Abstract
The striatum in the basal ganglia-thalamocortical circuitry is a key neural substrate that is implicated in motor balance and procedural learning. The projection neurons in the striatum are dynamically modulated by nigrostriatal dopaminergic input and intrastriatal cholinergic input. The role of intrastriatal acetylcholine (ACh) in learning behaviors, however, remains to be fully clarified. In this investigation, we examine the involvement of intrastriatal ACh in different categories of learning by selectively ablating the striatal cholinergic neurons with use of immunotoxin-mediated cell targeting. We show that selective ablation of cholinergic neurons in the striatum impairs procedural learning in the tone-cued T-maze memory task. Spatial delayed alternation in the T-maze learning test is also impaired by cholinergic cell elimination. In contrast, the deficit in striatal ACh transmission has no effect on motor learning in the rota-rod test or spatial learning in the Morris water-maze test or on contextual- and tone-cued conditioning fear responses. We also report that cholinergic cell elimination adaptively up-regulates nicotinic ACh receptors not only within the striatum but also in the cerebral cortex and substantia nigra. The present investigation indicates that cholinergic modulation in the local striatal circuit plays a pivotal role in regulation of neural circuitry involving reward-related procedural learning and working memory.
Memory consists of several separate entities that depend on different brain systems (1). Clinical and behavioral evidence suggests that the basal ganglia is involved in the procedural learning that leads to habit formation and in the performance of routine behaviors that have been acquired (2, 3). The striatum in the basal ganglia-thalamocortical circuitry receives input from all areas of the cerebral cortex (4) and comprises a major cell population of medium-sized spiny neurons that mediate neurotransmission via γ-aminobutyric acid (5). The neural activity of these projection neurons is dynamically regulated by nigrostriatal dopaminergic input (6), and impairment of dopaminergic transmission in patients with Parkinson's disease causes not only extrapyramidal motor imbalance but also habit-learning dysfunction (2, 3). Electrophysiological and morphological studies have indicated the existence of another minor but important neuronal subpopulation in the striatum (7). These neurons are tonically active, thus termed tonically active neurons. They respond to stimuli that serve to trigger a learned and rewarded motor task and are implicated in learning processes of the basal ganglia function (8–13). Accumulating evidence suggests that tonically active neurons are cholinergic interneurons (9–12) that involve dynamic modulation of the basal ganglia circuitry through rich synaptic connections with the medium-sized spiny neurons within the striatum (4). However, the behavioral role of the striatal cholinergic interneurons in different categories of learning remains poorly understood, due to the lack of animal models that allow for investigation of the function of cholinergic interneurons in learning behaviors.
In our previous study, we investigated the role of acetylcholine (ACh) in the striatal circuit by selectively ablating striatal cholinergic neurons with use of immunotoxin (IT)-mediated cell targeting (14). This investigation revealed that ACh regulates the striatal circuit concertedly but oppositely to dopamine and that selective cholinergic cell ablation causes severe impairment of motor balance (14). The present investigation concerns how the deficit of cholinergic modulation in the striatal circuit influences different categories of learning. Here, we report that elimination of the cholinergic neurons in adult striatum causes impairment of reward-related procedural learning and working memory. Furthermore, we report that cholinergic cell ablation adaptively increases nicotinic ACh receptors (nAChRs) in not only the striatum but also the frontal cortex and substantia nigra. These findings suggest that the cholinergic modulation in the local striatal circuit exerts profound and global effects on the circuitry involving procedural and working memories.
Materials and Methods
Animals and IT Treatment. The IG17 line of heterozygous transgenic mice expressing the fusion protein of human IL-2 receptor (hIL-2R) α/GFP and their WT littermates (15) was used for all IT-mediated cell-targeting experiments. The IT, which was composed of the Fv portion of mAb reacting with hIL-2R fused to a 38-kDa fragment of Pseudomonas exotoxin (2.5 ng in 0.5 μl of PBS), was injected into one or both sides of the striatum as described (14). On one side of the striatum, IT was injected at 11 sites with the following coordinates determined from the bregma as a reference (16): sites 1 and 2, anterior (A) 1.5, lateral (L) 1.5, ventral (V) 2.5 and 3.5; site 3, A 1.0, L 1.5, V 2.5; sites 4 and 5, A 1.0, L 2.3, V 2.0 and 3.0; sites 6 and 7, A 0.3, L 1.5, V 2.0 and 3.0; sites 8 and 9, A 0.3, L 2.5, V 2.0 and 3.0; sites 10 and 11, posterior 0.3, L 2.5, V 2.0 and 3.0 (mm each). Behavioral and immunoblot analyses were carried out 2 weeks after IT injection. All procedures were performed according to the guidelines of the Kyoto University Faculty of Medicine.
