Abstract
Little is known concerning the mechanisms by which auxin and cytokinin exert their effects on proliferation and differentiation. Cyclin-dependent kinases (CDKs) are major regulators of the eukaryotic cell cycle, thus they are assumed to control cell differentiation as well as proliferation in response to phytohormone signals. Here, we overexpressed rice R2 cDNA, which encodes a CDK-activating kinase, in tobacco leaf explants by using the glucocorticoid-mediated transcriptional induction system. Transient expression of R2 during the first 7 days of culture triggered callus formation in the absence of cytokinin. This phenotype was enhanced by higher expression of R2 or coexpression of cyclin H, and suppressed by treatment with roscovitine, a CDK inhibitor. R2 expression at a later stage did not prevent cells from differentiation into roots, suggesting a restricted period for sensing CDK activities that control differentiation fate of cells during organogenesis.
Organogenesis occurs in various plant tissue cultures in response to exogenously added phytohormones, mainly auxin and cytokinin (1). High auxin/cytokinin ratios in the medium usually induce root formation whereas low auxin/ cytokinin ratios promote shoot formation. On the other hand, media containing intermediate auxin/cytokinin ratios promote disorganized cellular proliferation and callus formation. Previous studies have shown that induction of shoots or roots from explants could be divided into three stages (2–4). In the first stage, the cells acquire competence for subsequent cell proliferation and differentiation; during the second stage, the developmental fate of competent cells is determined; and the third stage is devoted for differentiation and development of determined organs. However, the molecular mechanism(s) that govern the developmental fate of cells during organogenesis, remains poorly understood.
For the continuous operation of meristematic organization during plant development, cell division activity must be tightly controlled by machinery that regulates the cell cycle. The major regulators of eukaryotic cell cycle are cyclin-dependent kinases (CDKs) and their regulatory partner cyclins. We previously showed that reduced activity of CDK in planta resulted in differentiation of root initial cells before cessation of cell division (5). This finding suggested that the indeterminate state of initial cells is controlled independent of cell division, and the level of CDK activity might define the differentiation state of cells to coordinate cell division and differentiation in the meristem.
Activity of CDKs is also regulated by phosphorylation. CDK-activating kinase (CAK) phosphorylates CDKs at a conserved threonine residue on the T-loop region and activates their enzyme activities. In vertebrate and fission yeast, catalytic subunit of CAK is a member of the CDK family, termed CDK7/p40MO15 (6–8), which is activated by making a complex with cyclin H (9–11) and the stabilizing factor MAT1 (12–14). Rice R2 is closely related to CDK7 (15). Previously, we showed that R2 has CDK kinase activity (16), and rice cyclin H, termed Os;CycH;1, specifically interacts with R2 and activate its kinase activity. This finding suggested that R2 is a functional homologue of vertebrate-type CAKs (17).
Here, to investigate how cell fate is determined during in vitro organogenesis in terms of CDK activity, we overexpressed R2 cDNA in tobacco leaf explants by using the glucocorticoid-inducible system. We found that up-regulation of CDK activities converted root organogenesis on an auxin-rich medium into disorganized cellular proliferation. Our data indicate that the level of CDK activity at the early phase of organogenesis controls the differentiation fate of leaf cells. We propose that CDK activity is the major determinant of cell differentiation to accomplish proper development of organs.
Materials and Methods
Plant Transformation. The coding region of R2 cDNA (16) was cloned into XhoI–SpeI sites of pTA7001 vector (18) in sense orientation and introduced into tobacco (Nicotiana tabacum cv Petite Havana SR1) via Agrobacterium-mediated transformation. Transgenic seedlings were selected on Murashige and Skoog (MS) plates (19) containing 40 μg/ml hygromycin. Assays were performed on homozygous T2 progeny. The ORF of Os;cycH;1 was cloned into XbaI–SacI sites of 5× GAL4 vector, which contains five repeated GAL4 DNA-binding sequence of 23 bp (gifted from Peter Doerner, University of Edinburgh, Edinburgh). The resultant plasmid was digested with SpeI and SacI, and the 5× Gal-Os;cycH;1 fragment was subcloned into XbaI–SacI sites of pSPTV20 vector (20). This plasmid was introduced into the R2 transgenic line R1-1, and transgenic seedlings carrying both R2 and Os;cycH;1 cDNAs were selected on MS plates containing 40 μg/ml hygromycin and 50 μg/ml kanamycin. Plants were grown at 27°C under normal greenhouse conditions.
