Abstract
Using functional magnetic resonance imaging, brain activation patterns were examined in response to hearing one’s own first name in contrast to hearing the names of others. There are several regions in the left hemisphere that show greater activation to one’s own name, including middle frontal cortex, middle and superior temporal cortex, and cuneus. These findings provide evidence that hearing one’s own name has unique brain functioning activation specific to one’s own name in relation to the names of others.
Keywords: self-awareness, self referential, fMRI
1. Introduction
Self representation in children begins in the first year of life (Butterworth, 1992; Lewis, 1995; Meltzoff, 1990) and further developments emerge in the middle of the second year including self recognition, pretend play and use of personal pronouns (Lewis and Brooks-Gunn, 1979; Lewis and Ramsay, 2004). Quantitative measures of brain maturation (Carmody et al, 2004) applied to infants and young children have shown the relations between brain maturation of the left temporo-parietal and right medial frontal cortex and the emergence of self representational behavior (Lewis and Carmody, 2006). It is interesting to note that the finding of the maturity of the left temporo-parietal junction is consistent with others who have also found the left side to be most involved in self referential behavior (Saxe and Kanwisher, 2003) and is involved in reasoning about the beliefs of others (Samson et al., 2004).
While studies of facial familiarity including the self can be done easily with adults and cooperative older children, it is more difficult to obtain neuroimaging measures on infants and young children due to subject movement and to poor cooperation with task demands. Often, neuroimaging of infants and young children is performed under sedation (Kain et al., 1994; Merola et al., 1995), and functional magnetic resonance imaging (fMRI) studies of sedated children show that words activate specific brain regions (Altman and Bernal, 2001; Souweidane et al. 1999). In addition, we have shown that a child under sedation responds selectively to the sound of her own first name (Carmody et al., in press).
Our interest in looking at brain functioning of young children while they undergo developmental changes in self representational behavior requires us to have a reference of mature functioning. Therefore, it is of importance to us to determine how the developed adult brain responds to hearing one’s own name in order to determine the brain regions that are active to this unique stimulus. Having an adult end point allows us to study not only the development of self referential behavior but allows us to study brain activation in children who fail to show a normal developmental course.
Neuroimaging of adults using positron emission tomography (PET) and fMRI has shown that specific brain region activation is associated with self representational behaviors. For example, a network of brain regions involved in the ability to attribute mental states to self and others, known as “mentalizing”, include the medial prefrontal cortex and the temporal-parietal junction near the anterior portions of the superior temporal gyrus (Frith, 2001). In addition, the left superior temporal gyrus and the left medial frontal gyrus are activated when subjects engage in a theory-of-mind task relative to reading sentences (Fletcher et al., 1995; Gallagher et al., 2000; Mitchell et al., 2002), and activation occurs in left superior temporal cortex (Brodmann area 22) and left inferior parietal cortex when subjects judge whether adjectives are relevant to themselves (Fossati et al., 2003; Macrae et al., 2004). Using fMRI, the medial surface of the superior frontal gyrus is activated when calling a subject’s own name relative to calling the names of others (Kampe et al., 2003), and the right frontal cortex, including superior, middle, and inferior regions, is activated when subjects identified the faces of self and famous others (Platek et al., 2004). There is general agreement that self representational behaviors activate regions near the temporo-parietal junction although other studies suggest activation of the medial frontal cortex as well (Fletcher et al., 1995; Kampe et al., 2003).
In the studies of self recognition and self representation, brain activation was assessed in subjects experiencing several demands, such as deciding if lists of adjectives were descriptive of themselves and responding with a hand movement. It is also important to note that the task of theory of mind requires reflecting on both self and others. Our interest in the current study is to identify brain regions that are activated when hearing one’s own name without demanding specific attention to targets or responses from participants. We expected that the regions that increase in activation when hearing one’s own name also would be related to the areas identified in the tasks involved in judgments of self. In addition, we examined how hearing one’s own name differs from hearing the names of others in terms of unique patterns of brain activity.
2. Results
Brain regions were identified that were activated when hearing one’s own name relative to hearing the names of others. Figure 1 illustrates the regions of activation at four different axial levels (Z values of +10, 15, 20, and 25mm) for all participants based on the Talairach atlas (Talairach and Tournoux, 1998) when own name is compared to all other names. As shown in the figure, there are both anterior and posterior regions involved when hearing one’s own name. The data indicate a predominantly posterior network that includes the middle temporal cortex (BA 39), left superior temporal cortex (BA 22), middle occipital gyrus (BA 19) and cuneus (BA 18). An anterior network of activation was found in middle frontal cortex (BA 9, 10, 46) and superior frontal cortex (BA 10).
