Abstract
During embryonic development, gonadal steroid hormones (androgens and estrogens) are thought to organize the sexual differentiation of the brain in the heterogametic sexes of higher vertebrates (males in mammals, females in birds). Brain differentiation of the homogametic sexes is thought to proceed by default, not requiring sex hormones for sex-specific organization. In gallinaceous birds such as the Japanese quail, female brain organization is thought to develop via estrogen-dependent demasculinization of a default male brain phenotype. We performed male donor-to-female host (MF), female-to-male (FM), male-to-male (MM), and female-to-female (FF) isotopic, isochronic transplantation of the forebrain primordium in Japanese quail embryos before gonadal differentiation had occurred; brain chimeras had a forebrain (including the hypothalamus) originating exclusively from donor cells. MM, FF, and MF chimeras all showed sexual behavior governed by the genetic sex of the host. In contrast, FM chimeras (genetically female forebrain, all other tissues genetically male) showed no mounting and only rudimentary crowing behavior. Although MM, FF, MF, and FM chimeras all showed host-typical production of steroid hormones during embryonic life, only FM chimeras were hypogonadal, had atypical low levels of circulating testosterone in adulthood, and showed reduction (crowing) or absence (mounting) of reproductive behaviors. Morphological features of the medial preoptic nucleus (a sexually dimorphic brain area) also were not male-like in FM males. These data demonstrate a brain-intrinsic, genetically determined component that organizes the sex-typical production of gonadal hormones in adulthood and call for a reevaluation of the mechanisms underlying brain sexual differentiation in other higher-vertebrate species.
Sexual differentiation of the brain and behavior of higher vertebrates is thought to be a stepwise process. In the current canonical model, a primary genetic program determines the development of the gonad. Gonadal sex determination leads to sex-typical release of gonadal steroid hormones (androgens and estrogens) according to either a male or a female pattern. Epigenetic action of androgens and estrogens then specifies the male or female differentiation of the brain, which is initially monomorphic (1–3). Gonad-hormone-dependent organization of brain sex occurs in birds and mammals in the heterogametic sex (males in mammals, females in birds), whereas brain sex is thought to be default, not requiring gonadal hormones for sex-specific organization, in the homogametic sex (1–3). The “organizational” action of hormones leads to both sex-specific and sex-typical phenotypes (4, 5). Sex-specific phenotypes cannot be induced hormonally in adults of the opposite sex, whereas sex-typical phenotypes frequently are shown by only one sex but can occur spontaneously under certain physiological conditions and are hormone-inducible in adulthood in the opposite sex (5). To become overt, sexual phenotypes depend on the production of gonadal hormones via the hypothalamus–pituitary–gonad (HPG) axis in adulthood (4). The HPG axis also coordinates displays of sexual behavior with reproductive physiology. Sexual brain development therefore defines the ability of brain areas, including the neural part of the HPG axis, to respond to particular physiological signals in adulthood (6).
Doubts about the adequacy of the canonical concept of gonadal hormone-dependent determination of brain sex and behavior have been raised by recent work on the vocal system of the zebra finch (7–9), where partial sex reversal of the female gonad failed to induce a male-typical vocal control system (7, 8). Other examples suggesting brain-intrinsic sex-determining mechanisms include the sexually dimorphic development of midbrain dopaminergic neurons of rodents in vitro (10) and incomplete sex-reversals of behavior after hormone treatments during early development (11–15). These examples remain inconclusive because of the impossibility of exactly matching experimental procedures (the exact dosage or timing of endocrine treatments used to modify the hormonal environment during brain development) to individual variation in developmental processes. Endocrine manipulations of embryonic development also are just as likely to act on the brain as they are on the gonad and other steroid-sensitive organs, making it difficult to disentangle the roles played by the brain and gonad during brain sexual differentiation.
To circumvent these problems, this study used a nonendocrine manipulation involving the transfer of a male or female brain primordium to an undisturbed hormonal environment before the sexual differentiation of the gonad. Male donor-to-female host (MF), female-to-male (FM), male-to-male (MM), and female-to-female (FF) isotopic, isochronic transplantation of the brain primordium rostral to the otic capsules was performed on embryonic day 2 (E2) in Japanese quails. In these brain chimeras, the forebrain (including the hypothalamus) originates from the donor (16, 17). The primordia of the two other components of the HPG axis, Rathke's pouch (adenopituitary) and the gonadal anlage (gonad), are not transplanted and are of host origin. Normal sexual differentiation in Japanese quail has been studied extensively (18–20); the gonadal anlage starts to produce estrogens around E6 in females only. These estrogens direct the undifferentiated gonad to form an ovary (18, 19). Estrogen treatment of male quail embryos is demasculinizing, and inhibition of estrogen production of female embryos masculinizes their brain and behavior if treatments are before E14 (20). Ovarian estrogen production thus is thought to demasculinize default male brain development in females.
