Abstract
Neuropathy target esterase (NTE) is inhibited by several organophosphorus (OP) pesticides, chemical warfare agents, lubricants, and plasticizers, leading to OP-induced delayed neuropathy in people (>30,000 cases of human paralysis) and hens (the best animal model for this demyelinating disease). The active site region of NTE as a recombinant protein preferentially hydrolyzes lysolecithin, suggesting that this enzyme may be a type of lysophospholipase (LysoPLA) with lysolecithin as its physiological substrate. This hypothesis is tested here in mouse brain by replacing the phenyl valerate substrate of the standard NTE assay with lysolecithin for an “NTE-LysoPLA” assay with four important findings. First, NTE-LysoPLA activity, as the NTE activity, is 41–45% lower in Nte-haploinsufficient transgenic mice than in their wild-type littermates. Second, the potency of six delayed neurotoxicants or toxicants as in vitro inhibitors varies from IC50 0.02 to 13,000 nM and is essentially the same for NTE-LysoPLA and NTE (r2 = 0.98). Third, the same six delayed toxicants administered i.p. to mice at multiple doses inhibit brain NTE-LysoPLA and NTE to the same extent (r2 = 0.90). Finally, their in vivo inhibition of brain NTE-LysoPLA generally correlates with delayed toxicity. Therefore, OP-induced delayed toxicity in mice, and possibly the hyperactivity associated with NTE deficiency, may be due to NTE-LysoPLA inhibition, leading to localized accumulation of lysolecithin, a known demyelinating agent and receptor-mediated signal transducer. This mouse model has some features in common with OP-induced delayed neuropathy in hens and people but differs in the neuropathological signs and apparently the requirement for NTE aging.
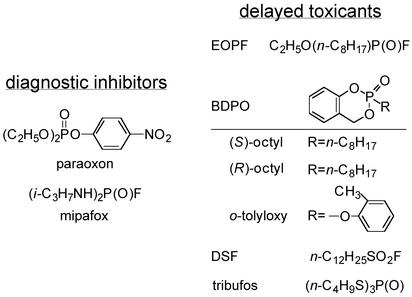
Organophosphorus (OP) esters are the principal class of insecticides (1) and chemical warfare agents (2). Their acute lethal action is attributed to inhibition of acetylcholinesterase by phosphorylation of its catalytic site (3). The second most important toxic effect of these compounds and some related pesticides, lubricants, and plasticizers is OP-induced delayed neuropathy (OPIDN) (4–7). The principal causal agent for the >30,000 cases of human paralysis is tri-o-tolyl phosphate as an adulterant in beverages around 1930 and in cooking oil in 1959 (5–7), but concern continues because it is still a common component in commercial jet oils (8). The delayed neurotoxicity of this triaryl phosphate involves bioactivation to the o-tolyloxy derivative of benzodioxaphosphorin oxide (BDPO; ref. 9). Other OP delayed neurotoxicants are the insecticide mipafox, which is no longer used (4, 5), and the cotton defoliant tribufos (10). Their structures are given in Fig. 1.
Fig. 1.
Structures of OP diagnostic inhibitors and delayed toxicants or neurotoxicants.
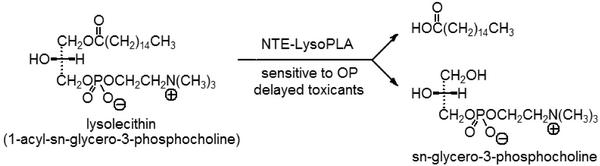
The target for OPIDN is a nerve protein with esteratic activity that is phosphorylated and inhibited by the delayed neurotoxicant (4). Neuropathy target esterase (NTE) is present in human, hen, and mouse brain and is defined as the paraoxon-resistant and mipafox-sensitive esterase with phenyl valerate-hydrolyzing activity (4–6, 11–14). NTE inhibitors of varying potency are o-tolyloxy-BDPO, mipafox, and tribufos (indicated above) and ethyl octylphosphonofluoridate (EOPF; refs. 13 and 14), the octyl-BDPO enantiomers (14, 15), and dodecanesulfonyl fluoride (DSF; see ref. 16 for the octane analog) designed for potency (Fig. 1). An aging reaction also is required for OPIDN; e.g., O,O-diisopropylphosphoryl-NTE is O-dealkylated to O-isopropylphosphoryl-NTE (see Discussion). The NTE assay is fully validated for toxicological relevance to OPIDN (11, 12) and served as the monitoring procedure in NTE isolation (17, 18). When the esterase domain of NTE (residues 727–1216) is expressed as the recombinant polypeptide (designated as NEST; ref. 19), the preferred substrate is 1-palmitoyl-sn-glycero-3-phosphocholine (also known as 1-palmitoyllysophosphatidylcholine, a lysolecithin) with Km ≈ 50 μM (20). NEST has sequence similarity in the active site region to calcium-independent phospholipase A2 (20), known to have lysophospholipase (LysoPLA) activity (21). Lysolecithin therefore becomes a candidate for the physiological substrate (ref. 20; Fig. 2).
