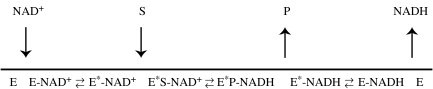Abstract
High hydrostatic pressure is a neglected tool for probing the origins of isotope effects. In chemical reactions, normal primary deuterium isotope effects (DIEs) arising solely from differences in zero point energies are unaffected by pressure; but some anomalous isotope effects in which hydrogen tunnelling is suspected are partially suppressed. In some enzymatic reactions, high pressure completely suppresses the DIE. We have now measured the effects of high pressure on the parallel 13C heavy atom isotope effect of yeast alcohol dehydrogenase and found that it is also suppressed by high pressure and, similarly, suppressed in its entirety. Moreover, the volume changes associated with the suppression of both deuterium and heavy atom isotope effects are virtually identical. The equivalent decrease in activation volumes for hydride transfer, when one mass unit is added to the carbon end of a scissile C–H bond as when one mass unit is added to the hydrogen end, suggests a common origin. Given that carbon is highly unlikely to undergo tunnelling, it follows that hydrogen is not doing so either. The origin of these isotope effects must lie elsewhere. We offer protein domain motions as a possibility.
Keywords: yeast alcohol dehydrogenase, hydrostatic pressure, deuterium isotope effect, heavy atom isotope effect, hydrogen tunnelling, protein domain motion
1. Introduction
Chemical systems respond to high hydrostatic pressure in accordance with Le Chatelier's principle, which states that systems at equilibrium subjected to a stress will adjust to relieve the stress and restore equilibrium. For example, using a smaller typeface to indicate a smaller volume, the equilibrium,
| (1.1) |
will shift to the right under pressure according to the exponential function,
| (1.2) |
where Kp is the equilibrium constant at elevated pressure, K0 is the equilibrium constant at zero or atmospheric pressure (the difference is negligible), ΔVK is the change in partial molar volumes between A and B, p is the pressure in bar (0.98692 standard atmospheres), R is the gas constant at 83.13 ml bar mol−1 K−1 and T is the temperature in Kelvin (Glastone et al. 1941).
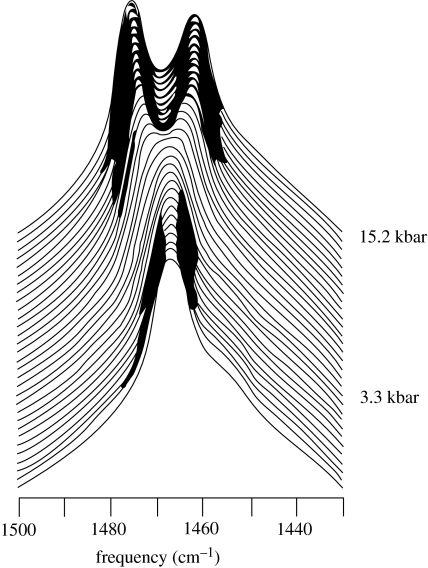
There are several points that one must keep in mind when dealing with pressure. First, the application of pressure to chemical systems does not ‘squeeze’ anything; it simply shifts equilibria that were already present at atmospheric pressure. Nothing new is being added including conformations that were not present in the absence of applied pressure. Second, the volume changes while reported in millilitres per mole need not represent real space. Pressure can change such things as viscosity, dielectric constants and ionization constants. Third, one must always remember that measured volume changes are systems volumes, and not just the specific species explicitly identified in a given equilibrium. This is particularly true with proteins, where most of the volume changes associated with changes in protein folding and solvation are in the solvent, and most of the solvent changes are entropic. Pressure forces water into the interior of folded globular proteins causing an increase in the solvation of hydrophobic groups and salt bridges. Finally, most of the physical chemists who use pressure as an independent variable in their chemical systems under study are not actually interested in what happens at high pressure, or why it happens. Rather, pressure is just a tool that makes it possible to deconstruct complex systems. For example, figure 1 shows a portion of infrared spectrum of a protein subjected to pressure. What appears initially to be a single absorption band is actually a pair of separate peaks, which diverge at hydrostatic pressures above approximately 5 kbar. Spectroscopists fit data such as these to appropriate algorithms to extract the spectra of the individual absorption components at atmospheric pressure. Subjecting enzymatic reactions to high pressure and collecting kinetic data make it possible similarly to extract separate kinetic components that are overlapping or otherwise obscured at atmospheric pressure.