If male brain development is default and female development occurs in the presence of ovarian hormones, then female brains in male bodies should develop into male brains and male brains in female bodies should develop into female brains (host-typical development). Although embryonic gonad formation and embryonic hormone production were normal in the brain chimeras studied here, gonadally male Japanese quails with female brains did not develop entirely male-like neural and behavioral phenotypes.
Materials and Methods
Production of Chimeras. Japanese quail eggs were obtained from commercial sources and incubated within 24 h of laying in a forced-draft incubator at 37.5°C and 80% relative humidity, with periodic turning of the eggs. Experimental eggs were incubated for 36–45 h and transferred to small incubators in the operating room. The transplants were carried out between two quails as detailed previously for quail–chick chimeras (16, 17). All surgical operations consisted of isochronic, isotopic transplants of the entire neural tube rostral to the otic capsules. Of all chimeras produced, 52 survived at least until E10. A total of 17 chimeras hatched successfully. Fifteen of the hatched chimeras survived until posthatching day 50 for testing of adult sexual behavior. Experiments were in accordance with animal research protocol 211253140/92 of the Upper State of Bavaria, Germany.
Sex Determination. Because the transplants were done without knowledge of the genetic sex of the eggs, postmortem sex determination to identify the genetic sex of the host and donor tissue was performed by using DNA techniques and following published protocols (18, 19). For the animals killed between E11 and E13, the hypothalamic–preoptic area, the lower spinal cord, and the breast muscle were analyzed. Each tissue of each animal yielded either male or female results (there were no mixed or ambiguous cases). Spinal cord and muscle tissue were always of the same genetic sex. The genetic sex of the hypothalamic–preoptic tissue of seven animals (four FM and three MF) differed from the spinal cord and breast muscles. This control experiment shows that the transplants performed always resulted in a genetically homogenous forebrain including the hypothalamus, as expected from quail–chicken chimeras (16, 17).
The chimera type of the animals used for behavioral studies (n = 15) was analyzed postmortem by inspection of the gonads and DNA–sex determination. For the latter, we used the same procedure as above but replaced hypothalamic tissue with neostriatal tissue to allow morphometric analysis of the hypothalamus. In seven cases, the neostriatal sex differed from the other tissues. In total, four MF, three FM, five MM, and three FF chimeras were analyzed.
Behavior. Hatched animals were maintained in heated cages on a 16-h-light/8-h-dark photoperiod for 2 weeks and then raised in an aviary under the same gonadal stimulatory photoperiod. From posthatching day 50 on, the chimeras and the control animals were housed individually in the same photoperiod. Vocalizations were recorded with a computer-based system during 3 h per day for 5 successive days. To test male sexual behavior, each chimera and male control were introduced into the middle of an observation aviary (3 × 5 m in size), in which four adult females were housed. Animals were observed under these conditions for 1 h every second day for a period of 10 days. To test female sexual behavior, each chimera and control female were placed in the observation aviary together with an intact male for 1 h per day every second day for a period of 10 days.
Hormone Measurements. Measurements of testosterone and 17β-estradiol were taken with commercial radioimmunoassays (DPC Biermann, Bad Nauheim, Germany). From the adult chimeras and six adult male and female controls, blood samples were taken from the wing vein before killing. From juvenile chimeras (E11–E13) and six male (E12) and six female (E12) controls, blood samples were taken from the heart at killing. The detection limit was 30 pg/ml for 17β-estradiol and 45 pg/ml for testosterone. The intraassay variation was 5.3–10.2%.
PCR for Androgen Receptors. Brain primordia (n = 10) were collected at E2 for amplification of the quail androgen receptor by using a PCR procedure applied successfully in birds (22).
Neuroanatomy. All subjects were administered lethal doses of isofluorane before perfusion. Subjects were perfused by intracardial perfusion with 0.9% saline, followed by ice-cold 4% paraformaldehyde in 0.025 M PBS at pH 7.3, followed by ice-cold 10% sucrose in PBS. Brains were removed and immersed in 30% sucrose in PBS for 12 h. The brains were cut with a freezing microtome in 30-μm coronal sections and collected in PBS. One series of sections was immunostained for aromatase, one for gonadotropin-releasing hormone I (GnRH-I), and one for vasotocin under free-floating conditions, and then all were mounted on Superfrost slides. Immunostaining of aromatase, vasotocin, and GnRH-I followed published protocols employing the same antibodies (23–25). Control staining (using hypothalamic sections of other age-matched quails) omitting the primary antibody resulted in no cell labeling. The aromatase antibody was a gift from J. B. Hutchison (Cambridge University, Cambridge, U.K.), the GnRH-I antibody was a gift from P. Sharp (Roslin Institute, Edinburgh), and the vasotocin antibody was provided by E. R. de Kloet (Leiden University, Leiden, The Netherlands).