Fig. 2.
Proposed biochemical lesion induced by OP delayed toxicants or neurotoxicants involving inhibition of NTE-LysoPLA, which normally hydrolyzes lysolecithin as its physiological substrate. The classical substrate for NTE is phenyl valerate, based on ease of assay (4, 12) rather than mechanistic considerations. NTE-LysoPLA and NTE in brain are resistant to paraoxon but sensitive to mipafox and other delayed toxicants or neurotoxicants.
Biochemical studies indicated above suggest that NTE may be a LysoPLA acting on lysolecithin. This hypothesis is tested here in mice by replacing the phenyl valerate substrate of the standard NTE assay with lysolecithin for a different type of “NTE-LysoPLA” assay (Fig. 2). Mouse brain was selected because OP-induced delayed toxicity in mice correlates with NTE inhibition (14, 16) and Nte-haploinsufficient (Nte+/-) mice are hyperactive and more sensitive to the delayed toxicant EOPF (22). As with NTE, the paraoxon-resistant and mipafox-sensitive LysoPLA activity was considered most important. A simple and specific assay was developed for NTE-LysoPLA. Brain preparations from wild-type and Nte+/- mice first were examined for activity with lysolecithin as the substrate. Then a series of OP toxicants of diverse structures (Fig. 1) and potencies was examined. NTE-LysoPLA and NTE should be similarly inhibited by these compounds in vitro and in vivo if they are in fact the same enzyme. The dose–response effects, as in vivo enzyme inhibitors, should also correlate with delayed toxicity. These are severe tests for the possible assignment of mouse brain NTE as a LysoPLA.
Materials and Methods
Materials. Lysolecithin (primarily palmitate and stearate esters, derived from egg yolk), sn-glycero-3-phosphocholine phosphodiesterase (mold), choline oxidase (Alcaligenes sp.), peroxidase (horseradish), 3-(N-ethyl-3-methylanilino)-2-hydroxypropanesulfonic acid sodium salt, and 4-aminoantipyrine were obtained from Sigma. Paraoxon, mipafox, and six delayed toxicants or neurotoxicants (Fig. 1) were available from previous studies in this laboratory.
Mice and Brain Enzyme Preparations. Male Swiss–Webster mice (≈25 g) were from Harlan Laboratories (Indianapolis). Nte+/- and the corresponding wild-type (+/+) mice (129S6/SvEvTac, male, ≈4 months old) were from the Salk Institute (22). The brain (freshly obtained or from storage at -80°C) was homogenized at 20% (wt/vol) in 50 mM Tris buffer (pH 8.0) containing 0.2 mM EDTA at 4°C. Enzyme assays used the supernatant fraction (700 × g, 10 min) at 4°C within 6 h after preparation.
Enzyme Activity and Inhibition Assays. The substrate used for NTE-LysoPLA was lysolecithin and that for NTE was phenyl valerate. In both assays only the paraoxon-resistant and mipafoxsensitive portion of the activity is relevant. NTE-LysoPLA was assayed at 25°C, and NTE was assayed at 37°C, in each case after a 20-min pretreatment with paraoxon at 40 μM and mipafox at 0 or 50 μM. For IC50 determinations, inhibitors were added in dimethyl sulfoxide (5 μl) with a 15-min incubation before the introduction of paraoxon/mipafox. Unless specified otherwise, the data are averages of four to eight replicates (NTE-LysoPLA) or one to two determinations (NTE), reflecting the greater variability of the former method.
NTE-LysoPLA Activity Assays. LysoPLAs are OP-sensitive enzymes that hydrolyze lysolecithin to sn-glycero-3-phosphocholine (Fig. 2; refs. 23 and 24). The assay procedure is modified from a method for analysis of lysolecithin in human serum and plasma (25). LysoPLA activity is monitored continuously in an enzyme-coupled microplate assay in which only the first step is OP-sensitive. The reaction proceeds to form choline, hydrogen peroxide, and a colored derivative from the sequential action of three added enzymes (sn-glycero-3-phosphocholine phosphodiesterase, choline oxidase, and peroxidase) and two chromogenic agents. The enzyme assayed is either a LysoPLA or a phospholipase functioning as a LysoPLA.