Leaf Section Assay. Tobacco mature leaves were sterilized in 1% (vol/vol) sodium hypochlorite solution for 10 min. After washing with sterilized water, leaves 5–7 mm square were cultured on Linsmaier–Skoog (LS) medium (21) containing various concentrations of naphthaleneacetic acid (NAA), kinetin, dexamethasone (DEX) (Sigma), or 100 μM roscovitine (Calbiochem) at 25°C under 16 h dark and 8 h light (≈3,000 lux) conditions. Leaf discs (6 mm in diameter) were cut out by using cork bowler, and their weight was measured after a 4-week culture. Cytokinin contents of leaf sections were measured as described by Miyazawa et al. (22).
Expression Analysis. Immunoblotting was conducted with anti-R2 antibody as described (16). Total RNA (1 μg) was used as template for RT-PCR with specific primers for R2, 5′-GGAATTCAGCTTCCAAGGCCACCACCT-3′ and 5′-GGAATTCTTACTCTGTGTACCAAATTCTG-3′, and for Os;cycH;1, 5′-GAAAATCATGTTTCTGCTGAG-3′ and 5′-TCCTTCGATAGATCGATACGG-3′, respectively. The conditions for direct amplification with Ready-To-Go RT-PCR Beads (Amersham Pharmacia) were 1 cycle at 42°C for 15 min and at 95°C for 5 min; 25 cycle at 95°C for 1 min, at 55°C for 1 min, and at 72°C for 45 s. As a control, specific primers for 18S rRNA (Quantum RNA Plants 18S, Ambion, Austin, TX) were used. CDK2 kinase activities of the immunoprecipitates with anti-R2 antibody were assayed as described by Yamaguchi et al. (16). Protein extracts (50 μg) from rice suspension cells were incubated with p13Suc1-agarose (Calbiochem) in 200 μl of extraction buffer (16) for 2 h at 4°C. The proteins bound to p13Suc1-agarose were assayed for histone H1-kinase activity as described by De Azevedo et al. (23).
Results and Discussion
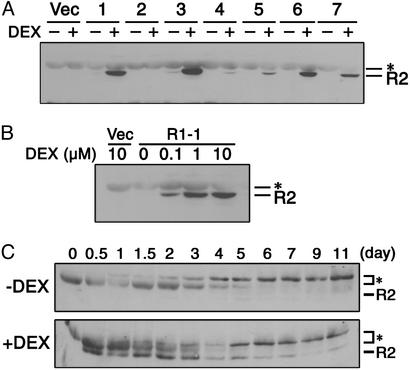
Overexpression of R2 in Tobacco Leaf Explants Caused Callus Formation in the Absence of Cytokinin. We generated transgenic tobacco plants that overexpressed sense mRNA of R2 by using the glucocorticoid-mediated transcriptional induction system (18). In this system, a glucocorticoid derivative, DEX, activates a transcription factor called GVG (18), which in turn induces R2 expression. Out of seven independent transgenic lines in which R2 expression was examined, six lines showed an increase of the R2 protein level after treatment with 1 μM DEX for 24 h (Fig. 1A). Because line R1-1 showed a higher expression, which was absolutely dependent on DEX treatment, homozygous T2 progeny of this line was mainly used for further analysis. Note that leaf sections of the other R2-expressing lines produced almost the same results as described below.
Fig. 1.
Expression analysis of R2 protein in transgenic tobacco plants. (A) Ten micrograms of proteins extracted from transgenic seedlings treated with (+) or without (-)1 μM DEX for 24 h were immunoblotted with anti-R2 antibody. Vector control line (Vec) and R2 transgenic lines R1-1 (lane 1), R2-2 (lane 2), R6-5 (lane 3), R9-3 (lane 4), R13-2 (lane 5), R16-2 (lane 6), and R19-1 (lane 7) are shown. (B) Transgenic seedlings of the vector control line (Vec) and the transgenic line R1-1 were treated with the indicated concentrations of DEX for 24 h, and 10 μg of protein extracts was immunoblotted with anti-R2 antibody. (C) Leaf sections of the transgenic line R1-1 were cultured on media containing 2.0 μg/ml NAA with (+) or without (-) 1 μM DEX for indicated days, and 30 μg of protein extracts was immunoblotted with anti-R2 antibody. Asterisks indicate endogenous tobacco proteins showing nonspecific cross-reaction with the R2 antibody.