Quantitative measurements of the sexually dimorphic nucleus of the quail preoptic area (POM) were done with a Leica (Deerfield, IL) microscope connected to a computer-based image-analysis system (Imatec, Munich). Volume calculations of the POM were based on measurements of the area of each fifth section multiplied by the section thickness and intersection distance. The extent of the POM on each section was calculated by manually tracing the area, following its boundary as indicated on the aromatase-stained sections. Aromatase-immunolabeled neurons were counted in three sections per animal covering 300 μm of the POM just rostral to the anterior commissure. The subregion of the POM containing vasotocinergic fibers and cells was measured in the digitized sections adjacent to the aromatasestained sections as described (26). First, the outlines of the POM were superimposed on the digitized vasotocin-immunostained section. Then, the fraction of the immunostained total surface of the POM was calculated. Using a similar procedure, the number of GnRH-I-immunolabeled neurons in the POM (M2 of ref. 27) was counted.
Statistics. Data were checked for normality. Unpaired t test was used for comparisons of the developmental data. Parametric ANOVA followed by Tukey's honestly significant difference post hoc test was used for comparison of all adult data sets. Data are presented as mean ± SE.
Results
Gonad Development and Hormone Production of the Chimeras. As detailed in Materials and Methods, all chimeras developed gonads according to the genetic sex of the host. Hormone measurements of embryonic chimeras and age-matched controls resulted in testosterone (male, 112 ± 51 pg/ml; female, 146 ± 69 pg/ml; MF, 101 ± 34 pg/ml; FM, 92 ± 18 pg/ml) and 17β-estradiol (undetectable in all genetic males; females, 86 ± 31 pg/ml; MF, 95 ± 24 pg/ml) plasma levels that were similar between control males and FM chimeras (t test, P = 0.131) and control females and MF chimeras (t test, P = 0.182). Hormone values were in the range previously published for quail embryos (28). These data indicate normal autogenous production of gonadal steroids, indicating normal gonadal differentiation in chimeric embryos. PCR amplification of the androgen receptor in the brain primordium at the time of transplantation was negative.
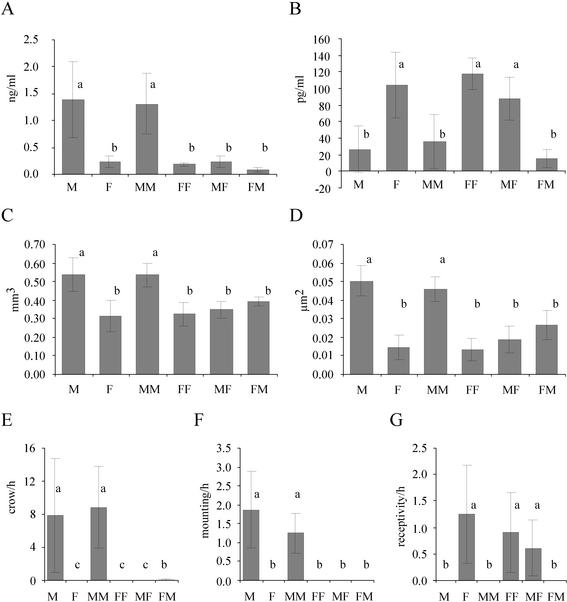
Adult FM chimeras had very small testes (113 ± 36 mg) compared with control (4,510 ± 421 mg) and MM (4,248 ± 287 mg) males (ANOVA followed by Tukey's test, P < 0.0001) whereas MF females developed ovaries and three of four MF females laid fertile eggs. The ovary of the fourth MF chimera, which did not lay eggs, did not contain developed follicles at the time of killing. The adult FM males produced significantly lower levels of circulating testosterone compared with control and MM males (ANOVA followed by Tukey's test, P < 0.013). Although 17β-estradiol levels of control, FF, and MF females (P > 0.19) were similar, they differed from FM males (P < 0.001) (Fig. 1 A and B).
Fig. 1.
Diagrams of the testosterone plasma levels (A), the 17β-estradiol plasma levels (B), the volume of the POM (C), the area of vasotocin-immunostained structures of the POM (D), crowing activity (E), mounting attempts (F), and the receptivity (G) of male controls (M), female controls (F), MM chimeras, MF chimeras, FF chimeras, and FM chimeras in adulthood. Similar letters indicate statistical similarity between these groups. Different letters indicate significant differences between the groups. For statistical details, see the text. FM males differed from other males in testosterone production, POM volume, vasotocin staining in the POM, crowing activity, and mounting behavior. FM males differed from females in estradiol production, crowing activity, and receptivity. Data are means ± SE.
