Abstract
Organic production of one of the most popular botanical supplements, Echinacea, continues to expand in the U.S. Echinacea seeds typically show a high degree of dormancy that can be broken by ethephon or gibberelic acid (GA), but these methods are currently disallowed in organic production. In order to determine the efficacy of non-chemical seed treatments, we evaluated the effect of varying seed source and supplying light, with and without cold-moist stratification, on seed germination of the three most important medicinal species of Echinacea, E. angustifolia DC, E. purpurea (L) Moench, and E. pallida (Nutt.) Nutt. Treatments included cold-moist stratification under 24 h light, 24 h dark, and 16/8 h light/dark to break seed dormancy. We found that germination was greater in the E. purpurea and E. pallida seeds from a commercial organic seed source compared to a public germplasm source. When seeds were not cold-moist stratified, 16–24 h light increased germination in E. angustifolia only. Echinacea angustifolia, E. purpurea, and E. pallida seeds that were cold-moist stratified under 16–24 h of light for 4 wk had a significantly greater percentage and rate of germination compared to seeds germinated in the dark. Therefore, cold-moist stratification under light conditions is recommended as a method to break seed dormancy and increase germination rates in organic production of Echinacea.
Market sales of Echinacea-derived supplements reached more than $39 million in the U.S. in 1998 (Coltrain, 2001). Traditionally, Echinacea has been used to treat the common cold, coughs, bronchitis, upper respiratory infections, and some inflammatory conditions (Percival, 2000). Echinacea roots and leaves have also been reported to stimulate the immune system and assist in wound healing (Schulthess et al., 1991). The increase in consumption of botanical supplements has paralleled the growing importance of organic herb production in the U.S., which has increased from 2448 ha in 1997 to 5910 ha in 2001 (USDA-ERS, 2005). Organic production of medicinal herbs is governed by a set of rules (USDA-AMS, 2005) that prescribes avoidance of synthetic chemicals, commonly used to increase seed germination. Non-chemical, alternative seed treatments have been cited as a research need by organic producers (Walz, 2003).
Commercial production of Echinacea is traditionally seed-based, with seed source and seed quality reported to affect germination of E. purpurea (Hassel et al., 2004; Li, 1998; Wartidiningsih and Geneve, 1994) and E. angustifolia (Hassel et al., 2004), but not E. pallida (Hassel et al., 2004). There has been extensive experimentation on chemical, environmental and mechanical methods, such as scarification and seed priming, to break Echinacea and other species' seed dormancy (Feghahati and Reese, 1994; Pill and Haynes, 1996; Samfield et al., 1991; Steadman, 2004; Wees, 2004; Yamauchi et al., 2004) in order to synchronize emergence rates and improve seedling production efficiency. Ethephon pre-treatment in conjunction with cold-moist stratification provided optimum Echinacea seed germination (Sari et al., 1999), while GA (Feghahati and Reese, 1994; Macchia et al., 2001) and BA (6-benzylaminopurine) were found to be effective treatments for E. angustifolia (Chuanren et al., 2004). Qu et al. (2004) found that E. pallida and E. angustifolia seeds treated with ethephon under dark conditions had similar or greater germination percentages than seeds germinated with light.
While pre-chilling treatments alone increased both percentage and rate of germination in E. angustifolia (Feghahati and Reese, 1994; Macchia et al., 2001), cold-moist stratification increased Echinacea germination rates compared to seeds under a dry-cold treatment (Steadman, 2004; Wartidiningsih et al., 1994). Humidity during the stratification period appeared to be an important factor in releasing seeds from dormancy, as Echinacea germination was increased to 80% with cold-moist stratification compared to 1% in dry, cold-treated seeds (Shalaby et al., 1997). Yamauchi et al. (2004) found that Arabidopsis seeds stratified at 4 °C for 48 h exhibited increased levels of AtGA3ox, a cold-inducible BA biosynthesis gene directly involved in GA metabolism. Regarding ideal periods for cold-moist stratification, Chuanren et al. (2004) obtained optimum germination of E. angustifolia seeds at 18 days, while Baskin et al. (1992) recommended two to twelve weeks. Four weeks was adequate to break dormancy in E. purpurea (Bratcher et al., 1993); thus, the general recommendation for breaking seed dormancy in Echinacea spp. is 4–6 weeks of cold-moist stratification (Li, 1998).