NTE-LysoPLA activity is proportional to the paraoxonresistant and mipafox-sensitive increase in absorbance at 570 nm. More specifically, unless indicated otherwise, all reactants were added in 100 mM Tris buffer (pH 8.0) containing 1 mM calcium chloride and 0.01% Triton X-100. Reagent A contains 3-(N-ethyl-3-methylanilino)-2-hydroxysulfonate (3 mM), peroxidase (10 units/ml), sn-glycero-3-phosphocholine phosphodiesterase (0.0001 units/ml), and choline oxidase (10 units/ml). Reagent B contains 5 mM 4-aminoantipyrine. Reagents A (120 μl) and B (80 μl) were added to individual chambers containing Tris buffer as above (45 μl) in a 96-well polystyrene plate. Brain homogenate (15 μl) was added, followed by paraoxon in acetone (5 μl) and mipafox in 50 mM Tris–citrate (5 μl) to give 40 and 50 μM final concentrations, respectively. After a 20-min incubation, lysolecithin (250 μM final concentration) is introduced in Tris buffer (50 μl). The NTE-LysoPLA activity is measured by kinetic assay of absorbance at 570 nm for 10 min at 25°C by using a microplate reader (Molecular Devices). The activity was linear with regard to protein level and time.
NTE Activity Assays. The procedure is a modification for mouse brain (14) of a standard method for hen brain (12). Brain homogenate (50 μl) was added to Tris–EDTA buffer (as above; 950 μl). Paraoxon and mipafox were introduced as before but in 10 μl of solution to give 40 and 50 μM final concentrations, respectively. After a 20-min incubation, phenyl valerate (1.4 mM final concentration) was introduced in 0.03% Triton X-100 in Tris–EDTA buffer (1 ml) and incubated for 15 min. The reaction was stopped with 1% SDS and 0.025% 4-aminoantipyrine in water (1 ml). Addition of 0.04% potassium ferricyanide in water (0.5 ml) allowed colorimetric determination at 490 nm of phenol liberated by NTE.
In Vivo Inhibition of NTE-LysoPLA and NTE Activities and Poisoning Signs. Swiss–Webster mice were treated i.p. with the test compound in dimethyl sulfoxide (30–100 μl) or the carrier solvent alone as a control. They were killed after 4 h, and the brains were assayed for NTE-LysoPLA and NTE activities on the same preparations. Other mice in the treated group were rated for delayed toxic or neurotoxic signs (14) or death at 0.3–6 days (16).
Results
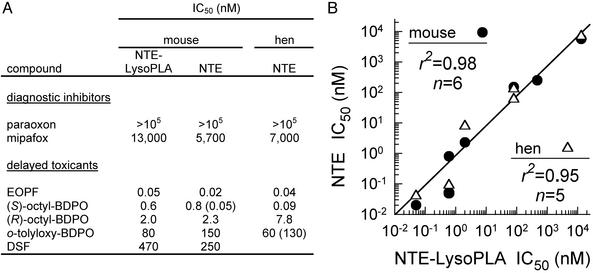
Diagnostic Inhibitors for Brain NTE-LysoPLA and NTE. Mouse brain NTE-LysoPLA and NTE are both insensitive to paraoxon (no inhibition at 105 nM) and moderately sensitive to mipafox (IC50 5,700–13,000 nM; Fig. 3). These OP compounds therefore are used as diagnostic inhibitors, determining the paraoxon (40 μM)-resistant and mipafox (50 μM)-sensitive portion of lysolecithin and phenyl valerate hydrolysis, respectively. The Lyso-PLA activity values [milliabsorbance units (mAU)/min, n = 13, mean ± SD] for brain from Swiss–Webster mice are 18.4 ± 3.2 with paraoxon and 15.3 ± 1.9 with paraoxon plus mipafox, giving NTE-LysoPLA activity of 3.1 (17% of the total lysolecithinhydrolyzing activity). The NTE activity values (AU, n = 15, mean ± SD) for Swiss–Webster mice are 0.60 ± 0.09 with paraoxon and 0.44 ± 0.06 with paraoxon plus mipafox, giving an NTE activity of 0.16 [10% of the total activity with no inhibitors, compared with 12% in our earlier report (14)]. These conditions therefore were standardized in assaying the mouse brain NTE-LysoPLA and NTE activities.
Fig. 3.