Tone-Cued T-Maze Memory Task. The tone-cued T-maze task was assessed according to the procedures described by Jog et al. (9). Mice were trained daily to respond to auditory instruction cues, a 1- or 8-kHz tone (75 dB), which indicated whether chocolate was in the left or right arm. A training session consisted of 20 trials per day. The tone remained on until the mice reached the goal or for a maximum of 20 s. The criterion for tone-cued memory acquisition was defined as >80% correct turns in the trials for each session.
Spatial Alternation Training and Delay Tests. Spatial alternation training and delay tests were performed in the T-maze apparatus according to the procedures described by Doré et al. (17). Each daily session consisted of 11 trials. On the first trial of each session, both goals were baited with chocolate. For the next 10 trials, the reward was placed in the arm opposite to that chosen by the mouse on the previous trial. The criterion for successful completion of training was defined as >80% correct turns averaged over 2 consecutive days. A maximum of 20 sessions was allowed for each training. Once the criterion was reached, spatial delayed alternation was tested by interposing 30- or 90-s delay between trials. Each delay was used for 2 consecutive days.
Rota-Rod Motor Learning Task. The rota-rod motor learning test was performed according to the procedures described by Gerlai et al. (18). A mouse was placed on a rotating roller (4 cm in diameter), and the time it remained on the roller was measured. The training consisted of 16 sessions, 3 sessions a day and 3 trials per session. A maximum of 30 s was allowed for each trial per animal. The rotation speed of the rota-rod was set to 25 rpm in the first session. When the mouse was able to stay on the rotating rod for a cumulative duration of at least 60 s during a three-trial session, the rotation speed was increased by 5 rpm for the next session. When the mouse accumulated <60 s but >30 s on-rod time, no increase in speed was made. When the mouse could not accumulate 30 s on-rod time, the rotation speed was decreased by 5 rpm. The rotation speed mastered by the mouse was recorded and statistically analyzed.
Contextual and Cued Conditioning Fear Responses. Fear responses were measured according to the procedures described by Zeng et al. (19) with some modifications. Each mouse was placed in an electric shock chamber on the training day (day 1); 2 min later, three repeated tone-shock pairs were given at 2-min intervals. Each tone-shock pair consisted of a 30-s white noise tone followed by a 3-s foot shock at 0.3 mA. The mouse remained in the chamber for 60 s before being returned to its home cage. On day 2, the mouse was placed back in the electric shock chamber for 6 min (contextual freezing responses). One hour later, the mouse was put in the home cage, and freezing responses during the first tone-free 2 min, the next tone-delivered 2 min, and the following tone-free 2 min were measured as pretone, tone, and posttone responses, respectively (tone-cued freezing responses).
Water-Maze Task. The water-maze task was carried out as described (20). Briefly, the training consisted of nine sessions, three sessions per day and four trials per session with inter-trial intervals of 30–60 min. In each trial, the mouse swam until it found the platform or up to a maximum of 90 s, at which point the mouse was guided to the platform. On day 4, the platform was removed from the pool, and each mouse was tested on a probe trial for 60 s. On day 5, the platform was placed at the opposite location, and the mouse was retrained in four sessions. On day 6, the platform was removed, and a probe trial was performed for evaluation of reversal learning.
Locomotor Activity. Animals were placed in a new chamber for 60 min and then habituated by daily placing in the same chamber for 60 min. Locomotor activity was assessed for a 60-min period on the first day (exploratory phase) and 3 days after habituation (habituated phase) with an IR activity monitor (MED Associates, St. Albans, VT).