The light-mediated phytochrome system was also found to be involved in regulation of GA biosynthesis in some seeds (Yamauchi et al., 2004). The effect of light was associated with the increase of mRNA in GA-3-oxidase, the enzyme that catalyzes the final steps of the biosynthetic pathway of bioactive GA (Yamaguchi and Kamiya, 2001). Light following pre-chilling did not affect germination in Echinacea (Wartidiningsih and Geneve, 1994) but Macchia et al. (2001) found that E. angustifolia seed germination was increased with a pre-chilling treatment in darkness when ethephon was not used. When ethephon was used in conjunction with a pre-chilling treatment, however, E. angustifolia seed germination was enhanced by providing light (Feghahati and Reese, 1994; Macchia et al., 2001). Baskin et al. (1992) also obtained higher rates of E. angustifolia germination when light was used with pre-chilling. No information on the effect of light during cold-moist stratification is available for E. purpurea and E. pallida.
Because synthetic chemicals are disallowed in organic production (USDA-AMS, 2005), seed germination without synthetic inputs is considered an important issue for organic Echinacea producers. Here we evaluated the effect of seed source, light during germination, and light during cold-moist-stratification on E. angustifolia, E. purpurea, and E. pallida seed germination. Methods developed from this research could provide useful guidelines for organic herb producers and others interested in non-chemical methods for improving seed germination.
Materials and Methods
Three experiments were conducted to examine the effects of non-chemical methods for increasing seed germination in Echinacea. Three species of Echinacea (E. angustifolia DC, E. purpurea (L.) Moench, and E. pallida (Nutt.) Nutt. were evaluated in each experiment. Seeds were obtained from two sources: Johnny's Selected Seeds (Albion, Maine), an organic seed company, and the North Central Regional Plant Introduction Station (NCRPIS) in Ames, Iowa. The cultivars and seed lots of the species obtained from Johnny's Seeds were E. angustifolia (Lot 17882), E. pallida (Lot 16481), and E. purpurea (Lot 19096). The Plant Introduction Station accession numbers and seed lots were E. angustifolia var. angustifolia PI 631285 (Lot 00ncai01), E. pallida PI 631293 (Lot 00ncai01), and E. purpurea PI 631307 (Lot 00ncai01). Seeds were placed in 100 × 15-mm Petri dishes containing two pieces of Whatman filter paper. Filter paper was soaked with deionized water before the experiment started as described in previous research (Wartidiningsih and Geneve, 1994). Each Petri dish contained 50 seeds and represented one replicate of each of the treatments. In each experiment, each treatment had three replicates. The three experiments were conducted in 2003 and repeated in 2004.
Experiment 1: Effect of seed source on germination
The objective of this experiment was to determine if greater Echinacea seed germination occurs in seeds from a commercial organic seed source compared to a regional public germplasm source. Because organic seed is required wherever commercially available in certified organic operations (USDA-AMS, 2005), seeds from Johnny's Selected Seeds (Albion, Maine) were compared with seeds from the North Central Regional Plant Introduction Station (Ames, Iowa) in this study. Eighteen Petri dishes containing 50 seeds each were placed in a growth chamber at 25 °C under 16/8 h light/dark conditions with cool-white fluorescent lamps providing a light intensity of 32 μmol· m−1· s−1. Because maximum seed germination of Echinacea had been shown to occur nine days following the stratification period (Li, 1998; Shalaby et al., 1997), germination was determined by counting the number of germinated seeds on days 3 and 10 after initiation of the experiment. Seeds were considered germinated if at least 2 mm of radicle had emerged.