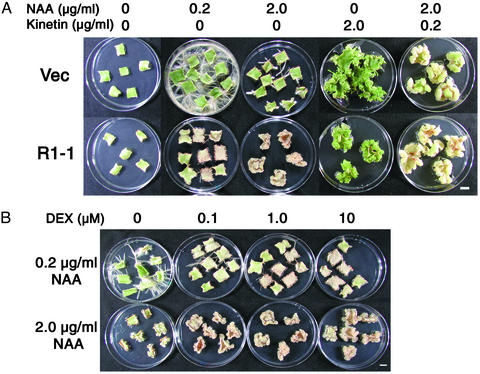
To examine the effect of exogenously added hormones on in vitro organogenesis, we cultured leaf sections of the transgenic plants on media containing 1 μM DEX and auxin (naphthaleneacetic acid, NAA) and/or cytokinin (kinetin). Control leaf sections carrying the empty vector produced roots or shoots in the presence of 0.2 μg/ml NAA or 2.0 μg/ml kinetin, respectively, whereas in the presence of 2.0 μg/ml NAA and 0.2 μg/ml kinetin, calli were formed (Fig. 2A). On R1-1 leaf sections, production of roots was markedly inhibited in media containing 0.2 μg/ml NAA, whereas shoot and callus formation was not as severely retarded as in vector control sections. Surprisingly, R1-1 explants produced calli in the presence of a higher concentration (2.0 μg/ml) of NAA (Fig. 2 A). These calli could be maintained for extended periods of subcultures in the same condition (data not shown). The cytokinin-independent induction of calli was not caused by an inherently higher cytokinin level in R2-expressing explants, as there was no difference in the content of isoprenoid-type cytokinins between samples cultured with and without DEX (data not shown). In contrast, vector control sections cultured in the presence of 2.0 μg/ml NAA produced fewer slowly growing roots (Fig. 2 A).
Fig. 2.
Callus induction on R2-expressing leaf explants in the absence of cytokinin. (A) Leaf sections of the vector control line (Vec) and the transgenic line R1-1 were cultured for 4 weeks on media containing 1 μM DEX and indicated concentrations of NAA and/or kinetin. (Bar, 1 cm.) (B) Leaf sections of the transgenic line R1-1 were cultured for 4 weeks on media containing the indicated concentrations of NAA and DEX. (Bar, 1 cm.)
We also cultured leaf explants in the presence of different concentrations of DEX (Fig. 2B). On a medium containing 0.2 μg/ml NAA, the R1-1 sections produced roots in the absence of DEX, whereas root formation was inhibited in the presence of high concentrations of DEX. In contrast, the growth of calli on media containing 2.0 μg/ml NAA was enhanced by increasing the concentration of DEX. The R2 protein level depended on DEX concentration (Fig. 1B), suggesting that ectopic R2 expression caused callus formation in auxin-rich media.
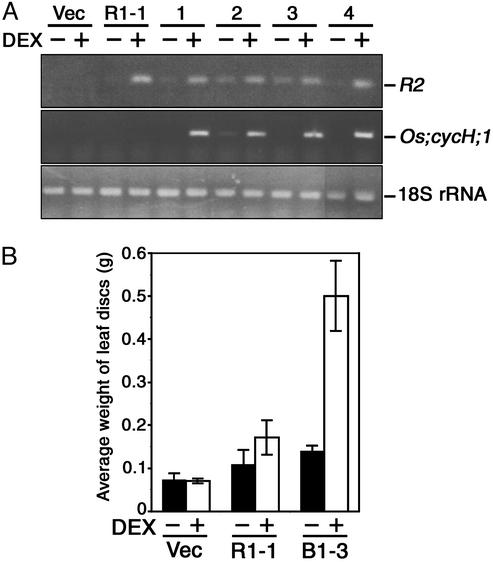
Coexpression of R2 and Os;cycH;1 Enhanced the Growth of Calli. We then expressed the cDNA of rice cyclin H (Os;cycH;1), which activates R2 by direct interaction (17). To obtain transgenic plants expressing both R2 and Os;cycH;1 in a DEX-dependent manner, we inserted the Os;cycH;1 cDNA under the GAL4 binding sequence, and introduced it into the transgenic line R1-1 in which GVG transcription factor could induce the expression of Os;cycH;1 as well as R2. The results of RT-PCR showed that both R2 and Os;cycH;1 mRNA accumulated in four independent lines by treatment with 1 μM DEX for 24 h (Fig. 3A). Leaf discs (6 mm in diameter) of transgenic plants were cultured on media containing 2.0 μg/ml NAA with or without 1 μM DEX, and their weight was measured after 4 weeks. As shown in Fig. 3B, coexpression of R2 and Os;cycH;1 enhanced the growth of calli compared with the solely R2-expressing line R1-1. This finding suggests that the kinase activity of R2, which was elevated by Os;cycH;1 expression, was responsible for callus induction in auxin-rich media.