Western Blotting. IT was injected into the left striatum, and 2 weeks after IT injection, animals were killed after deep anaesthetization. Samples were punched out from coronal slices (500 μm thick) of the frozen brain by using a 14-gauge syringe needle and homogenized in lysis buffer containing 1% SDS and proteinase inhibitor mixture Complete (Roche Diagnostics). The primary antibodies used for immunoblotting were goat polyclonal Ab against choline acetyltransferase (ChAT) (1:100; Chemicon); mouse mAbs against nAChR α4 subunit (1:2,000), nAChR α7 subunit (1:5,000), 160-kDa neurofilament (1:10,000) (all from Sigma), and NR1 (1:5,000, PharMingen); rat mAb against nAChR β2 subunit (1:2,000, Sigma); and rabbit polyclonal Abs against NR2B (1:5,000), GluR1 (1:1,000), GluR2/3 (1:2,000), mGluR1a (1:5,000), tyrosine hydroxylase (1:500), 68-kDa neurofilament (1:100,000) (all from Chemicon), NR2A (1:5,000), mGluR5 (1:5,000) (both from Upstate Biotechnology, Lake Placid, NY), and actin (1:5,000, Sigma). Rabbit polyclonal Ab against preprotachykinin (1:1,000) and guinea pig polyclonal Ab against preproenkephalin (1:100) were gifts from T. Kaneko, Kyoto University. Immunoblots were incubated with horseradish peroxidase-conjugated secondary antibody and developed with ECL reagents (Amersham Biosciences). Signal intensities of immunoreactive bands were quantified with a GS-710 calibrated imaging densitometer (Bio-Rad).
Results
For IT-mediated cell targeting, we generated transgenic mice in which the expression of the hIL-2R/GFP fusion protein was driven by the promoter function of metabotropic glutamate receptor subtype 2 (15). In these mice, hIL-2R/GFP was specifically expressed in cholinergic neurons within the cell population of the striatum (14, 21). The IT is composed of the Fv portion of mAb reacting with hIL-2R fused to a 38-kDa fragment of Pseudomonas exotoxin (15). To extensively ablate cholinergic cells in the striatum, IT was stereotaxically injected into 11 sites on one side of the striatum. Two weeks after IT injection, ChAT immunostaining showed a reduction of ≈80% of striatal cholinergic neurons. No such reduction was observed for other striatal cell types such as somatostatin-positive, parvalbuminpositive, and calbindin D-28k-positive neurons, or for cholinergic neurons in IT-treated WT mice (14, 21).
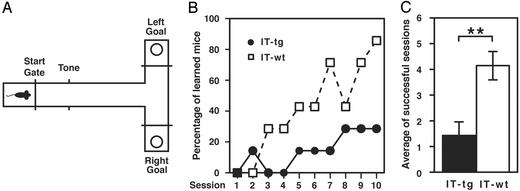
We tested a conditioned T-maze paradigm to assess the role of striatal cholinergic neurons in procedural learning (9). IT was bilaterally injected into the striatum of WT and transgenic mice. Two weeks after IT injection, the animals were trained to move through a T-maze and to gain a reward by responding to auditory instruction of two different tones that indicated whether they should turn into the right or left arm to gain a chocolate reward (Fig. 1A). A daily training session consisted of 20 trials, and the criterion of memory acquisition was defined as >80% correct turns in a session. IT-treated WT mice evenly visited two arms at the initial two sessions and progressively learned how to gain a reward through daily practices (Fig. 1B). IT-treated transgenic mice occasionally exceeded the learning criterion, but the success rate of learning for IT-treated transgenic mice was significantly less than that of IT-treated WT mice throughout the course of successive training (Fig. 1B). When individual mice were examined during conditioning, IT-treated WT mice exceeded the learning criterion at least twice, and more than half reached this criterion more than four times. In contrast, three of seven IT-treated transgenic mice never reached the learning criterion. The average number of successful sessions was significantly lower in IT-treated transgenic mice than in IT-treated WT mice (Fig. 1C). These results indicate that cholinergic cell elimination in the striatum impairs the ability to acquire procedural learning.
Fig. 1.
Impairment of procedural learning in the tone-cued T-maze test. (A) The procedure of the tone-cued T-maze test is indicated. (B) The percentage of mice that passed the learning criterion in each session is indicated. The number of learned mice was significantly lower in cholinergic cell-eliminated mice than in IT-treated WT mice (n = 7 each; Wilcoxon-signed-ranks test, P < 0.05). (C) The number of successful sessions that exceeded the learning criterion in 10 sessions was averaged. Columns and error bars represent mean ± SEM, respectively. **, P < 0.01.