Relationship between in vitro sensitivities of brain NTE-LysoPLA and NTE activities. NTE-LysoPLA and NTE were assayed with lysolecithin and phenyl valerate, respectively. Compound structures are shown in Fig. 1. (A) The data for mouse NTE-LysoPLA for all compounds and mouse NTE for paraoxon, (S)-octyl-BDPO, and DSF are from this study with Swiss–Webster mice. Other data for mouse NTE are from this laboratory (14, 16) or Veronesi et al. (26) for o-tolyloxy-BDPO. The hen NTE data are from Wu and Casida (13, 15, 27), Johnson (11), and Veronesi et al. (26). (B) Correlation for sensitivity of NTE-LysoPLA from mouse brain versus NTE from mouse brain (•) and hen brain (▵). Correlation coefficients (r2) do not include tabulated values for paraoxon (>105 nM) or those in parentheses, which were determined by a different investigator (0.05 nM for mouse NTE) or a different laboratory (130 nM for hen NTE).
Relationship Between Brain NTE-LysoPLA and NTE Activities of NTE-Deficient Mice. Brain NTE-LysoPLA and NTE activities are somewhat higher for the wild-type 129S6/SvEvTac mice than for the Swiss–Webster mice given above. The LysoPLA activity values (mAU/min, n = 7, mean ± SD) for the +/+ mice are 14.3 ± 1.9 with paraoxon and 10.2 ± 1.4 with paraoxon plus mipafox, giving NTE-LysoPLA activity of 4.1 (≈29% of the total lysolecithin hydrolysis). The NTE activity values (AU, n = 22, mean ± SD) for the +/+ mice are 0.85 ± 0.07 with paraoxon and 0.53 ± 0.04 with paraoxon plus mipafox, giving an NTE activity of 0.32 (≈38% of the total phenyl valerate hydrolysis).
The brains of Nte+/- mice have 59% of the NTE-LysoPLA and 55% of the NTE activities of their wild-type littermates, i.e., highly significant reductions (P < 0.01) in both cases (Table 1). The similar reduction in activity for both NTE-LysoPLA and NTE with a single gene deletion indicates that the same enzyme is involved.
Table 1. Relationship between brain NTE-LysoPLA and NTE activities of NTE-deficient mice.
| Genotype* | NTE-LysoPLA, mAU/min† | NTE, AU† |
|---|---|---|
| Absolute activity | ||
| +/+ | 4.09±0.22 | 0.321±0.018 |
| +/- | 2.43±0.19‡ | 0.176±0.045‡ |
| Relative activity, % | ||
| +/-÷+/+ | 59 | 55 |
Nte heterozygous 129S6/SvEvTac (Nte+/-) transgenic mice and their wild-type littermates
NTE-LysoPLA and NTE assayed with lysolecithin and phenyl valerate, respectively. n = 7 for +/+ and 4 for +/- in each case as the average of four assays for NTE-LysoPLA and two for NTE. Data are mean ± SE
Significant difference (P < 0.01) for both NTE-LysoPLA and NTE (comparison of +/+ with +/-)
Relationship Between in Vitro Sensitivities of Brain NTE-LysoPLA and NTE Activities. Inhibitor specificity profiles provide a powerful method for differentiating one enzyme from another or establishing their identity. Six delayed toxicants or neurotoxicants, including mipafox, were examined along with the inactive paraoxon (Fig. 3A). Two of them [EOPF and (S)-octyl-BDPO] are highly potent OP inhibitors in both assays (IC50 0.02–0.8 nM). The same enantiomeric specificity of octyl-BDPO is evident with both NTE-LysoPLA and NTE; i.e., the S enantiomer is ≈3-fold more potent than the R enantiomer when determined with mouse brain in the same experiment. o-Tolyloxy-BDPO is of intermediate potency, and DSF is less potent but still similar in both assays. The data are conveniently compared as a correlation plot for NTE-LysoPLA versus NTE of mouse brain (r2 = 0.98, n = 6) or NTE-LysoPLA of mouse brain versus NTE of hen brain (r2 = 0.95, n = 5) (Fig. 3B), providing evidence that NTE-LysoPLA is identical to NTE under the assay conditions.
Relationship Between in Vivo Inhibition of Brain NTE-LysoPLA and NTE Activities. Six delayed toxicants or neurotoxicants were administered to mice, and the inhibition of NTE-LysoPLA activity was compared with that of NTE activity 4 h later (Table 2). This comparison included EOPF, three BDPO derivatives, and DSF, which act directly, and tribufos, which undergoes bioactivation (28) and therefore was not included in the in vitro assays above. The six compounds, three at multiple doses, inhibited NTE-LysoPLA and NTE activities to the same extent (r2 = 0.90, n = 13). Importantly, the high potency of EOPF and the same stereospecificity for (R)- and (S)-octyl-BDPO were observed for both assays, providing further evidence that NTE-LysoPLA and NTE are very similar or identical.