Fig. 3.
Coexpression of R2 and Os;cycH;1 in tobacco leaf sections. (A) Amplification of R2 and Os;cycH;1 cDNAs by RT-PCR. Transgenic seedlings were treated with (+) or without (-)1 μM DEX for 24 h, and 1 μg of total RNA was used for RT-PCR with specific primers for R2, Os;cycH;1, or 18S rRNA. The vector control line (Vec), the R2 transgenic line R1-1, and lines transformed with both R2 and Os;cycH;1 cDNAs, B1-1 (lane 1), B1-3 (lane 2), B1-9 (lane 3), and B1-12 (lane 4) are shown. (B) Enhancement of callus growth by coexpression of Os;cycH;1 with R2. Leaf discs (measuring 6 mm in diameter) carrying the empty vector (Vec), R2 cDNA (R1-1), or both R2 and Os;cycH;1 cDNAs (B1-3) were cultured for 4 weeks on media containing 2.0 μg/ml NAA with (open bars) or without (solid bars) 1 μM DEX, and their weight was measured. Data are mean ± SD of 18 samples.
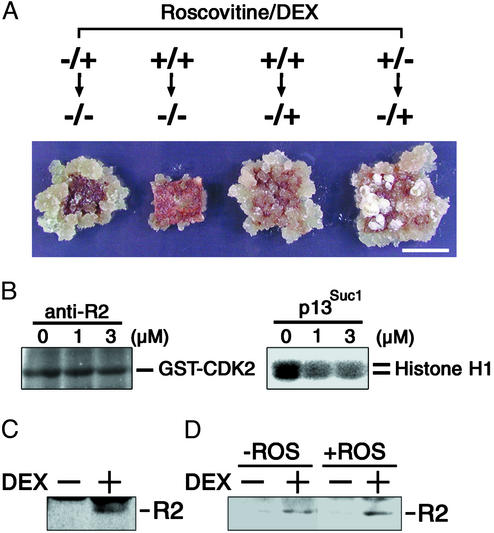
Up-Regulation of CDK Activities at the Early Phase of Culture Was Necessary and Sufficient for Callus Induction. To examine R2 expression in leaf cells, we performed immunoblot analysis of the R1-1 leaf sections. As shown in Fig. 1C, R2 protein was detectable after 12 h of DEX treatment, remained at a constant level until 48 h, and then gradually disappeared, whereas it was never detected in leaf sections cultured without DEX. Considering that callus became visible after ≈10 days of DEX treatment, the transient expression of R2 at the early phase of culture might induce callus detectable in the later stage. To examine whether transient expression of R2 is sufficient for callus formation, we took advantage of the glucocorticoid-inducible system. When leaf sections cultured on a DEX-containing medium for 7 days were transferred to a DEX-free medium, calli were produced as in the case of continuous DEX treatment (Fig. 4A). This finding clearly shows that R2 expression during the first 7 days at the longest was necessary and sufficient for callus induction. Similar results were obtained by cytokinin application instead of R2 expression. Namely, the first 5 days culture with 0.2 μg/ml kinetin was sufficient for calli formation from leaf explants (see Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org).
Fig. 4.