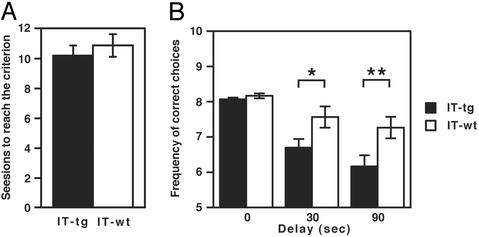
We next tested the ability of animals to learn spatial alternation in the T-maze to gain a reward. In this test, animals were trained to choose the alternative arm by replacing the chocolate reward at the arm opposite to the one selected on the previous trial. Each daily session consisted of 11 trials, and the criterion of successful habitual acquisition was defined as >80% correct choices averaged over 2 consecutive days. Although the basal ganglia has been implicated in achievement of spatial alternation (22), the two groups of mice showed no statistical difference in the number of sessions to reach the learning criterion (Fig. 2A). This observation may be accounted for by the relatively simple learning of spatial alternation as compared with the more complex tone-cued T-maze learning that needs to associate procedural learning with auditory conditioning. We then tested for spatial delayed alternation in the T-maze. Once animals were trained to reach the learning criterion, their ability to achieve spatial alternation was examined by delaying a spatial alternation trial 30 and 90 s after the previous trial. Both IT-treated WT and transgenic mice showed reduction in correct choices by interposing delays of 30 and 90 s between trials (Fig. 2B). Importantly, cholinergic cell-eliminated mice more significantly reduced spatial delayed alternation than did WT mice (Fig. 2B). The results indicate that ACh in the striatum contributes to working memory-related learning.
Fig. 2.
Spatial alternation test in the T-maze. (A) The number of sessions mice needed to reach the learning criterion in the spatial alternation task is indicated. Columns and error bars represent mean ± SEM, respectively. n = 15 each; statistically not different (P = 0.51). (B) The number of correct choices in the spatial delayed alternation test is indicated. The correct choice in delays of 30 and 90 s was more significantly reduced in IT-treated transgenic mice than in IT-treated WT mice (repeated-measure ANOVA, F1,28 = 11.8, P < 0.01; **, P < 0.01; *, P < 0.05).
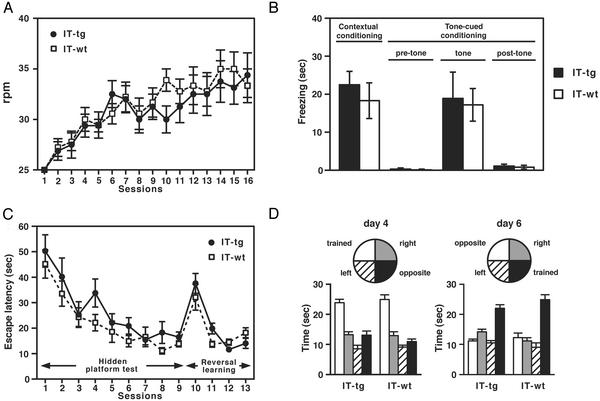
We addressed whether cholinergic cell elimination impairs other categories of learning by several different behavioral analyses. The chronically cholinergic cell-eliminated mice showed no abnormal motor behaviors (14). We performed a rota-rod test to examine whether cholinergic cell elimination influences the ability to acquire motor skill learning. Both IT-treated WT and transgenic mice progressively improved their skill in the rota-rod test (Fig. 3A). The time course and extents of improvement in the rota-rod test were indistinguishable between WT and cholinergic cell-eliminated mice (Fig. 3A). These results suggest that ACh depletion in the striatum has no direct effect on motor skill learning.
Fig. 3.
Behavioral analyses of different learning categories. (A) Motor learning test using a rota-rod. The points represent the mean rotation speed the animal reached with daily practices. The error bars represent mean ± SEM. IT-tg, n = 6; IT-wt, n = 7; statistically not different (repeated-measure ANOVA, F1,15 = 0.25, P = 0.63). (B) The freezing time in contextual and tone-cued fear-conditioning tasks [n = 7 each; not statistically different in both fear responses (Student's t test, contextual, P = 0.40; tone-cued, P = 0.20)]. (C) IT-injected WT and transgenic mice (n = 9 each) improved performance in both standard water-maze task (repeated-measure ANOVA, IT-tg, F8,64 = 9.48, P < 0.0001; IT-wt, F8,64 = 13.5, P < 0.0001) and spatial reversal task (repeated-measure ANOVA, IT-tg, F3,24 = 28.7, P < 0.0001; IT-wt, F3,24 = 9.86, P = 0.0002). Escape latencies of both tests were not statistically different (repeated-measure ANOVA, standard, F1,16 = 1.55, P = 0.23; reversal, F1,16 = 0.64, P = 0.44). (D) The time spent in the trained quadrant was not statistically different (Student's t test: standard, P = 0.59; reversal, P = 0.18).