Figure 1.
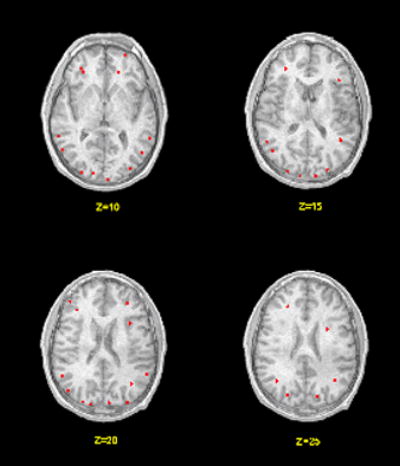
Brain activation maps when hearing names. The left side of the image corresponds to the left side of the brain. The red areas are associated with hearing one’s own name relative to hearing the names of others. The numbers below the slices indicate the z-axis Talairach coordinates.
While Figure 1 represents the summary of active areas across all participants, Table 1 presents the regions activated by three or more of the four participants at the levels of the four axial images with the nearest Brodmann areas (BA) and Talairach coordinates (x, y, and z) of the centers of clusters of voxels. While the Talairach coordinates are exact, the Brodmann areas are approximate estimates. Using the criteria of three or more participants for inclusion, the regions of activation are in the left hemisphere in frontal, temporal, and occipital cortex.
Table 1.
Cerebral Regions Activated When Hearing One’s Own Name Relative to Hearing the Names of Others, Nearest Brodmann Areas (BA) and Talairach Coordinates (x, y, z) of the Centers of Clusters of Voxels
| Cerebral Region | Hemisphere | BA | x | y | z |
|---|---|---|---|---|---|
| Middle Frontal Cortex | Left | 10 | −30 | 42 | 10 |
| 46 | −45 | 43 | 20 | ||
| Frontal Subgyral White Matter | Left | None | −28 | 37 | 10 |
| None | −31 | 38 | 15 | ||
| None | −35 | 30 | 20 | ||
| None | −25 | 34 | 25 | ||
| Middle Temporal Cortex | Left | 39 | −56 | −58 | 10 |
| 39 | −48 | −63 | 15 | ||
| 39 | −49 | −71 | 20 | ||
| 39 | −40 | −64 | 25 | ||
| Superior Temporal Cortex | Left | 22 | −61 | −39 | 10 |
| 22 | −56 | −49 | 15 | ||
| 22 | −55 | −51 | 20 | ||
| Cuneus/ Fusiform gyrus | Left | 17 | −15 | −86 | 10 |
| 18 | −11 | −94 | 15 | ||
| 18 | −15 | −87 | 20 | ||
| 19 | −26 | −83 | 25 |
Note: Probability of activation for each participant set at p < .01 at the voxel level with a 5 voxel spatial extent for regional activation. Criterion for inclusion in the table was 3 or more participants showing activation.
3. Discussion
Our interest was whether auditory name recognition is associated with a unique pattern of brain activation. We found brain regions that showed differences in the hemodynamic response between hearing one’s own name and hearing the names of others. The results differ from those of other studies for two reasons. First, participants heard only names without the requirement of completing other tasks, such as judging the relevance of adjectives and providing a motor response such as a key press. Second, the contrasts are made between hearing one’s first name with the names of others, and not to a silent, rest period. A review follows of the similarities and differences between the findings of this study and others.
3.1. Activation of anterior regions
Activation of anterior regions included left middle frontal cortex (BA 10 and BA 46) as well as left subgyral white matter. These patterns of activation are similar to the patterns reported in self referential tasks by others. For example, left medial prefrontal activation was found when individuals engaged in self referential processing contrasted with letter recognition, (Fossati et al., 2003) and inferior frontal gyrus activation occurred when participants heard their own name and reacted to the presence of auditory target names by pressing a button, a task that required explicit attention by participants (Kampe et al., 2003). Using a visual task that instructed participants to look at the image and think about whom it was (i.e., subjects were asked to mentally identify the face being presented), right frontal cortex was activated for own face images relative to famous face images (Platek et al., 2004). In a review of neuroimaging studies of theory-of-mind tasks, Frith (2001) found that the medial prefrontal cortex is part of a neural circuit involved in the ability to attribute mental states to self and others, known as “mentalizing.”
While many studies assessed brain activation involved in self referential tasks, the participants in those studies experienced several demands, such as deciding if a list of adjectives were descriptive of themselves, responding with a hand movement, or reflecting on both self and others, we have shown that simply hearing one’s own name also activates the medial prefrontal region, without the additional task demands.