Table 2. Relationship between in vivo inhibition of brain NTE-LysoPLA and NTE activities and delayed toxicity.
| Enzyme inhibition, %*
|
|||
|---|---|---|---|
| Toxicant and dose, mg/kg | NTE-LysoPLA | NTE | Delayed toxicity |
| EOPF | |||
| 1 | 18 ± 15 | 0 ± 0 | -† |
| 2 | 89 ± 12 | 89 ± 2 | |
| 3 | 100 ± 0 | 78 ± 5 | +† |
| 10 | 99 ± 1 | 95 ± 4 | +† |
| (S)-octyl-BDPO | |||
| 5 | 71 ± 8 | 92 ± 7 | +† |
| (R)-octyl-BDPO | |||
| 5 | 7 ± 8 | 6 ± 7 | -† |
| o-Tolyloxy-BDPO | |||
| 3 | 20 ± 17 | 8 ± 4 | - |
| 10 | 70 ± 4 | 55 ± 13 | - |
| 30 | 89 ± 7 | 94 ± 7 | -‡ |
| 100 | 87 ± 16 | 100 ± 0 | -‡ |
| DSF | |||
| 100 | 92 ± 9 | 100 ± 0 | + |
| Tribufos | |||
| 30 | 7 ± 8 | 11 ± 9 | -§ |
| 100 | 85 ± 16 | 100 ± 0 | +§ |
Compounds were administered i.p. to Swiss-Webster mice with determinations of enzyme activities at 4 h and delayed toxicity at 3 days
NTE-LysoPLA and NTE were assayed with lysolecithin and phenyl valerate, respectively. n = 3 in each case as the average of duplicate assays for NTE-LysoPLA and single determinations for NTE. Data are mean ± SE
Ref. 14
Cholinergic poisoning signs at 100, but not 30, mg/kg
Ref. 16
Relationship Between in Vivo NTE-LysoPLA and NTE Inhibition and Delayed Toxicity. Swiss–Webster mice in 12 groups were examined for a possible relationship between NTE-LysoPLA and NTE inhibition and delayed toxicity (Table 2). Five sets of treatments gave 71–100% NTE-LysoPLA inhibition and 78–100% NTE inhibition with delayed toxicity. Another five sets gave enzyme inhibition of 7–70% for NTE-LysoPLA and 0–55% for NTE without delayed toxicity. An apparent exception is o-tolyloxy-BDPO at 30 and 100 mg/kg, with 87–100% NTE-LysoPLA and NTE inhibition and cholinergic signs at the higher dose. Importantly, EOPF and (S)-octyl-BDPO were the most effective NTE-LysoPLA and NTE inhibitors both in vitro and in vivo (Table 2; Fig. 3), and they are also the most potent delayed toxicants. Thus, in vivo inhibition of NTE-LysoPLA and NTE activities is generally correlated with delayed toxicity.
Discussion
Assignment of Mouse Brain NTE as NTE-LysoPLA. Four lines of evidence support the assignment of mouse brain NTE as a type of LysoPLA, designated here as NTE-LysoPLA: (i) Nte+/- transgenic mice are similarly deficient in NTE-LysoPLA activity, (ii) the potency of delayed toxicants or neurotoxicants as in vitro inhibitors is essentially the same for NTE-LysoPLA and NTE, (iii) these compounds inhibit NTE-LysoPLA and NTE activities in vivo to the same extent, and (iv) toxicant-induced in vivo inhibition of NTE-LysoPLA is generally predictive of delayed toxicity. Based on the same OP pharmacological profile as NTE in vitro and in vivo, NTE-LysoPLA seems to play a key role in OP-induced hyperactivity and delayed toxicity in mice.
LysoPLAs: A Large Family of OP-Sensitive Enzymes. The human proteome contains >100 lipid hydrolases (29). Within this, the LysoPLAs are a large family of enzymes (21). Characterized isoforms are either small (≈25 kDa) or large (>50 kDa), and several have been cloned from mouse, rat, human, and rabbit sources (21). They are present in many tissues, and more than one isoform can exist in a single cell (23). The relative roles these and other LysoPLAs play in regulating lysolecithin and other lysophospholipid levels in cells are largely unknown (21). Lyso-PLAs are the principal enzymes for removing lysophospholipids from cell membranes, including in human brain (30). LysoPLAs act on substrates within nerve membranes (21), and the activity of NTE requires a membrane environment (19). NTE-LysoPLA represents only a small portion of the total LysoPLA activity. It has an apparent molecular mass, based on NTE, of ≈155 kDa (31, 32), which differs from previously identified LysoPLAs (21). LysoPLAs of ≈25 kDa are inhibited by OPs such as diisopropyl fluorophosphate for enzyme from mouse macrophage-like cells (IC50 5 mM; ref. 23) and methyl arachidonyl fluorophosphonate (IC50 600 nM) for human brain recombinant LysoPLA (24). Much higher sensitivity is observed in the present study to octylphosphonates EOPF and (S)-octyl-BDPO (IC50 < 1 nM) for NTE-LysoPLA from mouse brain. NTE-LysoPLA hydrolyzes lysolecithin (21) and thus determines its localization and persistence. The localization in nerve of NTE-LysoPLA is probably the same as that of NTE as defined by immunohistochemical techniques for mouse (22) and hen (33), histochemical demonstration of NTE esterase activity (34), and autoradiographic detection of [3H]octyl-BDPO-labeled NTE for hen (35).