Brain Differentiation of the Chimeras. The POM is involved in the control of appetitive and consummatory (e.g., mounting) male sexual behavior (30). The POM volume and number of aromatase-containing neurons are sex-typical characteristics in adult, reproductively active quails (31–33). Testosterone induces sexual dimorphism in both features in adulthood via its androgenic and estrogenic metabolites (32). The POM volume of MF chimeras was similar to that of FF and control females (ANOVA followed by Tukey's test, P = 0.35) but different from control males and MM chimeras (P < 0.00012) (Fig. 1C). FM males differed from control and MM males (P < 0.002) but not from control and FF females and MF females (P = 0.19) (Fig. 1C). Because POM volume and the number of aromatase-immunolabeled POM neurons were highly correlated, aromatase data were corrected for POM volume for group statistical comparisons. The corrected number of aromatase-labeled cells in this case was similar between all groups (ANOVA, P > 0.1 for all comparisons). In summary, POM volume and number of aromatase-containing neurons were female-like in FM males (Fig. 2).
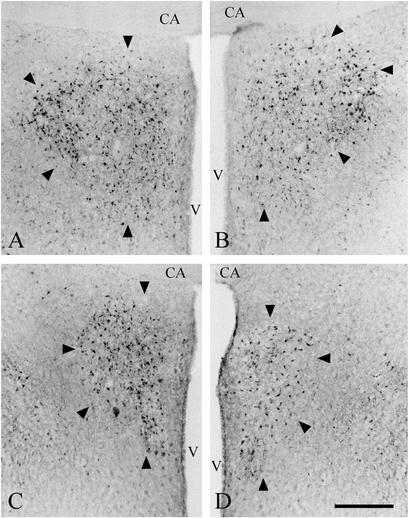
Fig. 2.
Photomicrographs of coronal sections of the aromatase-stained POM (arrowheads). The POM of a control male (A), of a MM chimera (B), of a FM chimera (C), and of a control female (D) are shown. All photomicrographs are taken at the level of the anterior commissure (CA). Note that the size of the POM of the FM male is similar to that of a control female but smaller than that of control and MM males. The POM of females and MM males had a smaller medial–lateral extension. Aromatase-stained neurons appear darkly. V, III ventricle. (Bar = 100 μm.)
The density of vasotocinergic fibers and neurons has been interpreted as an organizational, sex-specific feature of the quail POM that becomes overt after elevated testosterone secretion in adult males (33). Because POM size is positively correlated with the size of the subregion showing vasotocinergic staining, the latter were corrected for POM size for group statistical comparisons. The corrected vasotocin-immunostained area of FM chimeras was different from MM and male controls (ANOVA followed by Tukey's test, P < 0.001) (Fig. 1D). Comparison of FM with FF and female controls also resulted in a difference in the opposite direction (P < 0.03) that disappeared, however, after Bonferroni correction. All other chimeras had vasotocinergic staining of the POM that was similar to controls of the same genetic sex. Anecdotally, the one MF chimera that showed low sexual activity (see below) had the strongest vasotocin immunostaining in the POM of all females including controls and FFs.
GnRH-I is the main hypothalamic releasing factor that activates the adenopituitary in birds (27, 34, 35). The number of GnRH-I-immunolabeled neurons in the POM was similar in all groups (e.g., control males, 743 ± 187; FM, 663 ± 216; control females, 822 ± 142; ANOVA, P > 0.1 for all comparisons). A sex difference in the small number of GnRH-I-containing cells in the anterior lateral POM of the control animals (27) unfortunately was not apparent and could not be used to determine the GnRH-I phenotype of the FM chimeras.
Behavioral Differentiation of the Chimeras. Crowing is a testosterone-dependent, male-typical behavior; receptivity is an estrogen-dependent, female-typical behavior; and mounting is a testosterone-dependent, male-specific behavior (20). MM males behaved similar to control males (ANOVA followed by Tukey's test, P = 0.87) (Fig. 1 E and F). The MF females were as receptive as control and FF females (P = 0.21) (Fig. 1G). The one MF female that did not lay eggs was the least receptive. The three FM males neither courted females nor showed mounting attempts (Fig. 1F), nor were they receptive (Fig. 1G). The crowing activity of FM males was significantly lower compared with MM and control males (P < 0.0001) (Fig. 1F; two of these males crowed occasionally).
In summary, FM male, but not MF female, chimeras developed atypical sexual behavior. FM males showed neither male sex-specific behavior (mounting) nor female sex-typical behavior (receptivity) and only rudimentary sex-typical male behavior (crowing). At the physiological level, FM chimeras differed from adult control and MM males in gonad weight, testosterone production, POM volume, the number of aromatase-containing POM neurons, and the size of the vasotocinergic subregion of the POM. Furthermore, FM chimeras differed from adult control, FF, and MF females because of the presence of testes and low estrogen production. The likely consequence of this hypogonadism of the FM males is that both testosterone- and estrogen-dependent, sex-typical phenotypes are lacking. Whether the lack of sex-specific male phenotypes (POM area of vasotocinergic structures, mounting) of FM males results from a difference in the developmental organization of the POM or a difference in the organization of the HPG axis (see below) that leads to low adult testosterone production is unclear. The latter possibility is suggested by the more male-like vasotocinergic pattern of FM males compared with females, but this difference did not reach statistical significance. Future studies treating adult FM males with testosterone will be required to distinguish among these possibilities.