We next subjected IT-treated WT and transgenic mice to a fear-conditioning learning paradigm. Animals received electrical shock paired with tone cues, and on the next day, freezing fear responses were measured by placing the animals in the electric shock chamber (contextual) or delivering the tone in the home chamber (tone-cued). Both groups of animals displayed similar levels of freezing to the context and to the tone (Fig. 3B). In control, no freezing responses were observed before or after the tone delivery at the home chamber (Fig. 3B). This finding indicates that cholinergic cell elimination does not impair either contextual or cued fear conditioning.
Transgenic mice that overexpressed acetylcholinesterase in various brain regions showed up-regulation of nAChR mRNAs in the striatum, cerebral cortex, and hippocampus (23) and displayed impairments in spatial learning with the Morris watermaze test (24). We examined effects of striatal cholinergic cell elimination on spatial learning with the Morris water-maze test (Fig. 3C). Both WT and cholinergic cell-eliminated mice decreased their escape latencies through nine training sessions and showed no difference in the escape latency (Fig. 3C). In a probe trial conducted after the ninth session, WT and cholinergic cell-eliminated mice spent significantly more time in the target quadrant than in the other three quadrants, and no statistical difference was observed between the two groups of animals (Fig. 3D). To assess the spatial learning flexibility, these mice were subsequently given a four-session reversal training in which the hidden platform was moved to the opposite quadrant (Fig. 3C). In the reversal test, the WT and cholinergic cell-eliminated mice showed no difference in latencies to find the new platform location (Fig. 3C) or in the time spent in the newly trained quadrant (Fig. 3D). These observations demonstrate that the deficit of ACh transmission in the striatum does not hinder spatial learning in the water-maze test.
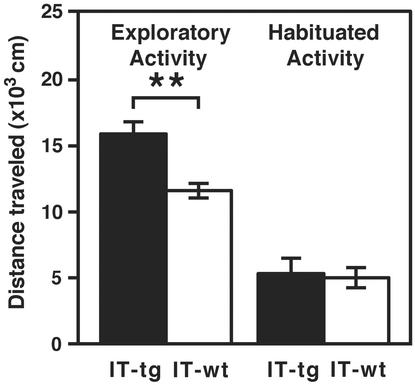
The transgenic mice that overexpressed acetylcholinesterase also showed increase in locomotor activity at the novel environment but not at the familiar environment (25). We examined locomotor activity at the exploratory and habituated phases of cholinergic cell-eliminated transgenic mice. These mice showed an increase in the locomotor activity at the exploratory phase but not at the habituated phase as compared to WT mice (Fig. 4). This finding indicates that reduction of ACh in the striatum is sufficient for stimulating locomotion at the new environment.
Fig. 4.
Exploratory hyperlocomotion of cholinergic cell-eliminated mice. The locomotion of cholinergic cell-eliminated mice was significantly higher than that of WT mice at the exploratory phase but not at habituated phase (IT-tg, n = 9; IT-wt, n = 7). Columns and error bars indicate mean ± SEM, respectively (Student's t test: **, P < 0.01).