Lysolecithin, Receptor-Mediated Signal Transduction, and Demyelination. Lysophospholipids play an essential role in phospholipid metabolism, and in vivo levels are critical for cell survival and function (21). They are normally present at low concentrations in membranes (0.5–6% of total lipid weight), but under pathological conditions lysolecithin constitutes up to 40% of the total lipid, e.g., in atherogenic lipoproteins (21). Increased lysophospholipid levels are associated with a host of diseases such as hyperlipidemia, inflammation, and lethal dysrhythmias in myocardial ischemia (21). Lysolecithin is a major nerve constituent with several types of neuroactivity (23). It acts directly at G protein-coupled receptors and induces receptor-mediated signal transduction (36, 37). Most relevant here, lysolecithin causes demyelination of neuronal sheaths (also typical for human multiple sclerosis), often accompanied by axonal lesions as a rapid and localized effect (38–42). Interestingly, the OP delayed neurotoxicant diisopropyl fluorophosphate produces somewhat similar effects, inducing “chemical transection of the axon” and demyelination (5, 43), and direct treatment of one sciatic artery or nerve produces localized unilateral neuropathy (44, 45).
Relevance of Mouse Brain NTE-LysoPLA Model to OPIDN. Advances in determining NTE structure and function have come from studies with several species and levels of organization. Despite many common features, there appear to be species differences in the sequence of events between NTE inhibition and neurotoxicity. The Drosophila neurodegeneration gene swiss cheese encodes a neuronal protein involved in glial hyperwrapping and brain degeneration and homologous to human NTE, possibly relating it to OPIDN (46, 47). The mouse NTE protein is 96% identical to the human NTE protein (46). The primary neuropathological lesion of OPIDN in hens is a bilateral degenerative change in distal levels of axons and their terminals, mainly affecting larger/longer myelinated central and peripheral nerve fibers, leading to breakdown of neuritic segments and their myelin sheaths (5). Demyelination of nerve sheaths occurs in adult hens, whereas more restricted lesions are evident in rats (5). Mice respond somewhat differently than hens and humans to OP delayed neurotoxicants, with more rapid action (in 3–5 versus 10–14 days) and less pronounced neuropathy, i.e., delayed toxicity rather than OPIDN (5). However, mice have obvious advantages in mechanisms research (14, 22). The model considered here relates OP-induced hyperactivity (22) and delayed mortality (14) to inhibition of mouse brain NTE-LysoPLA activity. This relationship in turn may result in a localized increase in lysolecithin level, which induces a cell signaling cascade, possibly leading to hyperactivity (or demyelination in sensitive species).
This mouse model has some features in common with OPIDN in hens and people but differs in the neuropathological signs and apparently the requirement for NTE aging. Unresolved questions (4–6) include the mechanism of species differences, not only in the time delay and neurotoxic/neuropathic signs, but also in the importance of dealkylation (aging) of the inhibited enzyme, possibly with a “toxic gain of function” in hens (4, 6, 48) but apparently not in mice (22), and the observation that some potent inhibitors are not neuropathic but protect against subsequent challenge with a neuropathic OP. The established relationships and the anomalies now can be viewed in a new light focusing on the inhibition of NTE-LysoPLA and localized elevation of lysolecithin as possible contributors to OP-induced delayed toxicity in mice or even to OPIDN in hens and people. Perhaps the LysoPLA activity of NTE is not the only OP-sensitive function of this 155-kDa protein.
Acknowledgments
Helpful comments were provided by Matthew Hemming (The Salk Institute) and Edward Dennis (University of California at San Diego, La Jolla). This study was supported by National Institute of Environmental Health Sciences Grant R01 ESOO8762 (to J.E.C.), Department of Defense (U.S. Army Medical Research and Material Command) Grant DAMD 17-99-1-9561 (to C.B.), and a fellowship from the Canadian Institute of Health Research (to C.J.W.).