Discussion
These data show first that both male and female quail brains are competent to respond to a female (heterogametic) endocrine environment. Second, the female (heterogametic) quail brain does not have the full capacity to respond to the male (homogametic) environment. This latter finding requires special discussion. Adult FM males develop hypogonadism (small testis, low testosterone production), suggesting low levels of gonadotropin secretion. Although gonadotropin secretion represents the integrated response of both the hypothalamus and adenopituitary, the following discussion is focused on the hypothalamus because only this part of the HPG axis was changed in the chimeras.
The HPG axis controls gonadal growth and hormone production via hormone-dependent feedback loops and signals from the body and the external environment. Because body weight (data not shown) and environmental conditions were similar for MM and FM chimeras, we can exclude these as explanatory factors. Given the normal pattern of development and behavior seen in the MM, FF, and MF chimeras, technical problems resulting from the surgery also can be ruled out. Although the number of GnRH-containing neurons is known to increase in the POM of reproductively inactive quails (35, 36), there were similar numbers in FM and control males. Thus, the hypogonadism cannot be due either to a lack of GnRH neurons or to a general reproductive refractory state in FM chimeras but might reflect a functional HPG axis that is tuned to work in a low-level range, producing low levels of circulating testosterone. The following scenario is proposed to explain the FM phenotype as an organizational difference within the sex-specific HPG axis. An early, embryonic, sex-specific organization of the HPG axis is suggested by previous work in quail (35, 36), chickens (37), and mammals (38–42). The demonstration of pharmacological sex reversal in birds also may indicate an early organization effect on the HPG axis, leading to atypical gonadal steroid production (8, 43–45).
The HPG axis is certainly functional in quails by the time they hatch. GnRH-I neurons migrate from the olfactory placode into the hypothalamic–septal area of quail before E12 (46). At E10/E11, estrogen receptors (17), androgen receptors (unpublished results), and testosterone-metabolizing enzymes (47) are expressed in the quail hypothalamus. The only known early sex difference in quail (present at the onset of, or preceding, the embryonic period of sexual brain differentiation) is the much higher activity of steroid reductases in the hypothalamus of female quail (47). Reductases convert testosterone into nonactive androgens (5β-reduced androgens) or nonaromatizable androgens. The 5β-reductase pathway is thought to protect the brain against high levels of neural estrogen production (48). Likewise, 5β-reduced androgens are endogenous inhibitors of aromatase activity (48). The activity of 5β-reductase appears insensitive to gonadal hormones (49, 50).
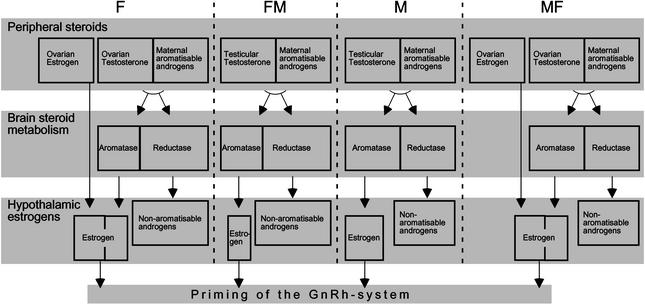
Assuming that the ontogenetic expression of the steroid reductases in the brain is determined genetically, the following scenario is suggested (Fig. 3). Female gonads make estrogen and testosterone. Male gonads make very little estrogen but some testosterone. To retain male brain organization, males need to convert testosterone to estrogen within the brain via aromatase activity (51). To retain female brain organization, females use their gonadal estrogens, as suggested in previous work with in ovo estrogen treatments (20), but shunt the production of additional estrogens in certain brain areas with high levels of steroid reductases in them. If a female brain is put into a male body, the aromatases can convert gonadal testosterone to estrogen in the brain, but the reductases in certain areas will shunt this estrogen into nonaromatizable products that also inhibit the activity of the aromatases (47). Local demasculinization therefore can occur in these areas from the lack of gonadal estrogens. The rest of the brain still will get enough estrogens from the aromatase to prevent demasculinization of pathways that do not contain sufficient reductase (in the circuit for crowing, for example). In adulthood, the demasculinized regions (including those leading to GnRH secretion) will respond according to a “female” pattern, releasing the female-appropriate pattern and dose of GnRH that is too small to support the reproductive activation of the male gonad.
Fig. 3.