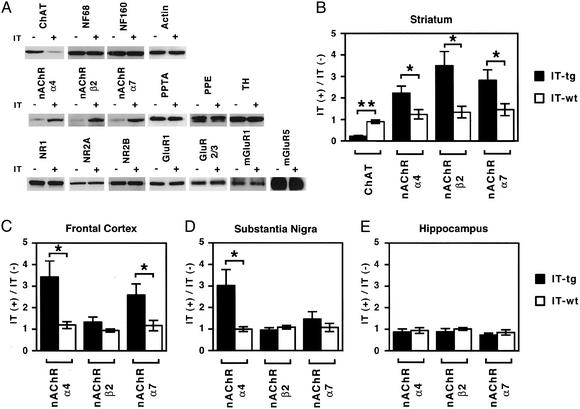
The striatal principal neurons receive glutamatergic input from the cerebral cortex and dopaminergic input from substantia nigra pars compacta and differentially express substance P and enkephalin, depending on their projecting targets (4, 26). We examined adaptive changes of nAChRs and glutamate receptors and other neuronal proteins in the chronically cholinergic cell-eliminated striatum. IT was unilaterally injected into the striatum, and homogenates of the IT-injected and uninjected sides of the striatum were subjected to immunoblotting with various antibodies. Levels of ChAT immunoreactivity decreased to ≈20% in the IT-injected side as compared with the uninjected side (Fig. 5 A and B). In control, the cytoskeleton proteins, actin and 68- and 160-kDa neurofilaments, remained unchanged after cholinergic cell elimination (Fig. 5A). The major species of the brain nAChRs, α4, β2, and α7 subunits (11, 27), all were expressed in the striatum, and these receptor subunits were up-regulated after cholinergic cell elimination (Fig. 5 A and B). The NR1, NR2A, and NR2B subunits of N-methyl-D-aspartate receptors, the GluR1, GluR2, and GluR3 subunits of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, and mGluR1 and mGluR5 of the mGluR family represent the predominant species of the respective glutamate receptor families in the striatum (28). None of these glutamate receptors showed any adaptive changes at the IT-injected sides of the striatum (Fig. 5A). In addition, consistent with our previous RNA hybridization analysis (14), the protein levels of preprotachykinin, preproenkephalin, and tyrosine hydroxylase remained unchanged at the chronic phase of cholinergic cell elimination (Fig. 5A).
Fig. 5.
Up-regulation of nAChRs in cholinergic cell-eliminated mice. (A) Expression levels of indicated proteins were determined by immunoblot analysis of homogenates obtained from the IT-injected (+) and uninjected (-) sides of the striatum of transgenic mice. Levels of ChAT and α4, β2, and α7 subunits of nAChRs were changed by cholinergic cell elimination, but no changes were observed for other proteins tested. (B–E) Levels of α4, β2, and α7 subunits of nAChRs and ChAT were quantified by immunoblot analysis of the indicated brain regions. Levels at the IT-injected side, relative to those of the IT-uninjected side, are indicated. Columns and error bars represent mean ± SEM, respectively. Each brain region was analyzed with at least five preparations (Student's t test: **, P < 0.01; *, P < 0.05).
We then examined possible adaptive changes in α4, β2, and α7 subunits of nAChRs in other brain regions. The α4 and α7 subunits, but not the β2 subunit of nAChRs, were significantly up-regulated at the IT-injected side of the frontal cortex (Fig. 5C). The α4 subunit was markedly increased at the IT-injected side of substantia nigra (Fig. 5D). In contrast, none of these subunits showed any changes in the hippocampus (Fig. 5E). The striatal cholinergic interneurons are restrictedly distributed within the striatum and form rich synaptic connections with the striatal projection neurons (4). This analysis has revealed that selective ablation of striatal cholinergic neurons induces adaptive changes of nAChRs not only within the striatum but also at the striatum-relevant brain regions. This finding suggests that ACh in the striatum plays a pivotal role in neurotransmission modulation in the basal gangliathalamocortical circuitry.
Discussion
This investigation has demonstrated that cholinergic cell elimination in the striatum impairs tone-cued procedural learning and spatial delayed alternation but has no effect on motor or spatial learning or on contextual- and tone-cued conditioning fear responses. The results of this investigation are consistent with the concept that the striatum is the main neural substrate implicated in procedural learning (1–3) and have provided compelling evidence that the cholinergic neurons in the striatum are essential for reward-related habit learning. In our previous study, we showed that cholinergic cell elimination in the nucleus accumbens, the ventral part of the striatum, increases the sensitivity to a low dose of cocaine and morphine to associate repeated exposure of these abusive drugs with the drug-exposed environment (21, 29). This enhancement of association is thought to be derived from dopaminergic hyperactivity on the principal projection neurons as a result of cholinergic cell-eliminated depletion of antagonistic ACh in the nucleus accumbens circuit. We also showed that unilateral cholinergic cell ablation in the striatum induces motor imbalance at the acute phase of cell ablation (14). ACh from the cholinergic interneurons thus plays a pivotal role in both motor balance and procedural learning. A number of electrophysiological studies have indicated that the cholinergic interneurons respond to sensory stimuli that trigger learned and rewarded motor tasks (8, 10, 11). The cholinergic neurons in the striatum are tonically active and generate action potentials in the absence of any synaptic input (12). Furthermore, the tonic firing of cholinergic neurons is paused by sensory stimuli in a learned and reward motor task (8, 11). Because the cholinergic input in the striatum ultimately depresses neuronal activities of the cerebral cortex (14), it is likely that stimulus-dependent suppression of tonically active cholinergic neurons facilitates information transmission to the cerebral cortex and contributes to procedural learning.