Experiment 2: Light conditions during germination
A second experiment was conducted to evaluate the effect of light on seed germination of seeds without cold-moist stratification to determine if light alone could enhance germination. Seeds (Johnny's Selected Seeds, Albion, Maine) that had not been stratified were used for these experiments. For this experiment, Petri dishes containing 50 seeds each of the three Echinacea species were placed in a growth chamber at 25 °C, with cool-white fluorescent lamps providing a light intensity of 32 μmol· m−1· s−1, under the following treatments: 24 h of light, 24 h of darkness, and 16/8 h light/dark. A total of 1,350 seeds in 27 Petri dishes were evaluated in this experiment. Seeds were considered germinated if at least 2 mm of radicle was present.
Experiment 3: Light during cold-moist stratification
In order to evaluate the effect of light during cold-moist stratification, 27 Petri dishes containing 50 seeds each were kept at 4 °C in a growth chamber for 4 wk under the following treatments: 24 h of light, 24 h of dark, and 16/8 h light/dark. Cool-white fluorescent lamps provided a light intensity of 32 μmol· m−1· s−1 in the light periods during cold-moist stratification. Humidity was checked daily and water was added with a squeezable bottle as needed. After 4 wk of cold-moist stratification, Petri dishes were transferred to a growth chamber to observe seed germination. During the seed germination period, the temperature was 25 °C and light intensity was the same as during the cold-moist stratification period. Light was supplied for 16 h during this phase of the experiment based on the results of Experiment 2. A control treatment was included to compare germination in conditions without stratification. Seeds in the control treatment included the three Echinacea species that were not stratified. The control treatment was placed in the growth chamber under temperatures of 25 °C and 16 h of light. Germination was determined by counting the number of seeds with 2 mm or more of radicle growth.
Experiment design and data analysis
The first set of experiments (Experiments 1–3) was conducted from 10 February–22 March 2003, and the second set of experiments from 25 May–4 July 2004. A completely randomized design with three replicates of each treatment was used for each of the three experiments. Seed germination was evaluated with the general linear model procedure of the Statistical Analysis System®, and Fisher's test was used for mean treatment separation (SAS Institute, 2001).
Results and Discussion
Experiment 1: The effect of seed source on seed germination
Because of similar results in the 2003 and 2004 experiments (P = 0.5474), data were combined into a single analysis for this discussion. Seed source greatly affected the percentage of E. purpurea and E. pallida seed that germinated (Table 1). Germination was not observed on day 3 (data not presented), but by day 10, E. purpurea and E. pallida seeds from the commercial organic source exhibited higher percentages of germination compared to seeds from the regional public germplasm source (Table 1). Echinacea angustifolia germination, however, was equivalent between seed sources (Table 1). Germination, ranging from 66–71%, was statistically equivalent among the three species from the commercial organic seed source (Table 1), while E. angustifolia germination was greater than E. purpurea and E. pallida in public germplasm seeds (Table 1). Echinacea purpurea germination had been reported to be greater than E. angustifolia in previous studies (Shalaby et al., 1997), but in this study, E. purpurea and E. angustifolia germination rates were equivalent, with germination rates in E. angustifolia from both seed sources averaging 67%.
Table 1.
Seed germination comparison between two seed sources of three Echinacea species under 16 h of light when seeds were not cold-moist stratified.
|
Echinacea spp. |
||||
|---|---|---|---|---|
| Seed source | E. angustifolia | E. pallida | E. purpurea | |
| __________Germination (%)__________z | Significance: P valuey | |||
| Commercial organic | 68.2 ax | 70.7 a | 66.0 a | NS |
| Public germplasm | 66.2 a | 35.1 b | 20.9 b | < 0.0001 |
| Significance: P valuew | NS | < 0.0001 | < 0.001 | |
Germination was determined by counting the number of germinated seeds on day 10. Seeds were considered germinated if at least 2 mm of radicle were present.
P values in the last column represent comparisons among the different species within the same seed source.
Means within each column not followed by the same letter are significantly different at P = 0.05 according to Fisher's test.