Proposed brain-intrinsic mechanism of sexual differentiation of the HPG axis. Because of an innate higher activity of steroid reductases in the female brain, FM chimeras produce low levels of estrogens in the brain. This primes the GnRH system to regulate gonadal steroid production at a low working range. In females (F) and MF chimeras, ovarian estrogens compensate for this lower capacity of their embryonic brains to produce estrogens, whereas males' (M) embryonic gonads do not produce estrogens.
In agreement with this scenario, the male HPG axis controls circulating testosterone and the female HPG axis controls circulating 17β-estradiol, but the plasma concentrations differ 10-fold in adult male and female quails (Fig. 1 A and B), as well as in other birds (52) and mammals (53). In humans, the dose required to stimulate the secretion of gonadotropin is about 220-fold less for 17β-estradiol than for testosterone (54).
An alternative to this scenario is that the estrogen sensitivity of GnRH neurons or the circuits afferent to GnRH neurons is intrinsically different between males and females, and this difference does not require any organizing action by hypothalamic estrogens. The ultimate explanation for the phenotype of the FM chimeras also may involve a combination of these two mechanisms, which will require further research to elucidate fully.
The organization of the HPG axis to produce high levels of circulating estrogens is likely deleterious because of the effects of permanently elevated estrogen levels on nonneural body physiology such as immune function (55, 56). The high level of aromatizable androgens deposited in the yolk of bird eggs (57) would seem to require some mechanism to protect the developing brain from high neural estrogen production. Any explanation of the hyposexual development of FM males posits some kind of intrinsic sex difference in GnRH-producing hypothalamic circuits that has to respond to gonadal hormones to develop proper adult feedback control of the HPG axis. Indeed, the gonadal anlage (19) is not present in the quail embryos at the time of the transplants, and androgen (this study) and estrogen receptors (17) are not yet expressed in the brain primordium at this time.
The phenotype of the FM chimeras studied here suggests that there is no default brain sex, neither male nor female: male brains are male and female brains are female from the initiation of brain development. The epigenetic action of estrogens is necessary to retain (modulate) male sexual differentiation in males and female sexual differentiation in females.
Thus, the sexually dimorphic development of the vocal control area, nucleus hyperstriatalis ventrale, pars caudale (HVC), in the zebra finch no longer appears to be an isolated case (9). Further, sex reversal of gonads of female whiptail lizards does not lead to a sex reversal of limbic brain structures (58), and the higher expression of mouse genes located on sex chromosomes in female as compared with male mouse brains is suggestive (59). In humans, the development of transsexuality does not agree with the canonical concept of purely gonad-driven differentiation of brain and behavior (60). The existence of brain-intrinsic, genetically determined mechanisms of sexual differentiation remains to be verified in other vertebrate species, including humans.
Acknowledgments
I thank Dr. E. Balaban for teaching me the chimera technique and for his enthusiasm, Dr. A. ter Maat for help with the statistics, and S. Godsave for technical support. This work was done in part at the Max-Planck-Institut of Behavioral Physiology, Seewiesen, Germany.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HPG, hypothalamus–pituitary–gonad; En, embryonic day n; MF, male-to-female; FM, female-to-male; MM, male-to-male; FF, female-to-female; GnRH, gonadotropin-releasing hormone; POM, preoptic area.
References
- 1.Phoenix, C. H., Goy, R. W., Gerall, A. A. & Young, W. C. (1959) Endocrinology 65, 369-382. [DOI] [PubMed] [Google Scholar]
- 2.Whalen, R. E. (1968) in Reproduction and Sexual Behavior, ed. Diamond, M. (Indiana Univ. Press, Bloomington), pp. 303-340.
- 3.Jost, A. (1983) Psychoneuroendocrinology 8, 183-193. [DOI] [PubMed] [Google Scholar]
- 4.Breedlove, S. M., Cooke, B. M. & Jordan, C. L. (1999) Brain Behav. Evol. 54, 8-14. [DOI] [PubMed] [Google Scholar]
- 5.Gahr, M. (1994) in The Differences Between the Sexes, eds. Short, R. V. & Balaban, E. (Cambridge Univ. Press, Cambridge, U.K.), pp. 273-302.
- 6.Goy, R. W. & McEwen, B. S. (1980) Sexual Differentiation of the Brain (MIT Press, Cambridge, MA).