Effect of R2 expression and roscovitine on callus induction. (A) Leaf sections of the transgenic line R1-1 were cultured on a medium containing 2.0 μg/ml NAA. For the first 7 days, they were cultured in the presence (+) or absence (-) of 100 μM roscovitine and 1 μM DEX, and then cultured for 3 weeks on a roscovitine-free medium with (+) or without (-)1 μM DEX. (Bar, 1 cm.) (B) Specific inhibition of CDK activities by roscovitine. Protein extracts (50 μg) prepared from rice suspension cells were immunoprecipitated with anti-R2 antibody or precipitated with p13Suc1-agarose, and the immunoprecipitates or proteins bound to the p13Suc1-agarose were assayed for CDK2- and histone H1-kinase activities, respectively, in the presence of indicated concentrations (μM) of roscovitine. (C) Leaf sections of transgenic line R1-1 were cultured on a medium containing 2.0 μg/ml NAA and 100 μM roscovitine with (+) or without (-) 1 μM DEX for 24 h, and 30 μg of protein extracts were immunoblotted with anti-R2 antibody. (D) Leaf sections of the transgenic line R1–1 were cultured on a medium containing 2.0 μg/ml NAA with (+) or without (-) 100 μM roscovitine (ROS) for 7 days and then cultured for 24 h on a roscovitine-free medium containing 2.0 μg/ml NAA with (+) or without (-) 1 μM DEX, and 30 μg of protein extracts were immunoblotted with anti-R2 antibody.
The above results showed that R2 expression or cytokinin application during the early stage of culture converted root organogenesis on an auxin-rich medium into disorganized cellular proliferation, suggesting that CDK activities governed by phytohormones seem to regulate differentiation fate of leaf cells. This assumption is consistent with the fact that, in our assay, calli were formed in the presence of a higher concentration (2.0 μg/ml) of NAA, which might lead to an increase in CDKs transcript levels (24–27). It was, however, difficult to monitor the change of CDK activity during the early phase of organogenesis because the dedifferentiation process is initiated in a single differentiated cell within the vascular bundle (28). To overcome this problem, we used a CDK inhibitor, roscovitine, which specifically inhibits CDK activities in animals (23, 29) and kinase activities associated with yeast p13Suc1 in maize (30, 31). We confirmed that the CDK2-kinase activity of R2 immunoprecipitates was less sensitive to roscovitine than the CDK activities associated with p13Suc1 in rice suspension cells (Fig. 4B). Treatment of leaf explants with roscovitine for the first 7 days of culture suppressed callus formation in auxin-rich media (Fig. 4A), though it did not inhibit the DEX-induced R2 expression (Fig. 4C). These findings indicate that inhibition of CDK activities by roscovitine during the first 7 days prevented leaf cells from proliferation. However, when leaf sections cultured on auxin-rich media containing DEX and roscovitine were transferred to the same medium lacking roscovitine, they expressed R2 (Fig. 4D) and induced calli (Fig. 4A). This outcome was independent of R2 expression during the first 7 days (Fig. 4A). These results suggest that up-regulation of CDK activities was responsible for the conversion of root differentiation into callus formation during organogenesis.
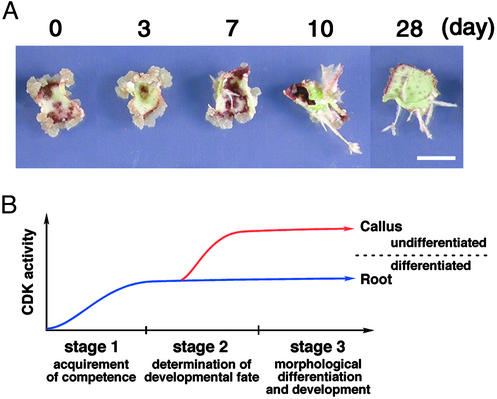
The CDK Activity Controls Differentiation Fate of Cells in a Restricted Period. Next, we examined when leaf cells become sensitive to CDK activity during the process of organogenesis. Transfer of leaf sections cultured on DEX-free media for 3 days to a DEX-containing medium resulted in callus formation (Fig. 5A). However, leaf explants cultured on DEX-free media for >7 days and then transferred to a DEX-containing medium produced short roots, but not calli (Fig. 5A). These results indicate that differentiation of roots was determined during a 7-day period, and subsequent R2 expression did not alter the cell fate. In other words, up-regulation of CDK activity at the early phase of root organogenesis seems to modulate the differentiation fate and result in disorganized cellular proliferation. According to the scheme of organogenesis proposed by Christianson and Warnick (2, 3), stage II, at which the competent cells are canalized and determined for root formation, is the period at which primed cells can sense CDK activity (Fig. 5B).
Fig. 5.