3.2. Activation of posterior regions
In our study, posterior regions that were activated in response to one’s own name included left middle temporal cortex (BA 39) and left superior temporal cortex (BA 22). These regions are similar to the activation at the temporo-parietal junction reported in an fMRI study when subjects perform a ‘theory of mind’ task (Gallagher et al., 2000), and activation in superior temporal cortex when making judgments about others (Mitchell et al., 2002). In addition, we found activation in left cuneus and fusiform gyrus (largely BA 18) similar to the activation of posterior cingulate when making judgments about others (Macrae et al., 2004) and to the activation of left fusiform gyrus in a recognition task of one’s own face (Kircher et al., 2000). However, participants in those two studies (Kircher et al., 2000; Macrae et al., 2004) viewed visual presentations of the stimuli, while the presentations in the current study were auditory. A brief review of neuroimaging findings in tasks that are not self referential lends an explanation of the activation of the cuneus.
In a study using PET that identified cerebral structures involved in music appreciation, preferential left hemisphere activation was found for familiarity, pitch tasks and rhythm (Platel et al. 1997). Activation was found in left cuneus/preceuneus (BA 18/19) for the pitch task, an unexpected finding for a task involving auditory presentations. The authors suggested that the participants used a strategy involving mental imagery to achieve the task. In a PET study of the cognitive component of olfactory processing, left cuneus was activated when participants named the odors (Qureshy et al., 2000). The authors suggest that the task involved some imagery to ascertain the name of the odor and indicated that the visual cortex is involved in mental imagery tasks (see also Kosslyn, 1988; Royet et al., 1999). In the current study, the salience of one’s own name may elicit activity in non-auditory brain regions.
The differences in activation patterns between our findings and those of other studies may be the result of the demands made of participants to pay attention for the presence of a target and to make a motor response. Even so, the areas that are common to this study and the tasks that demand attention and judgments are located in medial frontal cortex and superior temporal cortex near the temporo-parietal junction.
In addition, a network of brain regions involved in the ability to attribute mental states to self and others, known as “mentalizing”, include the medial prefrontal cortex and the temporal-parietal junction near the anterior portions of the superior temporal gyrus (Frith, 2001). We found greater activation in middle and superior frontal cortex, as well as the superior temporal cortex when hearing one’s own name than when hearing the names of others.
3.3. Locating the resting self
It has been suggested that the resting state of self is located in medial prefrontal cortex (Wicker et al., 2003). Many studies that require reflection on internal processes, such as theory-of-mind, emotion, or perspective taking, require subjects to use similar brain regions that are active when reflecting on self. As such, there may be few regions identified in these areas when comparing the resting state to the task state. We chose to make the contrast between hearing the two types of names rather than contrasting to a rest or silent condition because brain regions that are active in the resting state are, at times, more active and active in different ways, than when involved in cognitive tasks (Gusnard et al., 2001; Gusnard and Raichle, 2001; Raichle et al., 2001; Wicker et al., 2003).
An explanation for the increased activity at rest is that individuals vary, both between imaging sessions as well as within a single session, in their reflections of the self (Wicker et al., 2003). As such, there may be greater activation when reflecting on self during the resting state than when subjects direct their attention to external tasks. Therefore, in order to identify areas involved in processing one’s own name, it is necessary to use as a contrast an activity that is not the resting state. We chose to compare the brain activation when hearing one’s own name to brain activation when hearing the names of others. Moreover, we used a design in which all participants heard the same set of four names, one of which was their own.
3.4. Conclusions
The findings of this simple paradigm are consistent with the findings in the literature. There is unique brain activation specific to one’s own name in relation to the names of others. In addition, the patterns of activation when hearing one’s own name relative to hearing the names of others are similar to the patterns reported when individuals make judgments about themselves and their personal qualities, and include the regions of the medial frontal cortex and superior temporal cortex near the temporo-parietal junction. These results will enable us to study young children and even infants’ responses to their own names in order to see when self representation first occurs. Pediatric populations can be readily studied with this auditory task.
4. Experimental procedure
4.1. Participants
Four English-speaking, right-handed, male adults (ages 22, 30, 31, and 32 years) volunteered to serve as participants. In a preliminary interview, none indicated a history of psychiatric problems, brain injury, neurological impairments or learning disability, and all admitted to being right-handed. Each participant had the right to refuse participation, terminate the testing session, or not complete the task.