Nonsignificant at P > 0.05; P value stated otherwise.
P values in the last row represent comparisons between seed sources.
As previously shown, seed source affected germination rates in E. purpurea (Wartidiningsih and Geneve, 1994) but contrary to the results of Hassel et al. (2004), seed source also affected germination of E. pallida in our study. Because NCRPIS' objectives include producing sufficient quantities of seeds to conserve specific plant populations and to make seeds freely available to the research community (Widrlechner and McKeown, 2002), Echinacea plant and subsequent seed selection consisted of an unbiased, random collection of all individuals representing a single population of native plant material. The seeds from the NCRPIS were provided specifically for this research with the populations selected based on viability and other information in the germplasm database. The commercial source selects seeds on an annual basis and maintains specific germination criteria for commercial production purposes. With native Echinacea populations, the age of the mother plant (Hassel et al., 2004), environmental conditions during the growing season, seed dormancy, and seed lot storage time (Wartidiningsih and Geneve, 1994) also affect subsequent seed germination. Thus, the percentage of dormant seeds in NCRPIS seed lots may have been greater than in the commercial organic source. However, many growers prefer local ecotype seeds from regional germplasm collections and this study confirms that germination from public seed sources may be considerably more variable than from a commercial organic seed source.
Experiment 2: The effect of light during germination of non-stratified seeds
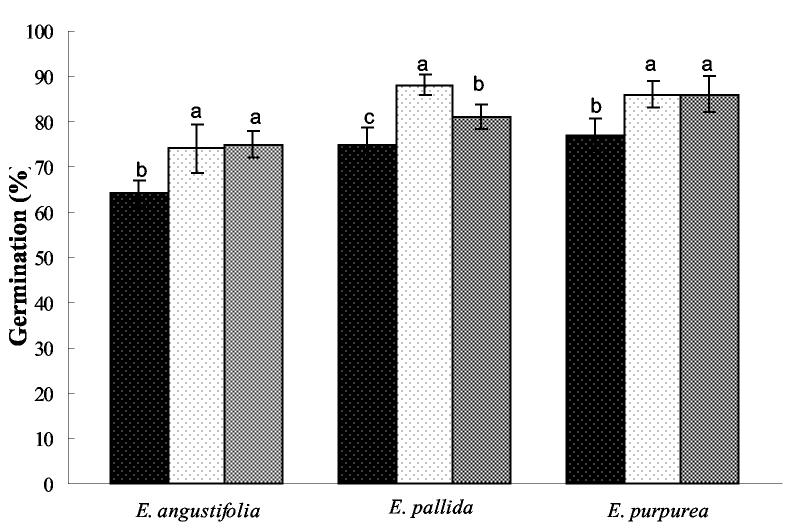
In determining the effect of light conditions on germination of non-stratified Echinacea seeds, we found a differential response among the three Echinacea species (P = 0.001) by day 10 (germination did not commence until after day 3, as previously observed). Germination of E. angustifolia increased by 10% under 16 or 24 h of light compared to dark conditions, while E. purpurea germination was 7% greater under dark conditions (Fig. 1). Echinacea pallida germination was equivalent under light and dark conditions (Fig. 1).
Figure 1.
Effect of light during germination of non-stratified E. angustifolia, E. pallida, and E. purpurea seeds. Germinated seeds were counted 10 days after initiation of the experiment. Treatments in were  24 h of dark,
24 h of dark,  24 h of light, and
24 h of light, and  16/8 h of light/dark at a constant temperature of 25 °C. Bars represent the standard error of the mean. Bars within each group with different letters are significantly different at P = 0.05 according to Fisher's test.
16/8 h of light/dark at a constant temperature of 25 °C. Bars represent the standard error of the mean. Bars within each group with different letters are significantly different at P = 0.05 according to Fisher's test.
This study suggested that Echinacea species show different levels of seed dormancy. These results are in concurrence with Yamauchi et al. (2004), where non-stratified seeds exposed for short periods of time to red light increased transcription levels of genes related to GA metabolism and seed germination. Thus, if cold-moist stratification is not feasible, light should be provided to E. angustifolia to improve germination rates.