The CDK activity controls differentiation fate of cells in a restricted period during organogenesis. (A) Leaf sections of the transgenic line R1-1 were cultured for the indicated days on a medium containing 2.0 μg/ml NAA without DEX, and then transferred to a medium containing 2.0 μg/ml NAA and 1 μM DEX until 28 days after starting the culture. (Bar, 1 cm.) (B) Schematic representation of requirement of CDK activity in the process of organogenesis on leaf explants.
We showed that NAA at 0.2 μg/ml with R2 expression, and a higher concentration of 2.0 μg/ml without R2 expression retarded root differentiation (Fig. 2 A). Ectopic expression of B2-type cyclin in tobacco leaf discs also interfered with auxin-induced root regeneration (32). Therefore, a moderate activation of CDKs might inhibit root differentiation even though it was not sufficient to cause callus formation. We have previously reported that overexpression of sense or antisense gene for Arabidopsis CAK1At caused a reduction of CDK activity and differentiation of initial cells in the root meristem (5). Because this phenotype was rescued by CDKA overexpression (M.U., unpublished data), a sufficient level of CDK activity might be required to prevent initial cells from differentiation. Taken together with the present results, we propose that CDK activity determines differentiation fate of cells to guarantee continuous development of tissues.
Riou-Khamlichi et al. (33) also demonstrated that Arabidopsis leaf explants expressing the d-type cyclin CycD3;1 produced calli in the absence of exogenous cytokinin. Constitutive overexpression of CycD3;1 in planta increased CycD3;1-associated kinase activity, and led to differentiation defects in leaf tissues (34). Because R2 and CycD3;1 can enhance CDK activity by phosphorylation and binding, respectively (16, 35), posttranslational regulation of CDKs may play a critical role in controlling cell differentiation as well as cell division. Further characterization of the protein(s) that regulates CDK activity in response to hormonal and developmental signals should unveil the molecular mechanism(s) underlying plant cell division and differentiation during organogenesis.
Supplementary Material
Acknowledgments
We thank Drs. Munetaka Sugiyama and Masami Sekine for valuable advice, Prof. Nam-Hai Chua for the pTA7001 vector, and Dr. Peter Doerner for the 5× GAL4 vector. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (Grant 14036212), and by Research for the Future from the Japan Society for the Promotion of Science.
Abbreviations: CDK, cyclin-dependent serine/threonine protein kinase; CAK, CDK-activating kinase; DEX, dexamethasone; NAA, naphthaleneacetic acid.
References
- 1.Skoog, F. & Millar, C. O. (1957) Symp. Soc. Exp. Biol. 11, 118-140. [PubMed] [Google Scholar]
- 2.Christianson, M. L. & Warnick, D. A. (1983) Dev. Biol. 95, 288-293. [DOI] [PubMed] [Google Scholar]
- 3.Christianson, M. L. & Warnick, D. A. (1985) Dev. Biol. 112, 494-497. [Google Scholar]
- 4.Sugiyama, M. (1999) Curr. Opin. Plant Biol. 2, 61-64. [DOI] [PubMed] [Google Scholar]
- 5.Umeda, M., Umeda-Hara, C. & Uchimiya, H. (2000) Proc. Natl. Acad. Sci. USA 97, 13396-13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fesquet, D., Labbé, J. C., Derancourt, J., Capony, J. P., Galas, S., Girard, F., Lorca, T., Shuttleworth, J., Dorée, M. & Cavadore, J. C. (1993) EMBO J. 12, 3111-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poon, R. Y. C., Yamashita, K., Adamczewski, J. P., Hunt, T. & Shuttleworth, J. (1993) EMBO J. 12, 3123-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon, M. J., Harper, J. W. & Shuttleworth, J., (1993) EMBO J. 12, 3133-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher, R. P. & Morgan, D. O. (1994) Cell 78, 713-724. [DOI] [PubMed] [Google Scholar]