4.2. Stimuli
The first names of the four participants were used as stimuli and the same four names were used for all participants (Dan, Jay, Mike, Saul). The names were not different spectrally. This allowed an analysis of the differences in brain activation between hearing their own name and the activation when hearing the names of others.
Names were recorded by a male voice in blocks of 15 seconds with the same name repeated every three seconds within the block, and were presented at the rate of 500 msec per name with a silent interstimulus interval of 2500 msec. A 12-second silent period followed each block. There were 6 blocks of each name for a total of 24 blocks arranged in a randomized order with the same order for all four participants. Each participant heard his own name 30 times and heard each of the other names 30 times. For the first interval of 30-seconds, no names were presented allowing participants to accommodate to the scanner noise. The entire task required 678 seconds to complete.
4.3. Procedure
The task was to listen to the names without making a motor response. Participants heard the auditory stimuli through headphones connected to a PC using software (E-Prime). As we already know from the literature, the cocktail party phenomena (Cherry, 1953) suggest that adults never tire of hearing their name and thus there is no reason to believe that there was a diminution of the participants’ interest to hearing their name in this brief setting. A questionnaire given following the scanning revealed that the subjects found the study interesting and that the sounds of their names elicited responses of ‘that’s me.’
4.4. fMRI recording
Scanning was performed with a 1.5 Tesla General Electric Scanner with echoplanar capability and a standard quadrature head coil. Participants were positioned supine with the head in a midline location in the coil. In addition to instructions to limit head motion, foam pads within the head coil helped secure head fixation and prevent motion. Participants were imaged with eyes closed in the darkened scanner room. Scanning began with a standard spin echo T1-weighted sequence positioned parallel to the line of the anterior and posterior commissures covering the prefrontal cortex and temporoparietal junction in four slices. This yielded axial slices of the brain for analyses. Imaging parameters were matrix size = 128 X 128; TR = 500 msec; TE = 60; FOV = 24 cm; NEX = 1; slice thickness = 4 mm, with 1 mm skip. T2*-weighted images were acquired using echo planar imaging (EPI) gradient echo sequence (matrix size = 128 X 128; TR = 3000 msec; TE = 60 msec; FOV = 24 cm; flip angle = 75 degrees; slice thickness = 4 mm, with 1 mm skip, interleaved, and 1 NEX) covering the same brain regions and in the same plane as the T1-weighted sequence. During each functional imaging sequence, 226 volumes of four axial sections were taken for each participant.
4.5. Measures of brain activation
Analyses of the brain activation were conducted at two levels. First, statistical parametric maps were created, followed by a regional analysis of the hemodynamic response. Brain regions were analyzed for each participant for activation slice-by-slice with the Analyses of Functional Neuroimages Package (AFNI) running under UNIX software (Cox, 1996; Cox and Hyde, 1997). The first ten image sets taken during a silent period were excluded in the analysis to ensure steady baseline measures. The data were realigned to the twentieth image set to minimize motion related artifacts. Inspection of the registration graphs indicated that no participant moved more than 1.5 mm in any direction over the course of the session. The images were smoothed with a filter (full width at half maximum 6 mm). The MRI signals were compared for differential activity when hearing the names. A cross-correlation analysis was applied on a voxel-by-voxel basis to determine if the MRI signal differed when hearing one’s own name relative to hearing the names of others. In the analysis, the MRI signal for own name was compared to the signal for others’ names, providing a set of voxels that were more active when hearing one’s own name relative to hearing the names of others. All voxels that passed the statistical threshold (p-value = 0.01, uncorrected) in the task-activated datasets were considered activated. In this way, we identified the voxels that correlated with the changes in task from periods when own name was present to periods when other names were present. This analysis provided statistical parametric maps for each participant of voxels of activation for the differences in activation between hearing one’s own name and hearing the names of others.
The T1 anatomical images were aligned to the EPI images to localize the regions of activation. A specific region was defined as active for a participant if five or more contiguous pixels were activated (Forman et al., 1995). Identification of the active brain regions was achieved by comparisons to the horizontal standard images available in the Talairach Daemon (Lancaster et al., 2000).
Acknowledgments
This research was supported by PHS grants to Michael Lewis from the National Institute on Drug Abuse (R01-DA07109) and the National Institute of Mental Health (R01-MH59391 and R01-MH64473).