Experiment 3: The effect of light during cold-moist stratification
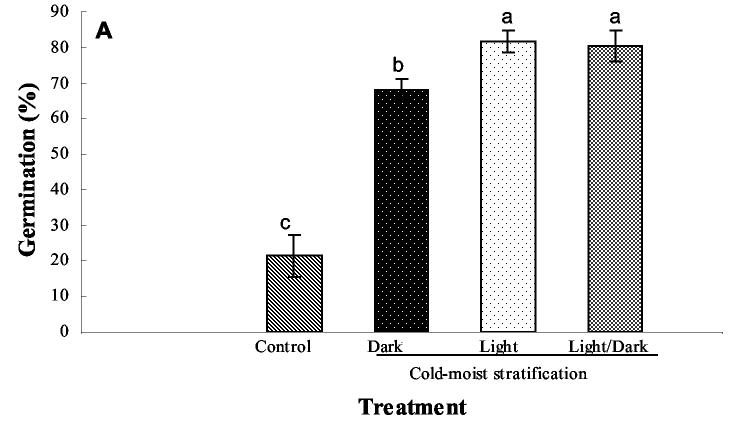
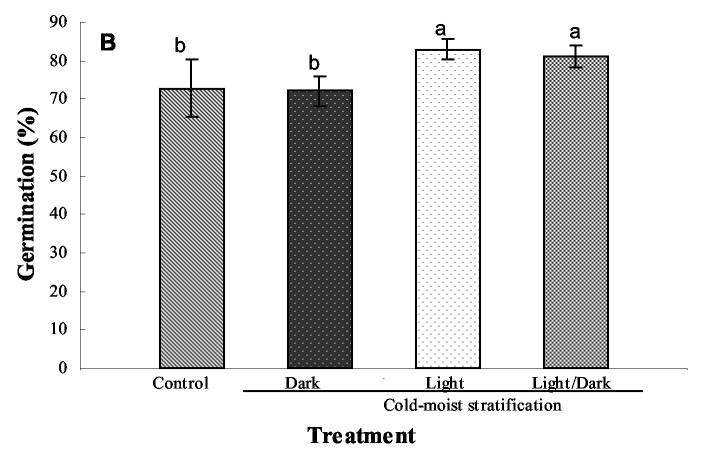
Germination rate and percentage of seeds germinated were improved by supplying light during cold-moist stratification of E. angustifolia, E. purpurea, and E. pallida. Enhancement of percentage and rate of seed germination in the three species of Echinacea was observed on day 3 (Fig. 2A) after the 4-wk stratification period (P = 0.0012) and on day 10 (P = 0.0029) (Fig. 2B). Overall germination rate was highest (82%) on the third day after the 4-wk cold-moist stratification period in seeds that were exposed to 24 h of light during the stratification period. The germination rate of seeds in the control treatment (ambient conditions) was only 21% on day 3 (Fig. 2A). Although the percentage of control seeds that germinated increased by day 10 to 72%, the greatest uniformity of seed germination occurred in the treatments receiving light for 16 or 24 h. By day 10, control seed germination was comparable to cold-moist stratified seeds under dark conditions (72%), but 10% less than the germination obtained in cold-moist stratified seeds under 16 or 24 h of light (Fig. 2B). This result is important for organic producers because, with the low cost of placing seeds in a refrigerated storage unit and providing water and light, greater uniformity in seedling emergence and subsequent synchronized transplanting, flowering, and harvest can be achieved.
Figure 2.