Our results have also demonstrated that cholinergic cell elimination impairs the ability to perform spatial delayed alternation in a T-maze. A number of lines of evidence have indicated that this memory is relevant to working memory and requires integrity of the prefrontal cortex function (30, 31). Interestingly, cholinergic cell elimination adaptively up-regulated the major species of the brain nAChRs not only within the striatum but also in the frontal cortex and substantia nigra. The cholinergic interneurons are restrictedly distributed within the striatum (4). Then, how does the local perturbation of ACh neurotransmission induce adaptive up-regulation of nAChRs in the regions located at a long distance? In the striatum, nAChRs are known to be expressed at the axonal terminals of both glutamatergic neurons of the cerebral cortex (32) and dopaminergic neurons of substantia nigra (33). One possible explanation is that the reduction of ACh in the striatum is informed from the axonal terminals to soma of these neurons in a retrograde manner that triggers induction of nAChRs at distantly located neurons. Alternatively, the striatum forms a circuitry loop with both the cerebral cortex and substantia nigra (4). The local perturbation of ACh neurotransmission may thus induce adaptive changes of nAChRs via these neural circuitry systems. Whatever the mechanisms, this investigation implies that the cholinergic synaptic modulation in the striatum exerts a profound influence not only on the striatal circuit but also the basal ganglia circuitry.
Acknowledgments
We thank Takeshi Kaneko for a gift of antibodies and Kumlesh K. Dev for his careful reading of this manuscript. This work was supported in part by research grants from the Ministry of Education, Science, and Culture of Japan and the International Resource Program of the National Cancer Institute.
Abbreviations: ACh, acetylcholine; nAChR, nicotinic ACh receptor; IT, immunotoxin; ChAT, choline acetyltransferase; hIL-2R, human IL-2 receptor.
References
- 1.Squire, L. R. & Zola, S. M. (1996) Proc. Natl. Acad. Sci. USA 93, 13515-13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salmon, D. P. & Butters, N. (1995) Curr. Opin. Neurobiol. 5, 184-190. [DOI] [PubMed] [Google Scholar]
- 3.Knowlton, B. J., Mangels, J. A. & Squire, L. R. (1996) Science 273, 1399-1402. [DOI] [PubMed] [Google Scholar]
- 4.Gerfen, C. R. (1992) Annu. Rev. Neurosci. 15, 285-320. [DOI] [PubMed] [Google Scholar]
- 5.Ribak, C. E., Vaughn, J. E. & Roberts, E. (1979) J. Comp. Neurol. 187, 261-283. [DOI] [PubMed] [Google Scholar]
- 6.Joel, D. & Weiner, I. (2000) Neuroscience 96, 451-474. [DOI] [PubMed] [Google Scholar]
- 7.Kimura, M., Kato, M. & Shimazaki, H. (1990) Exp. Brain Res. 82, 672-676. [DOI] [PubMed] [Google Scholar]
- 8.Graybiel, A. M., Aosaki, T., Flaherty, A. W. & Kimura, M. (1994) Science 265, 1826-1831. [DOI] [PubMed] [Google Scholar]
- 9.Jog, M. S., Kubota, Y., Connolly, C. I., Hillegaart, V. & Graybiel, A. M. (1999) Science 286, 1745-1749. [DOI] [PubMed] [Google Scholar]
- 10.Blazquez, P. M., Fujii, N., Kojima, J. & Graybiel, A. M. (2002) Neuron 33, 973-982. [DOI] [PubMed] [Google Scholar]
- 11.Zhou, F.-M., Wilson, C. J. & Dani, J. A. (2002) J. Neurobiol. 53, 590-605. [DOI] [PubMed] [Google Scholar]
- 12.Wilson, C. J., Chang, H. T. & Kitai, S. T. (1990) J. Neurosci. 10, 508-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apicella, P. (2002) Eur. J. Neurosci. 16, 2017-2026. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko, S., Hikida, T., Watanabe, D., Ichinose, H., Nagatsu, T., Kreitman, R. J., Pastan, I. & Nakanishi, S. (2000) Science 289, 633-637. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe, D., Inokawa, H., Hashimoto, K., Suzuki, N., Kano, M., Shigemoto, R., Hirano, T., Toyama, K., Kaneko, S., Yokoi, M., et al. (1998) Cell 95, 17-27. [DOI] [PubMed] [Google Scholar]
- 16.Franklin, K. B. J. & Paxinos, G. (1997) The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego).