References
- Altman NR, Bernal B. Brain activation in sedated children: auditory and visual functional MR imaging. Radiology. 2001;221:56–63. doi: 10.1148/radiol.2211010074. [DOI] [PubMed] [Google Scholar]
- Butterworth G. Origins of self-perception in infancy. Psychological Inquiry. 1992;3:103 – 111. [Google Scholar]
- Carmody DP, Dunn SM, Boddie-Willis AS, DeMarco JK, Lewis M. A quantitative measure of myelination development in infants, using MR images. Neuroradiology. 2004;46:781–786. doi: 10.1007/s00234-004-1241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody DP, Moreno R, Mars A, Seshadri K, Lambert GH, Lewis M. Brain activation to social words in a sedated child with autism. J Autism Dev Disorder. doi: 10.1007/s10803-006-0270-3. in press. [DOI] [PubMed] [Google Scholar]
- Cherry EC. Some experiments on the recognition of speech, with one and two ears. J Acoustic Soc of Am. 1953;25:975–979. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. In search of the emotional self: an FMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Frith U. Mind blindness and the brain in autism. Neuron. 2001;32:969–979. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Kain ZN, Gaal DJ, Kain TS, Jaeger DD, Rimar S. A first-pass cost analysis of propofol versus barbiturates for children undergoing magnetic resonance imaging. Anest Analg. 1994;79:1102–1106. doi: 10.1213/00000539-199412000-00013. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Frith U. “Hey John”: signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. J Neurosci. 2003;23:5258–5263. doi: 10.1523/JNEUROSCI.23-12-05258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, Simmons A, Williams SC, Bartels M, David AS. Towards a functional neuroanatomy of self processing: effects of faces and words. Brain Res Cogn Brain Res. 2000;10:133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM. Aspects of a cognitive neuroscience of mental imagery. Science. 1988;240:1621–1626. doi: 10.1126/science.3289115. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. Aspects of self: From systems to ideas . In: Rochat P, editor. The self in early infancy: Theory and research. Elsevier; North Holland: 1995. pp. 95–115. [Google Scholar]
- Lewis M, Brooks-Gunn J. Social cognition and the acquisition of self. Plenum; New York: 1979. [Google Scholar]
- Lewis M, Carmody DP. Self representation and brain development. Program for the Annual Convention of the Association for Psychological Science. 2006;18:220. [abstract] [Google Scholar]
- Lewis M, Ramsay D. Development of self-recognition, personal pronoun use, and pretend play during the 2nd year. Child Dev. 2004;75:1821–1831. doi: 10.1111/j.1467-8624.2004.00819.x. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. Foundations for developing a concept of self: The role of imitation in relating self to other and the value of social mirroring, social modeling, and self practice in infancy. In: Cicchetti D, Beeghly M, editors. The self in transition: Infancy to childhood. University of Chicago Press; Chicago: 1990. pp. 139–164. [Google Scholar]
- Merola C, Albarracin C, Lebowitz P, Bienkowski RS, Barst SM. An audit of adverse events in children sedated with chloral hydrate or propofol during imaging studies. Paediatr Anaesth. 1995;5:375–378. doi: 10.1111/j.1460-9592.1995.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci USA. 2002;99:15238–15243. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek SM, Keenan JP, Gallup GG, Jr, Mohamed FB. Where am I? The neurological correlates of self and other. Brain Res Cogn Brain Res. 2004;19:114–122. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Platel H, Price C, Baron JC, Wise R, Lambert J, Frackowiak RS, Lechevalier B, Eustache F. The structural components of music perception: A functional anatomical study. Brain. 1997;120 (Pt 2):229–243. doi: 10.1093/brain/120.2.229. [DOI] [PubMed] [Google Scholar]
- Qureshy A, Kawashima R, Imran MB, Sugiura M, Goto R, Okada K, Inoue K, Itoh M, Schormann T, Zilles K, Fukuda H. Functional mapping of human brain in olfactory processing: a PET study. J Neurophysiol. 2000;84:1656–1666. doi: 10.1152/jn.2000.84.3.1656. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le Bars D, Costes N, Vigouroux M, Farget V, Sicard G, Holley A, Mauguiere F, Comar D, Froment JC. Functional anatomy of perceptual and semantic processing for odors. J Cogn Neurosci. 1999;11:94–109. doi: 10.1162/089892999563166. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else’s belief. Nat Neurosci. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Souweidane MM, Kim KH, McDowall R, Ruge MI, Lis E, Krol G, Hirsch J. Brain mapping in sedated infants and young children with passive-functional magnetic resonance imaging. Pediatr Neurosurg. 1999;30:86–92. doi: 10.1159/000028768. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Mapping. Thieme; New York: 1988. [Google Scholar]
- Wicker B, Ruby P, Royet JP, Fonlupt P. A relation between rest and the self in the brain? Brain Res Brain Res Rev. 2003;43:224–230. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]