Effect of light during cold-moist stratification on Echinacea (combined E. angustifolia, E. pallida, and E. purpurea) seed germination. The cold-moist stratification treatment consisted of 4 wk at 4 °C under either  24 h of light,
24 h of light, 24 h of dark, or
24 h of dark, or  16/8 h of light/dark. Control treatment
16/8 h of light/dark. Control treatment  was not cold-moist stratified. Seed germination was conducted under 16/8 h of light/dark at 25 °C and determined (A) at 3 d and (B) 10 days after removal from the stratification treatment. Control represents seeds germinated under 16/8 h of light/dark at 25 °C but with no stratification. Bars represent the standard error of the mean. Bars with different letters are significantly different at P = 0.05 according to Fisher's test.
was not cold-moist stratified. Seed germination was conducted under 16/8 h of light/dark at 25 °C and determined (A) at 3 d and (B) 10 days after removal from the stratification treatment. Control represents seeds germinated under 16/8 h of light/dark at 25 °C but with no stratification. Bars represent the standard error of the mean. Bars with different letters are significantly different at P = 0.05 according to Fisher's test.
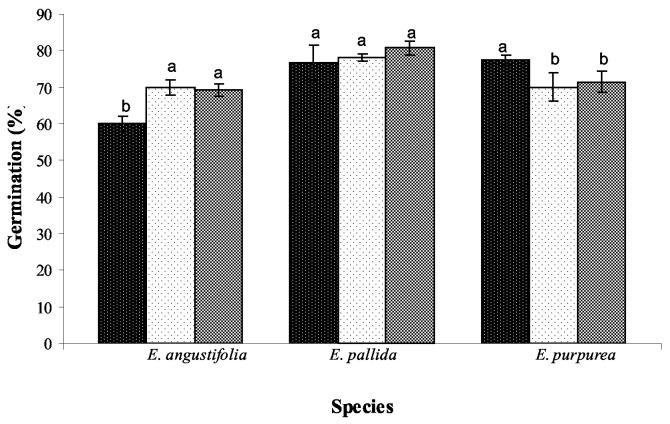
There were no differences in germination rates between E. pallida and E. purpurea seeds exposed to light during cold-moist stratification (Fig.3). Light conditions, on average, increased germination rates among all Echinacea species by 10% (Fig.3). The greatest germination percentage (88%) was obtained in E. pallida seeds under 24 h of light or 16/8 h light/dark conditions during cold-moist stratification. Seeds of E. angustifolia that were under 24 h of darkness during this period showed the lowest percentage of germination (64%), which was ≈10% less than seeds that had been exposed to light for 24 h during cold-moist stratification (Fig. 3).
Figure 3.
Effect of light during cold-moist stratification on seed germination of E. angustifolia, E. pallida, and E. purpurea. Germination was counted 10 d after removal from the stratification treatment. Treatments during cold-moist stratification were  24 h of dark,
24 h of dark,  24 h of light, and
24 h of light, and  16/8 h of light/dark. After the stratification period, seeds were placed to germinate under 16/8 h of light/dark at 25 °C. Bars represent the standard error of the mean. Bars within each group with different letters are significantly different at P = 0.05 according to Fisher's test.
16/8 h of light/dark. After the stratification period, seeds were placed to germinate under 16/8 h of light/dark at 25 °C. Bars represent the standard error of the mean. Bars within each group with different letters are significantly different at P = 0.05 according to Fisher's test.
As demonstrated by others, cold-moist stratification was shown in this experiment to be an effective method to break seed dormancy in E. purpurea (Bratcher et al., 1993; Wartidiningsih et al., 1994) and in E. angustifolia (Baskin et al., 1992; Macchia et al., 2001). Our results, unlike those reported by Macchia et al. (2001), demonstrated a positive effect from light during the cold-moist-stratification period. Germination percentages greater that 70% for all species of Echinacea were obtained after 4 wk of cold-moist stratification under 16–24 h of light. This rate is comparable to that obtained by Baskin et al. (1992) for E. angustifolia after 8 wk of cold-stratification. Based on our results, the time required to break seed dormancy may be reduced by up to 4 wk by supplying light during the cold-moist stratification period. As suggested by Yamauchi et al. (2004), light and cold temperature may have a synergetic effect on seed germination, with temperature being the most important factor to activate genes associated with GA metabolism. Based on Hilhorts (1998) hypothesis, when light is supplied during the stratification period, the germination process is accelerated, as seen in our experiments.
