnTBAP (200 μM), a cellpermeable superoxide dismutase (SOD) mimetic and peroxynitrite scavenger, was also used.
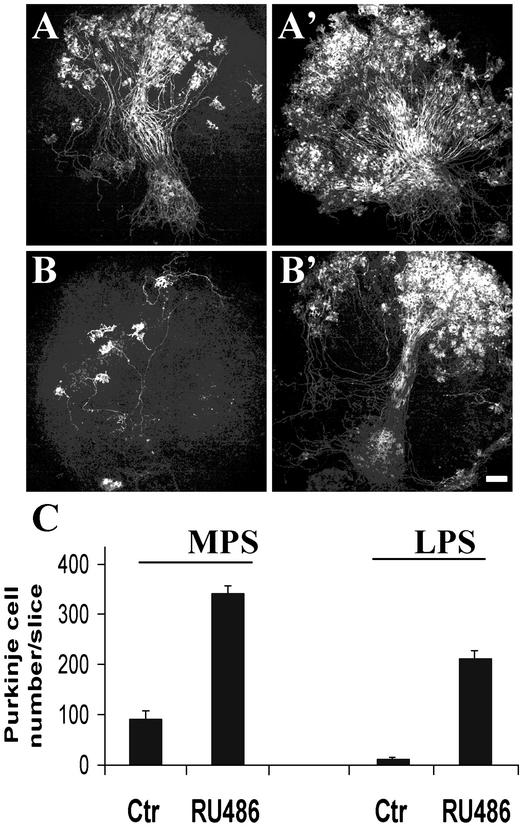
RU486 Prevents Purkinje Cell Apoptosis in Organotypic Cultures of Cerebellar Slices from pcd Mutant Mice. The Purkinje cell death that occurs in organotypic culture of postnatal rat and mouse cerebella was proposed to reflect the naturally developmental programmed cell death (7). To extend our observations, we wished to determine whether RU486 could exert a similar neuroprotective action in the cerebellar cultures of pcd/pcd mutant mice. This mutation is responsible for cerebellar hereditary ataxia because all Purkinje cells die between 2 and 4 wk of age (10). To determine whether Purkinje cells follow a similar death process in organotypic slice cultures than in vivo and particularly whether RU486 could prevent this degeneration, we compared Purkinje cell survival in slices from P0 mice, obtained by mating heterozygous (pcd) mice. In the homozygous (Hz) subgroup (25% of mice, see Materials and Methods), only a mean of 13 ± 3 of Purkinje cells survived per slice, with a maximum of 30 Purkinje cells (represented in Fig. 6C as LPS: low Purkinje cell survival). Whereas in the other subgroup (75% of mice, corresponding to WT and heterozygous mice), a mean of 94.18 ± 14.5 surviving Purkinje cells remained per slice (Fig. 6, medium Purkinje cell survival: MPS). Thus, after 4 wk in culture, Purkinje cells from pcd/pcd cerebella died in much greater proportions than in slices from WT and heterozygous mice (Fig. 6). In contrast, the addition of RU486 greatly enhanced Purkinje cell survival in all cerebellar slices (16.2- and 3.7-fold in homozygous and in WT-heterozygous subgroups, respectively; Fig. 6B). These experiments suggest that RU486 strongly promotes survival of Purkinje cells in cerebellar slices from pcd mutant mice.
Fig. 6.
RU486 prevents Purkinje cell degeneration in cerebellar slice cultures from P0 pcd mutant mice. P0 cerebellar slices were not treated (control; A and B) or treated with 20 μM RU486 (A′ and B′) for 1 wk and maintained in culture for 3 more wk. In control slices, two subgroups were distinguished: a subgroup (25% of mice) with low Purkinje cell survival (LPS), likely corresponded to homozygous mice (B and B′), and the other subgroup with medium Purkinje cell survival (MPS), which could correspond to WT and heterozygous mice (A and A′). (Scale bar = 250 μm.) (C) Histograms illustrating the mean number of surviving Purkinje cells ± SEM per slice.
Discussion
Using different steroids, we observed that only RU486, an antagonist of PR and GR (1), has the property to prevent Purkinje cell death that normally occurs in organotypic cultures of rat and mouse cerebellar slices. This powerful neuroprotective effect has been demonstrated in two different conditions: (i) in the age-dependent apoptosis occurring in slices taken from P3 to P7 mouse and rat cerebella, and (ii) in the massive cell death that occurs in slices taken from the cerebellum of newborn pcd mutant mice.