Figure 1.
Partial infrared spectra of a sulphogalactosylglycerolipid in a lipid bilayer as a function of increasing hydrostatic pressure.
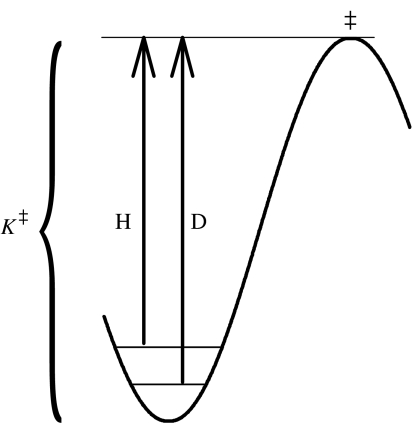
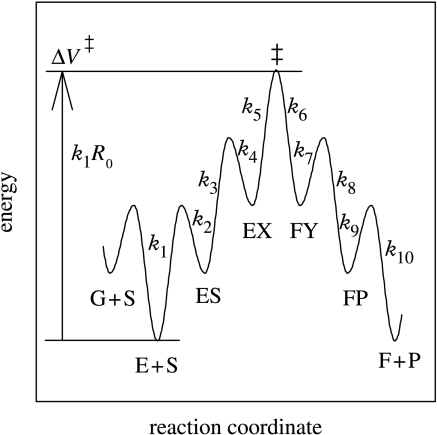
With regards to kinetic phenomena, pressure has a similar effect on rate constants as it does on equilibria, because absolute rate theory is based upon a quasi-equilibrium between the reactant state and the transition state (TS), K‡, as illustrated in figure 2. This quasi-equilibrium constant is part of the pressure-dependent portion of a rate constant:
| (1.3) |
where k0 is the rate constant for the chemical transformation under atmospheric conditions, κT the transmission coefficient, kB the Boltzmann's constant, h the Planck's constant and ΔV‡ the activation volume or volume difference between the reactant state and TS (Glastone et al. 1941).
Figure 2.
A portion of a reaction coordinate diagram showing the origin of the quasi-equilibrium constant K‡, as the difference between a reactant state and a transition state, plus the origin of normal primary deuterium isotope effects, as the difference between zero-point energy levels of H and D in the energy well associated with the reactant state.
The question arises as to whether normal isotope effects will be sensitive to high pressure. Semi-classical rate theory holds that such effects arise from differences in zero point energies, also illustrated in figure 2. These differences arise from differences in vibrational frequencies of isotopic and non-isotopic atoms as described by the Bigeleisen & Wolfsberg equation (1958):
| (1.4) |
Such frequencies are related to the stretching vibrations seen in infrared spectra and, as illustrated in figure 1, they are relatively insensitive to pressures of a few kilobars. Nevertheless, Isaacs (1984) examined a variety of normal primary deuterium isotope effects (DIE) in chemical reactions and confirmed that they were insensitive to pressures in the low kilobar range.
2. The effect of pressure on hydrogen tunnelling
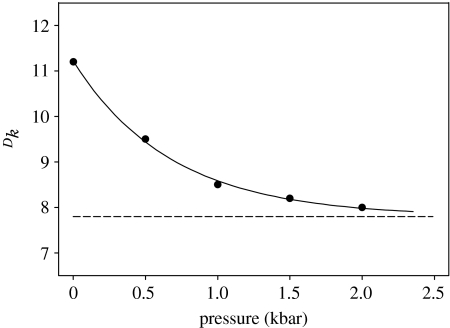
Isaacs also examined the effect of pressure on some abnormal DIEs, deemed ‘anomalous’ either because they were too large to be accounted for by differences in zero point energies or because of nonlinear Arrhenius plots, and found these were partially sensitive to pressure. For example, table 1 shows pressure and isotopic data for the kinetics of the hydride transfer between chloranil and leuko crystal violet (Isaacs et al. 1987).
Table 1.