- 7.Wade, J. & Arnold, A. P. (1996) Proc. Natl. Acad. Sci. USA 93, 5264-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong, A., Freking, F., Wingfield, J., Schlinger, B. A. & Arnold, A. P. (1999) Gen. Comp. Endocrinol. 115, 346-353. [DOI] [PubMed] [Google Scholar]
- 9.Gahr, M. & Metzdorf, R. (1999) J. Neurosci. 19, 2628-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reisert, I. & Pilgrim, C. (1991) Trends Neurosci. 14, 468-473. [DOI] [PubMed] [Google Scholar]
- 11.Wilson, J. A. & Glick, B. (1970) Am. J. Physiol. 218, 951-955. [DOI] [PubMed] [Google Scholar]
- 12.Adkins, E. K. & Adler, N. T. (1972) J. Comp. Physiol. Psychol. 81, 27-36. [DOI] [PubMed] [Google Scholar]
- 13.Döhler, K. D., Hancke, J. L., Srivastava, S. S., Hofmann, C., Shiryne, J. E. & Gorski, R. A. (1984) Prog. Brain Res. 61, 99-117. [DOI] [PubMed] [Google Scholar]
- 14.Sayag, N., Robionzon, B., Snapir, N., Arnon, E. & Grimm, V. E. (1991) Horm. Behav. 25, 137-153. [DOI] [PubMed] [Google Scholar]
- 15.Clifton, P. G. & Andrew, R. J. (1989) Horm. Behav. 23, 572-589. [DOI] [PubMed] [Google Scholar]
- 16.Balaban, E., Teillet, M. & Le Douarin, N. M. (1988) Science 241, 1339-1342. [DOI] [PubMed] [Google Scholar]
- 17.Gahr, M. & Balaban, E. (1996) Dev. Brain Res. 92, 182-189. [DOI] [PubMed] [Google Scholar]
- 18.Smith, C. A. & Sinclair, A. (2001) J. Exp. Zool. 290, 691-699. [DOI] [PubMed] [Google Scholar]
- 19.Bruggeman, V., Van As, P. & Decuypere, E. (2002) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 131, 839-846. [DOI] [PubMed] [Google Scholar]
- 20.Balthazart, J. & Adkins-Regan, E. (2002) in Hormones, Brain and Behavior, eds. Arnold, A. P., Etgen, A. M., Fahrbach, S. E. & Rubin, R. T. (Academic, Amsterdam), Vol. 4, pp. 223-302. [Google Scholar]
- 21.Griffiths, R., Double, M. C., Orr, K. & Dawson, R. J. (1998) Mol. Ecol. 7, 1071-1075. [DOI] [PubMed] [Google Scholar]
- 22.Godsave, S., Lohman, R., Vloet, R. & Gahr, M. (2002) J. Comp. Neurol. 453, 57-70. [DOI] [PubMed] [Google Scholar]
- 23.Kiss, J. Z., Voorhuis, T. A. M., van Eekelen, J. A. M., de Kloet, E. R. & de Wied, D. (1987) J. Comp. Neurol. 263, 347-364. [DOI] [PubMed] [Google Scholar]
- 24.Beyer, C., Tramonte, R., Hutchison, R. E., Sharp, P., Barker, P. J., Huskisson, N. S. & Hutchison, J. B. (1994) Brain Res. Bull. 33, 583-588. [DOI] [PubMed] [Google Scholar]
- 25.Wang, R. & Millam, J. R. (1999) Cell Tissue Res. 297, 223-228. [DOI] [PubMed] [Google Scholar]
- 26.Panzica, G. C. (1998) J. Neurobiol. 37, 684-699. [DOI] [PubMed] [Google Scholar]
- 27.Van Gils, J., Absil, P., Grauwels, L., Moons, L., Vandesande, F. & Balthazart, J. (1993) J. Comp. Neurol. 334, 304-323. [DOI] [PubMed] [Google Scholar]
- 28.Ottinger, M. A., Pitts, S. & Abdelnabi, M. A. (2001) Poult. Sci. 80, 795-799. [DOI] [PubMed] [Google Scholar]
- 29.Balthazart, J., Absil, P., Gerard, M., Appeltants, D. & Ball, G. F. (1998) J. Neurosci. 18, 6512-6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panzica, G. C., Viglietti-Panzica, C., Calcagni, M., Anselmetti, G. C., Schumacher, M. & Balthazart, J. (1987) Brain Res. 416, 59-68. [DOI] [PubMed] [Google Scholar]
- 31.Thompson, R. R. & Adkins-Regan, E. (1992) Dev. Brain Res. 70, 231-237. [DOI] [PubMed] [Google Scholar]
- 32.Balthazart, J., Foidart, A., Absil, P. & Harada, N. (1996) J. Steroid Biochem. Mol. Biol. 56, 185-200. [DOI] [PubMed] [Google Scholar]
- 33.Viglietti-Panzica, C., Aste, N., Balthazart, J. & Panzica, G. C. (1994) Brain Res. 657, 171-184. [DOI] [PubMed] [Google Scholar]