Cold-moist stratification not only increased the percentage of germinating seeds in this experiment, but also germination rate, as previously reported by Wartidiningsih et al. (1994). Seeds treated with low temperature and light began to germinate 48 h after the treatments, compared to untreated seeds which germinated on day 6. Germination was increased to levels comparable to those obtained when ethephon and cold treatments were applied at the same time (Feghahati and Reese, 1994) and resulted in a high percentage of seeds that germinated at the same time, which favors uniformity in plant size and time to transplanting. The germination percentages and rates were also similar to those obtained when seeds were primed (Samfield et al., 1991; Pill et al., 1994). In addition, seed source had a significant effect on E. pallida and E. purpurea seed germination.
In conclusion, based on these experiments, cold-moist stratification at 4 °C for 4 wk under 16–24 hr of light served as an effective, alternative method for breaking Echinacea seed dormancy that does not require extensive expertise or special equipment (Wartidiningsih et al., 1994). While germination of Echinacea has generally been reported in a lower range, we observed that seeds of the three most medicinally important Echinacea species from the commercial organic seed source had germination rates in the range of 66–82% in this study. Therefore, cold-moist stratification of commercial organic seeds under light conditions is recommended for optimal organic Echinacea production.
Acknowledgments
This publication was made possible by grant number P01 ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), NIH and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. We also thank Dr. Mark P. Widrlechner, USDA-ARS North Central Regional Plant Introduction Station, for providing the seeds for our experiments, Johnny's Selected Seeds, Albion, Maine, for organic seeds, and Dr. Rajeev Arora, Horticulture Department, Iowa State University, for use of cold rooms and advice on this project. We thank the Leopold Center for Sustainable Agriculture for providing additional support for this project.
Footnotes
This is a journal paper of the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa. This research has been supported by Hatch Act, State of Iowa and the National Institute of Health funds.
Additional index words. Botanical herbs, ethephon, non-chemical production, seed dormancy
Literature Cited
- Baskin CC, Baskin JM, Hoffman GR. Seed dormancy in the prairie for Echinacea angustifolia var. angustifolia (Asteraceae): Afterripening pattern during cold stratification. Int. J. Plant Sci. 1992;153:239–243. [Google Scholar]
- Bratcher CB, Dole JM, Cole JC. Stratification improves seed germination of five native wildflower species. HortScience. 1993;28:899–901. [Google Scholar]
- Chuanren D, Bochu W, Wanqian L, Jing C, Jie L, Huan A. Effect of chemical and physical factors to improve the germination rate of Echinacea angustifolia seeds. Colloids and Surfaces B: Biointerfaces. 2004;37:101–105. doi: 10.1016/j.colsurfb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Coltrain D. Economic issues with Echinacea. 2001 Kansas State University Extension Publication MF-2532. [Google Scholar]
- Feghahati SM, Reese RN. Ethylene-, light-, and prechill-enhanced germination of Echinacea angustifolia seeds. J. Amer. Soc. Hort. Sci. 1994;119:853–858. [Google Scholar]
- Hassel RL, Dufault R, Phillips T. Relationship among seed size, source and temperature on germination of Echinacea angustifolia, E. pallida and E. purpurea. Acta Hort. 2004;629:239–243. [Google Scholar]
- Hilhorst H. The regulation of secondary dormancy. Seed Science Research. 1998;8:77–90. [Google Scholar]