In organotypic cultures, Purkinje neurons die by apoptosis when the explants are taken from mouse or rat newborns at P1–P7 (6, 7). It was proposed that this in vitro age-dependent cell death reflects the developmental cell death observed in vivo. It provides therefore a good model for studying neuroprotection. We have shown that RU486 is able to rescue a large number of Purkinje cells from apoptotic death in P3–P7 cerebellar slices cultured for 5 DIV. The mechanism that underlies neuroprotective effects of RU486 on Purkinje cells was first envisioned on the basis of antagonism displayed at PR and GR levels. Because RU486 is an antagonist of PR, we treated slices with PROG in an attempt to counteract RU486 neuroprotective effect, but we did not observe any effect of PROG on Purkinje cell survival. These results were confirmed by using mice in which the PR gene is inactivated (PR-KO; ref. 15). Even in cerebellar slices of homozygous mice, the intensity of RU486-neuroprotective action was not diminished. Thus, RU486 exerts its protective effect by a PR-independent mechanism. However, it could involve GR, because RU486 is also an antagonist of GR. Abnormally increased glucocorticosteroid levels have deleterious effects on a number of maturational processes (16), and corticosteroids possibly could potentiate the programmed cell death of Purkinje neurons observed in this culture model. Indeed, dexamethasone, a synthetic GR agonist, induces the death of all Purkinje cells in these slice cultures. When applied together with RU486, a competition between these two steroids was observed. Thus, it could be suggested that the Purkinje cell apoptosis was a consequence of glucocorticosteroid action (i.e., glucocorticosteroids contained in horse serum) and that the neuroprotective effect of RU486 was mediated through GR. However, in this study, corticosterone was detected neither in rat cerebellum at P3, nor in cerebellar slices cultured in the presence or absence of RU486. Moreover, adding corticosterone alone or together with RU486 to culture media did not affect Purkinje cell survival and did not counteract the neuroprotective effect of RU486. We concluded that natural glucocorticosteroids were not involved in Purkinje cell apoptosis.
As most glucocorticosteroid effects are mediated by GR via activation or repression of gene expression (for review, see ref. 17), we investigated whether the steroid RU486 could act via these receptors. It is unlikely that GR contributes to Purkinje cell degeneration in organotypic culture of postnatal rat and mice cerebella because the inactivation of this receptor in neural and glial cells did not prevent RU486 effects and because the GR antagonist dexamethasone 21-mesylate did not reproduce the RU486 effect. Behl et al. (4) also showed that the protective effect of RU486 against oxidative stress-induced neuronal death was independent of the presence and activation of GR or PR. Why, in our model, dexamethasone alters the effect of RU486 differently from corticosterone remains obscure. Our finding that the steroid RU486 does not act via its classical receptors to protect Purkinje neurons from apoptosis raises questions about the mechanisms involved in this effect.
Recently, the steroid RU486 has been shown to exert an antioxidant effect, preventing the in vitro oxidation of low-density lipoproteins in a cell-free system (18) and protecting neuronal cells against oxidative stress-induced death (4). However, in our system, powerful antioxidant molecules, such as NAC, VitE, GSH, and MnTBAP, did not prevent Purkinje cell death, suggesting that RU486 does not exert its neuroprotective effect via its antioxidant activity. Our results show that the Purkinje cell apoptosis, occurring after organotypic culture of rat and mouse cerebellum, which is thought to be related to naturally developmental programmed cell death (7), is not due to oxidative stress-induced cell death.
During our study, we also found that RU486 could exert a protection to Purkinje cells in organotypic cerebellar slices from Purkinje cell degeneration (pcd) mice. The latter exhibit an intense death of cerebellar Purkinje cells between the second and fourth postnatal week caused by an autosomal recessive mutation in the Nna1 gene (13). The ability of RU486 to restore neural survival was observed after exposure of cerebellar tissue to RU486 at P0, before the occurrence of Purkinje cell degeneration.
In summary, we report that RU486 protects Purkinje neurons from age-dependent cell death in organotypic slice cultures of rat and mouse cerebellum. The protective effect of RU486 is unrelated to its PR- and GR-antagonistic activities and to its antioxidant properties. Considering also the beneficial effects of RU486 in preventing Purkinje cell degeneration in cerebellar slice cultures from pcd mutant mice, this steroid could be considered for use in some neurodegenerative diseases.