Abbreviations: OP, organophosphorus; OPIDN, OP-induced delayed neuropathy; BDPO, benzodioxaphosphorin oxide; NTE, neuropathy target esterase assayed with phenyl valerate as the substrate; EOPF, ethyl octylphosphonofluoridate; DSF, dodecanesulfonyl fluoride; LysoPLA, lysophospholipase; NTE-LysoPLA, NTE assayed with lysolecithin as the substrate; AU, absorbance units.
References
- 1.Casida, J. E. & Quistad, G. B. (1998) Annu. Rev. Entomol. 43, 1-16. [DOI] [PubMed] [Google Scholar]
- 2.Marrs, T. C., Maynard, R. L. & Sidell, F. R., eds. (1996) Chemical Warfare Agents: Toxicology and Treatment (Wiley, New York).
- 3.Taylor, P. (2001) in Goodman & Gilman's The Pharmacological Basis of Therapeutics, eds. Hardman, J. G., Limbird, L. E. & Gilman, A. G. (McGraw–Hill, New York), 10th Ed., pp. 175-191.
- 4.Johnson, M. K. & Glynn, P. (2001) in Handbook of Pesticide Toxicology, ed. Krieger, R. I. (Academic, San Diego), Vol. 2, pp. 953-965. [Google Scholar]
- 5.Ehrich, M. & Jortner, B. S. (2001) in Handbook of Pesticide Toxicology, ed. Kreiger, R. I. (Academic, San Diego), Vol. 2, pp. 987-1012. [Google Scholar]
- 6.Lotti, M. (2000) in Experimental and Clinical Neurotoxicology, eds. Spencer, P. S., Schaumburg, H. H. & Ludolph, A. C. (Oxford Univ. Press, New York), 2nd Ed., pp. 897-925.
- 7.Brown, M. A. & Brix, K. A. (1998) J. Appl. Toxicol. 18, 393-408. [DOI] [PubMed] [Google Scholar]
- 8.Winder, C. & Balouet, J.-C. (2002) Environ. Res. A 89, 146-164. [DOI] [PubMed] [Google Scholar]
- 9.Casida, J. E., Eto, M. & Baron, R. L. (1961) Nature 191, 1396-1397. [DOI] [PubMed] [Google Scholar]
- 10.Casida, J. E., Baron, R. L., Eto, M. & Engel, J. L. (1963) Biochem. Pharmacol. 12, 73-83. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, M. K. (1975) Arch. Toxicol. 34, 259-268. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, M. K. (1977) Arch. Toxicol. 37, 113-115. [DOI] [PubMed] [Google Scholar]
- 13.Wu, S.-Y. & Casida, J. E. (1995) Chem. Res. Toxicol. 8, 1070-1075. [DOI] [PubMed] [Google Scholar]
- 14.Wu, S.-Y. & Casida, J. E. (1996) Toxicol. Appl. Pharmacol. 139, 195-202. [DOI] [PubMed] [Google Scholar]
- 15.Wu, S.-Y. & Casida, J. E. (1994) Chem. Res. Toxicol. 7, 77-81. [DOI] [PubMed] [Google Scholar]
- 16.Quistad, G. B., Sparks, S. E., Segall, Y., Nomura, D. K. & Casida, J. E. (2002) Toxicol. Appl. Pharmacol. 179, 57-63. [DOI] [PubMed] [Google Scholar]
- 17.Glynn, P., Read, D. J., Guo, R., Wylie, S. & Johnson, M. K. (1994) Biochem. J. 301, 551-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lush, M. J., Li, Y., Read, D. J., Willis, A. C. & Glynn, P. (1998) Biochem. J. 332, 1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkins, J. & Glynn, P. (2000) J. Biol. Chem. 275, 24477-24483. [DOI] [PubMed] [Google Scholar]
- 20.van Tienhoven, M., Atkins, J., Li, Y. & Glynn, P. (2002) J. Biol. Chem. 277, 20942-20948. [DOI] [PubMed] [Google Scholar]
- 21.Wang, A. & Dennis, E. A. (1999) Biochim. Biophys. Acta 1439, 1-16. [DOI] [PubMed] [Google Scholar]
- 22.Winrow, C. J., Hemming, M. L., Allen, D. M., Quistad, G. B., Casida, J. E. & Barlow, C. (2003) Nat. Genet. 33, 477-485. [DOI] [PubMed] [Google Scholar]
- 23.Wang, A., Deems, R. A. & Dennis, E. A. (1997) J. Biol. Chem. 272, 12723-12729. [DOI] [PubMed] [Google Scholar]