- Li T. Echinacea: Cultivation and medicinal value. HortTechology. 1998;8:122–129. [Google Scholar]
- Macchia M, Angelini LG, Ceccarini L. Methods to overcome seed dormancy in Echinacea angustifolia DC. Scientia Horticulturae. 2001;89:317–324. [Google Scholar]
- Percival SS. Use of Echinacea in Medicine. Biochemical Pharmacology. 2000;60:155–158. doi: 10.1016/s0006-2952(99)00413-x. [DOI] [PubMed] [Google Scholar]
- Pill WG, Crossan CK, Frett JJ, Smith WG. Matric and osmotic priming of Echinacea purpurea (L.) Moench. seeds. Scientia Horticulturae. 1994;59:37–44. [Google Scholar]
- Pill WG, Haynes JG. Gibberellic acid during priming of Echinacea purpurea (L.) Moench. seeds improves performance after seed storage. J. Hort. Sci. 1996;71:287–295. [Google Scholar]
- Qu L, Wang X, Yang J, Hood E, Scalzo R. Ethephon promotes germination on Echinacea angustifolia and E. pallida in darkness. HortScience. 2004;39:1101–1103. [PMC free article] [PubMed] [Google Scholar]
- Samfield DM, Zajicek JM, Cobb BG. Rate and uniformity of herbaceous perennial seed germination and emergence as affected by priming. J. Amer. Soc. Hort. Sci. 1991;116:10–13. [Google Scholar]
- Sari AO, Morales MR, Simon JE. Echinacea angustifolia: an emerging medicinal. In: Janick J, editor. Perspectives on New Crops and New Uses. ASHS Press; Alexandria, VA: 1999. pp. 490–493. [Google Scholar]
- SAS Institute . SAS user's guide: Statistics. Version 8.2 ed. Statistical Analysis Service Institute Inc.; Cary, NC: 2001. [Google Scholar]
- Schulthess BH, Giger E, Baumann TW. Echinacea: Anatomy, phytochemical pattern, and germination of the achene. Planta Med. 1991;57:384–388. doi: 10.1055/s-2006-960123. [DOI] [PubMed] [Google Scholar]
- Shalaby AS, Agina EA, El-Gengaihi SE, El-Khayat AS, Hindawy SF. Response of Echinacea to some agricultural practices. Journal of Herbs, Spices & Medicinal Plants. 1997;4:59–67. [Google Scholar]
- Steadman K. Dormancy release during hydrated storage in Lolium rigidum seeds is dependent on temperature, light quality, and hydration status. J. of Experimental Botany. 2004;55:929–937. doi: 10.1093/jxb/erh099. [DOI] [PubMed] [Google Scholar]
- USDA-AMS (U.S. Department of Agriculture -Agriculture Marketing Service) National Organic Program. Final Rule: 7 CFR Part 205, USDA-AMS. Washington, D.C.: Jan 10, 2005. http://www.ams.usda.gov/nop 2005. [Google Scholar]
- USDA-ERS (U.S. Department of Agriculture–Economic Research Service) Organic Production Data. USDA-ERS. Washington, D.C.: Jan 10, 2005. http://www.ers.usda.gov/data/Organic/ 2005. [Google Scholar]
- Walz E. Final Results of the Fourth Biennial National Organic Farming Survey. Organic Farming Research Foundation; Santa Cruz, CA: 2003. [Google Scholar]
- Wartidiningsih N, Geneve RL. Seed source and quality influence germination in purple coneflower [Echinacea purpurea (L.) Moench] HortScience. 1994;29:1443–1444. [Google Scholar]
- Wartidiningsih N, Geneve RL, Kester ST. Osmotic priming or chilling stratification improves seed germination of purple coneflower. HortScience. 1994;29:1445–1448. [Google Scholar]
- Wees D. Stratification and priming may improve seed germination of purple coneflower, blue-flag iris and evening primrose. Acta Hort. 2004;629:391–394. [Google Scholar]
- Widrlechner MP, McKeown KA. Assembling and characterizing a comprenhensive Echinacea germplasm collection. In: Janick J, editor. Perspectives on New Crops and New Uses. ASHS Press; Alexandria, VA: 2002. pp. 506–508. [Google Scholar]
- Yamaguchi S, Kamiya Y. Gibberellins and light-stimulated seed germination. J. Plant Growth Regul. 2001;20:369–376. doi: 10.1007/s003440010035. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. The Plant Cell. 2004;16:367–378. doi: 10.1105/tpc.018143. [DOI] [PMC free article] [PubMed] [Google Scholar]