Acknowledgments
We thank Dr. B. O'Malley for providing the PR-KO mice; P. Leclerc for image analyzis; Dr. F. Cadepond, Dr. C. Massaad, and C. Ibanez for helpful comments; and Bernard Eychenne and M. P. Morel for technical assistance. This work was supported in part by an Artemis-Fondation Nationale de Gerontologie grant (to E.-E.B).
Abbreviations: PR, progesterone receptor; PR-KO, PR mutant; GR, glucocorticosteroid receptor; Pn, postnatal day n; pcd, Purkinje cell degeneration; MnTBAP, Mn(III)tetrakis(4-benzoic acid) porphyrin chloride; NAC, N-acetyl-L-cysteine; DIV, days in vitro; PROG, progesterone; VitE, vitamin E; GSH, reduced glutathione.
References
- 1.Baulieu, E. (1989) Science 245, 1351-1357. [DOI] [PubMed] [Google Scholar]
- 2.Silvestre, L., Dubois, C., Renault, M., Rezvani, Y., Baulieu, E. E. & Ulmann, A. (1990) N. Engl. J. Med. 322, 645-648. [DOI] [PubMed] [Google Scholar]
- 3.Baulieu, E. (1987) J. Cell. Biochem. 35, 161-174. [DOI] [PubMed] [Google Scholar]
- 4.Behl, C., Trapp, T., Skutella, T. & Holsboer, F. (1997) Eur. J. Neurosci. 9, 912-920. [DOI] [PubMed] [Google Scholar]
- 5.McCullers, D. L., Sullivan, P. G., Scheff, S. W. & Herman, J. P. (2002) Neuroscience 109, 219-230. [DOI] [PubMed] [Google Scholar]
- 6.Dusart, I., Airaksinen, M. S. & Sotelo, C. (1997) J. Neurosci. 17, 3710-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghoumari, A. M., Wehrle, R., Bernard, O., Sotelo, C. & Dusart, I. (2000) Eur. J. Neurosci. 12, 2935-2949. [DOI] [PubMed] [Google Scholar]
- 8.Ghoumari, A. M., Wehrle, R., De Zeeuw, C. I., Sotelo, C. & Dusart, I. (2002) J. Neurosci. 22, 3531-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tronche, F., Kellendonk, C., Kretz, O., Gass, P., Anlag, K., Orban, P. C., Bock, R., Klein, R. & Schutz, G. (1999) Nat. Genet. 23, 99-103. [DOI] [PubMed] [Google Scholar]
- 10.Mullen, R. J., Eicher, E. M. & Sidman, R. L. (1976) Proc. Natl. Acad. Sci. USA 73, 208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landis, S. C. & Mullen, R. J. (1978) J. Comp. Neurol. 177, 125-144. [DOI] [PubMed] [Google Scholar]
- 12.Stoppini, L., Buchs, P. A. & Muller, D. (1991) J. Neurosci. Methods 37, 173-182. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Gonzalez, A., La Spada, A. R., Treadaway, J., Higdon, J. C., Harris, B. S., Sidman, R. L., Morgan, J. I. & Zuo, J. (2002) Science 295, 1904-1906. [DOI] [PubMed] [Google Scholar]
- 14.Corpechot, C., Young, J., Calvel, M., Wehrey, C., Veltz, J. N., Touyer, G., Mouren, M., Prasad, V. V., Banner, C., Sjovall, J., Baulieu, E. E. & Robel, P. (1993) Endocrinology 133, 1003-1009. [DOI] [PubMed] [Google Scholar]
- 15.Lydon, J. P., DeMayo, F. J., Funk, C. R., Mani, S. K., Hughes, A. R., Montgomery, C. A. J., Shyamala, G., Conneely, O. M. & O'Malley, B. W. (1995) Genes Dev. 9, 2266-2278. [DOI] [PubMed] [Google Scholar]
- 16.De Kloet, E. R., Rosenfeld, P., Van Eekelen, J. A., Sutanto, W. & Levine, S. (1988) Prog. Brain Res. 73, 101-120. [DOI] [PubMed] [Google Scholar]
- 17.Reichardt, H. M., Tronche, F., Bauer, A. & Schutz, G. (2000) Biol. Chem. 381, 961-964. [DOI] [PubMed] [Google Scholar]
- 18.Parthasarathy, S., Morales, A. J. & Murphy, A. A. (1994) J. Clin. Invest. 94, 1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]