- 10.Labbé, J. C., Martinez, A. M., Fesquet, D., Capony, J. P., Darbon, J. M., Derancourt, J., Devault, A., Morin, N., Cavadore, J. C. & Dorée, M. (1994) EMBO J. 13, 5155-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mäkelä, T. P., Tassan, J. P., Nigg, E. A., Frutiger, S., Hugher, G. J. & Weinberg, R. A. (1994) Nature 371, 254-257. [DOI] [PubMed] [Google Scholar]
- 12.Devault, A., Martinez, A. M., Fesquet, D., Labbé, J. C., Morin, N., Tassan, J. P., Nigg, E. A., Cavadore, J. C. & Dorée, M. (1995) EMBO J. 14, 5027-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher, R. P., Jin, P., Chamberlin, H. M. & Morgan, D. O. (1995) Cell 83, 47-57. [DOI] [PubMed] [Google Scholar]
- 14.Tassan, J. P., Jaquenoud, M., Fry, A. M. Frutiger, S., Hughes, G. J. & Nigg, E. A. (1995) EMBO J. 14, 5608-5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata, S. (1991) FEBS Lett. 279, 149-152. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi, M., Umeda, M. & Uchimiya, H. (1998) Plant J. 16, 613-619. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi, M., Fabian, T., Sauter, M., Bhalerao, R. P., Schrader, J., Sandberg, G., Umeda, M. & Uchimiya, H. (2000) Plant J. 24, 11-20. [DOI] [PubMed] [Google Scholar]
- 18.Aoyama, T. & Chua, N. H. (1997) Plant J. 11, 605-612. [DOI] [PubMed] [Google Scholar]
- 19.Murashige, T. & Skoog, F. (1962) Physiol. Plant 15, 473-497. [Google Scholar]
- 20.Becker, D., Kemper, E., Schell, J. & Masterson, R. (1992) Plant Mol. Biol. 20, 1195-1197. [DOI] [PubMed] [Google Scholar]
- 21.Linsmaier, E. M. & Skoog, F. (1965) Physiol. Plant 18, 100-127. [Google Scholar]
- 22.Miyazawa, Y., Kato, H., Muranaka, T. & Yoshida, S. (2002) Plant Cell Physiol. 43, 1534-1541. [DOI] [PubMed] [Google Scholar]
- 23.De Azevedo, W. F., Leclerc, S., Meijer, L., Havlicek, L., Strnad, M. & Kim, S.-H. (1997) Eur. J. Biochem. 243, 518-526. [DOI] [PubMed] [Google Scholar]
- 24.Martinez, M. C., Jørgensen, J.-E., Lawton, M. A., Lamb, C. J. & Doerner, P. W. (1992) Proc. Natl. Acad. Sci. USA 89, 7360-7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemerly, A. S., Ferreira, P., de Almeida Engler, J., Van Montagu, M., Engler, G. & Inzé, D. (1993) Plant Cell 5, 1711-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao, G.-H., Hong, Z. & Verma, D. P. S. (1993) Proc. Natl. Acad. Sci. USA 90, 943-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, K., Letham, D. S. & John, P. C. L. (1996) Planta 200, 2-12. [DOI] [PubMed] [Google Scholar]
- 28.Attfield, E. M. & Evans, P. K. (1991) J. Exp. Bot. 42, 51-57. [Google Scholar]
- 29.Meijer, L., Borgne, A., Mulner, O., Chong, J. P. J., Blow, J. J., Inagaki, N., Inagaki, M., Delcros, J.-G. & Moulinoux, J.-P. (1997) Eur. J. Biochem. 243, 527-536. [DOI] [PubMed] [Google Scholar]
- 30.Planchais, S., Glab, N., Inzé, D. & Bergounioux, C. (2000) FEBS Lett. 476, 78-83. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez, M. P., Torres, A., Boniotti, M. B., Gutierrez, C. & Vázquez-Ramos, J. M. (2002) Plant Mol. Biol. 50, 167-175. [DOI] [PubMed] [Google Scholar]
- 32.Weingartner, M., Pelayo, H. R., Binarova, P., Zwerger, K., Melikant, B., de la Torre, C., Heberle-Bors, E. & Bögre, L. (2003) J. Cell Sci. 116, 487-498. [DOI] [PubMed] [Google Scholar]
- 33.Riou-Khamlichi, C., Huntley, R., Jacqmard, A. & Murray, J. A. H. (1999) Science 283, 1541-1544. [DOI] [PubMed] [Google Scholar]
- 34.Dewitte, W., Riou-Khamlichi, C., Scofield, S., Healy, J. M. S., Jacqmard, A., Kilby, N. J. & Murray, J. A. H. (2003) Plant Cell 15, 79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Healy, J. M. S., Menges, M., Doonan, J. H. & Murray, J. A. H. (2001) J. Biol. Chem. 276, 7041-7047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.