- 17.Doré, F. Y., Goulet, S., Gallagher, A., Harvey, P.-O., Cantin, J.-F., D'Aigle, T. & Mirault, M.-E. (2001) Neurotoxicol. Teratol. 23, 463-472. [DOI] [PubMed] [Google Scholar]
- 18.Gerlai, R., Millen, K. J., Herrup, K., Fabien, K., Joyner, A. L. & Roder, J. (1996) Behav. Neurosci. 110, 126-133. [DOI] [PubMed] [Google Scholar]
- 19.Zeng, H., Chattarji, S., Barbarosie, M., Rondi-Reig, L., Philpot, B. D., Miyakawa, T., Bear, M. F. & Tonegawa, S. (2001) Cell 107, 617-629. [DOI] [PubMed] [Google Scholar]
- 20.Yokoi, M., Kobayashi, K., Manabe, T., Takahashi, T., Sakaguchi, I., Katsuura, G., Shigemoto, R., Ohishi, H., Nomura, S., Nakamura, K., et al. (1996) Science 273, 645-647. [DOI] [PubMed] [Google Scholar]
- 21.Hikida, T., Kaneko, S., Isobe, T., Kitabatake, Y., Watanabe, D., Pastan, I. & Nakanishi, S. (2001) Proc. Natl. Acad. Sci. USA 98, 13351-13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mogensen, J., Iversen, I. H. & Divac, I. (1987) Acta Neurobiol. Exp. 47, 45-54. [PubMed] [Google Scholar]
- 23.Svedberg, M. M., Svensson, A.-L., Johnson, M., Lee, M., Cohen, O., Court, J., Soreq, H., Perry, E. & Nordberg, A. (2002) J. Mol. Neurosci. 18, 211-222. [DOI] [PubMed] [Google Scholar]
- 24.Beeri, R., Le Novère, N., Mervis, R., Huberman, T., Grauer, E., Changeux, J. P. & Soreq, H. (1997) J. Neurochem. 69, 2441-2451. [DOI] [PubMed] [Google Scholar]
- 25.Erb, C., Troost, J., Kopf, S., Schmitt, U., Löffelholz, K., Soreq, H. & Klein, J. (2001) J. Neurochem. 77, 638-646. [DOI] [PubMed] [Google Scholar]
- 26.Di Chiara, G., Morelli, M. & Consolo, S. (1994) Trends Neurosci. 17, 228-233. [DOI] [PubMed] [Google Scholar]
- 27.Wada, E., Wada, K., Boulter, J., Deneris, E., Heinemann, S., Patrick, J. & Swanson, L. W. (1989) J. Comp. Neurol. 284, 314-335. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi, S. & Masu, M. (1994) Annu. Rev. Biophys. Biomol. Struct. 23, 319-348. [DOI] [PubMed] [Google Scholar]
- 29.Hikida, T., Kitabatake, Y., Pastan, I. & Nakanishi, S. (2003) Proc. Natl. Acad. Sci. USA 100, 6169-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Santed, F., de Bruin, J. P., Heinsbroek, R. P. & Verwer, R. W. (1997) Behav. Brain Res. 84, 73-79. [DOI] [PubMed] [Google Scholar]
- 31.Romanides, A. J., Duffy, P. & Kalivas, P. W. (1999) Neuroscience 92, 97-106. [DOI] [PubMed] [Google Scholar]
- 32.Marchi, M., Risso, F., Viola, C., Cavazzani, P. & Raiteri, M. (2002) J. Neurochem. 80, 1071-1078. [DOI] [PubMed] [Google Scholar]
- 33.Zoli, M., Moretti, M., Zanardi, A., Mclntosh, J. M., Clementi, F. & Gotti, C. (2002) J. Neurosci. 22, 8785-8789. [DOI] [PMC free article] [PubMed] [Google Scholar]