- 34.Wilson, S. C., Knight, P. G. & Cunningham, F. J. (1983) J. Endocrinol. 125, 139-146. [DOI] [PubMed] [Google Scholar]
- 35.Foster, R. G., Panzica, G. C., Parry, D. M. & Viglietti-Panzica, C. (1988) Cell Tissue Res. 253, 327-335. [DOI] [PubMed] [Google Scholar]
- 36.Sayag, N., Snapir, N., Robinzon, B., Arnon, E., El Halawani, M. E. & Grimm, V. E. (1989) Physiol. Behav. 45, 1107-1112. [DOI] [PubMed] [Google Scholar]
- 37.Dewil, W., Buyse, J., Veldhuis, J. D., Mast, J., De Coster, R. & Decuypere, E. (1998) Domest. Anim. Endocrinol. 15, 115-127. [DOI] [PubMed] [Google Scholar]
- 38.Wood, R. I., Kim, S. J. & Foster, D. L. (1996) J. Neuroendocrinol. 8, 617-625. [PubMed] [Google Scholar]
- 39.Finn, P. D., McFall, T. B., Clifton, D. K. & Steiner, R. A. (1996) Endocrinology 137, 4767-4772. [DOI] [PubMed] [Google Scholar]
- 40.Grober, M. S., Winterstein, G. M., Ghazanfar, A. A. & Eroschenko, V. P. (1998) Gen. Comp. Endocrinol. 112, 356-363. [DOI] [PubMed] [Google Scholar]
- 41.Robinson, J. E., Forsdike, R. A. & Taylor, J. A. (1999) Endocrinology 140, 5797-5805. [DOI] [PubMed] [Google Scholar]
- 42.Sung-Ji, K., Foster, D. L. & Wood, R. I. (1999) Biol. Reprod. 61, 599-605. [DOI] [PubMed] [Google Scholar]
- 43.Mashaly, M. M. & Glick, B. (1979) Gen. Comp. Endocrinol. 38, 105-110. [DOI] [PubMed] [Google Scholar]
- 44.Etches, R. J. & Kagami, H. (1997) in Perspectives in Avian Endocrinology, eds. Harvey, S. & Etches, R. J. (Journal of Endocrinology Press, Bristol, U.K.), pp. 57-67.
- 45.Vaillant, S., Dorizzi, M., Pieau, C. & Richard-Mercier, N. (2001) J. Exp. Zool. 290, 727-740. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto, N., Uchiyama, H., Ohki-Hamazaki, H., Tanaka, H. & Ito, H. (1996) Dev. Brain Res. 95, 234-244. [DOI] [PubMed] [Google Scholar]
- 47.Schumacher, M., Hutchison, R. E. & Hutchison, J. B. (1988) Brain Res. 441, 98-110. [DOI] [PubMed] [Google Scholar]
- 48.Hutchison, J. B., Wozniak, A., Beyer, C. & Hutchison, R. E. (1996) J. Steroid Biochem. Mol. Biol. 56, 201-207. [DOI] [PubMed] [Google Scholar]
- 49.Schumacher, M. & Hutchison, J. B. (1986) Brain Res. 377, 63-72. [DOI] [PubMed] [Google Scholar]
- 50.Freking, F., Ramachandran, B. & Schlinger, B. A. (1998) J. Neurobiol. 36, 30-40. [DOI] [PubMed] [Google Scholar]
- 51.Ottinger, M. A., Thompson, N., Viglietti-Panzica, C. & Panzica, G. C. (1997) Brain Res. Bull. 44, 471-477. [DOI] [PubMed] [Google Scholar]
- 52.Dittami, J. P. (1981) Z. Tierpsychol. 55, 289-324. [DOI] [PubMed] [Google Scholar]
- 53.Döhler, K. D. & Wuttke, W. (1975) Endocrinology 97, 898-907. [DOI] [PubMed] [Google Scholar]
- 54.Hayes, F. J., Seminara, S. B., Decruz, S., Boepple, P. A. & William, F. C. (2000) J. Clin. Endocrinol. Metab. 85, 3027-3035. [DOI] [PubMed] [Google Scholar]
- 55.Al-Afaleq, A. I. & Homeida, A. M. (1998) Immunopharmacol. Immunotoxicol. 20, 315-327. [DOI] [PubMed] [Google Scholar]
- 56.Da Silva, J. A. P. (1999) Ann. N.Y. Acad. Sci. 876, 102-118. [DOI] [PubMed] [Google Scholar]
- 57.Groothuis, T. G. G. & Schwabl, H. (2002) Funct. Ecol. 16, 281-289. [Google Scholar]
- 58.Wennstrom, K. L., Blesius, F. & Crews, D. (1999) Brain Res. 838, 104-109. [DOI] [PubMed] [Google Scholar]
- 59.Xu, J., Burgoyne, P. S. & Arnold, A. P. (2002) Hum. Mol. Genet. 11, 1409-1419. [DOI] [PubMed] [Google Scholar]
- 60.Swaab, D. F. & Hofman, M. A. (1995) Trends Neurosci. 18, 264-270. [PubMed] [Google Scholar]