- 24.Wang, A., Yang, H.-C., Friedman, P., Johnson, C. A. & Dennis, E. A. (1999) Biochim. Biophys. Acta 1437, 157-169. [DOI] [PubMed] [Google Scholar]
- 25.Kishimoto, T., Soda, Y., Matsuyama, Y. & Mizuno, K. (2002) Clin. Biochem. 35, 411-416. [DOI] [PubMed] [Google Scholar]
- 26.Veronesi, B., Padilla, S., Blackmon, K. & Pope, C. (1991) Toxicol. Appl. Pharmacol. 107, 311-324. [DOI] [PubMed] [Google Scholar]
- 27.Wu, S.-Y. & Casida, J. E. (1992) Chem. Res. Toxicol. 5, 680-684. [DOI] [PubMed] [Google Scholar]
- 28.Hur, J. H., Wu, S.-Y. & Casida, J. E. (1992) J. Agric. Food Chem. 40, 1703-1709. [Google Scholar]
- 29.Bracey, M. H., Hanson, M. A., Masuda, K. R., Stevens, R. C. & Cravatt, B. F. (2002) Science 298, 1793-1796. [DOI] [PubMed] [Google Scholar]
- 30.Ross, B. M. & Kish, S. J. (1994) J. Neurochem. 63, 1839-1848. [DOI] [PubMed] [Google Scholar]
- 31.Williams, D. G. & Johnson, M. K. (1981) Biochem. J. 199, 323-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida, M., Tomizawa, M., Wu, S.-Y., Quistad, G. B. & Casida, J. E. (1995) J. Neurochem. 64, 1680-1687. [DOI] [PubMed] [Google Scholar]
- 33.Glynn, P., Holton, J. L., Noland, C. C., Read, D. J., Brown, L., Hubbard, A. & Cavanagh, J. B. (1998) Neuroscience 83, 295-302. [DOI] [PubMed] [Google Scholar]
- 34.Koelle, G. B., Thampi, N. S., Han, M. S. & Olajos, E. J. (1989) J. Histochem. Cytochem. 37, 589-596. [DOI] [PubMed] [Google Scholar]
- 35.Kamijima, M. & Casida, J. E. (1999) Neurosci. Lett. 273, 101-104. [DOI] [PubMed] [Google Scholar]
- 36.Kabarowski, J. H. S., Zhu, K., Le, L. Q., Witte, O. N. & Xu, Y. (2001) Science 293, 702-705. [DOI] [PubMed] [Google Scholar]
- 37.Xu, Y. (2002) Biochim. Biophys. Acta 1582, 81-88. [DOI] [PubMed] [Google Scholar]
- 38.Hall, S. M. (1972) J. Cell Sci. 10, 535-546. [DOI] [PubMed] [Google Scholar]
- 39.Morell, P. (1984) Myelin (Plenum, New York), 2nd Ed.
- 40.Martenson, R. E. (1992) Myelin: Biology and Chemistry (CRC, Boca Raton, FL).
- 41.Quarles, R. H., Morell, P. & McFarland, H. F. (1999) in Basic Neurochemistry: Molecular, Cellular and Medical Aspects, eds. Siegel, G. J., Agranoff, B. W., Albers, R. W., Fischer, S. K. & Uhler, M. D. (Lippincott Williams & Wilkins, Philadelphia), 6th Ed., pp. 783-801.
- 42.Jean, I., Allamargot, C., Barthelaix-Pouplard, A. & Fressinaud, C. (2002) NeuroReport 13, 627-631. [DOI] [PubMed] [Google Scholar]
- 43.Bouldin, T. W. & Cavanagh, J. B. (1979) Am. J. Pathol. 94, 241-252. [PMC free article] [PubMed] [Google Scholar]
- 44.Caroldi, S., Lotti, M. & Masutti, A. (1984) Biochem. Pharmacol. 33, 3213-3217. [DOI] [PubMed] [Google Scholar]
- 45.Carrera, V., Barril, J., Mauricio, M., Pellín, M. & Vilanova, E. (1992) Toxicol. Appl. Pharmacol. 117, 218-225. [DOI] [PubMed] [Google Scholar]
- 46.Moser, M., Stempfl, T., Li, Y., Glynn, P., Büttner, R. & Kretzschmar, D. (2002) Mech. Dev. 90, 279-282. [DOI] [PubMed] [Google Scholar]
- 47.Kretzschmar, D., Hasan, G., Sharma, S., Heisenberg, M. & Benzer, S. (1997) J. Neurosci. 17, 7425-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forshaw, P. J., Atkins, J., Ray, D. E. & Glynn, P. (2001) J. Neurochem. 79, 400-406. [DOI] [PubMed] [Google Scholar]