Effect of pressure on hydride transfer.
| pressure (bar) | kH/kD |
|---|---|
| 1 | 11.5 |
| 500 | 10.3 |
| 1000 | 9.0 |
| 1500 | 8.2 |
| 2000 | 8.0 |
Isaacs and coworkers examined eight such reactions, including hydrogen, proton as well as hydride transfers, and observed that in all the cases, the DIEs decreased somewhat with increasing pressure, meaning that ΔVD‡ was always more negative than ΔVH‡. Isaacs (1984) concluded that ‘If tunneling, the usual explanation for the former anomalies is correct, then the pressure effect may constitute a further criterion of non-classical behavior…’ It is curious that in the approximately 20 years since Isaacs work was published, this reference and conclusion has been cited only seven times, and six of those were by this laboratory. (It would seem that those attending this Discussion Meeting in particular ought to be aware of this phenomenon.)
Isaacs did not derive a rate equation to describe his findings. To do so, one can start with the formulation of Bell (1980):
| (2.1) |
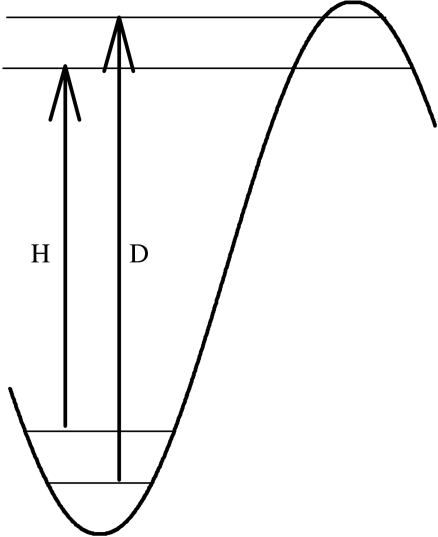
where kH/kD represents the portion of an isotope effect arising from differences in zero point energies in the reactant state and QH/QD is the tunnelling correction factor arising from apparent differences in the TS, illustrated in figure 3. In order to simplify the algebra of subsequent equations, equation (2.1) can be written in the following form, using the nomenclature of Northrop (1977):
| (2.2) |
Figure 3.
A portion of a reaction coordinate diagram showing an additional origin for deuterium isotope effects, wherein particle tunnelling at different reaction barrier widths near the transition state also contributes to the differences in energies of activation for H and D.
Applying the exponential pressure function given above to Bell's expression, one obtains:
| (2.3) |
where ΔVQ is the change in partial molar volumes of hydride versus deuteride transfer. However, equation (2.3) describes a function that decreases asymptotically with increasing pressure to a value of zero, which cannot be correct. In dealing with isotope effects either algebraically or mathematically, one must adjust for the absence of an isotope effect, which is unity, rather like subtracting the weight of the basket from a bushel of potatoes. Equation (2.2) can be rewritten as follows:
| (2.4) |
DQ−1 is the term that reduces to zero in the absence of hydrogen tunnelling, not DQ. Therefore, the correct equation for the effect of pressure is:
| (2.5) |
A fit of the data from table 1 is shown in figure 4, with excellent agreement between point and line. The values obtained by regression analysis are: ΔVQ=36.5±3.0 ml mol−1, Dk=7.8±0.1 and DQ=1.44±0.02 (Northrop 1999). Thus, 33±1% of the observed DIE at atmospheric pressure is sensitive to hydrostatic pressure, and would appear to originate in hydrogen tunnelling as postulated by Isaacs, leaving behind a pressure-insensitive component that is consistent with differences in zero point energies. This exercise illustrates the unique capability of pressure studies to quantify quantum mechanical tunnelling that goes far beyond what can be achieved by other methods. The technique really should be exploited more often.
Figure 4.
Effect of pressure on the deuterium isotope effect on hydride transfer from chloranil to leuko-crystal violet. A fit of the data in table 1 to equation (2.5).
3. The effect of pressure on deuterium isotope effects in enzymatic reactions
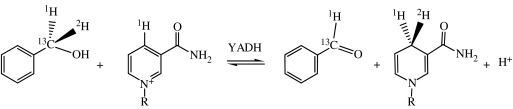
DIEs on the reaction catalysed by yeast alcohol dehydrogenase (YADH) were suspected to have a hydrogen-tunnelling component (Cha et al. 1989). In order to test this hypothesis, Northrop & Cho (2000a) examined the effects of high pressure on the primary isotope effect for hydride transfer, 2H in scheme 1, from benzyl alcohol to nicotinamide adenine dinucleotide (NAD+). Their experiments were performed in the non-competitive mode, which means that deuterated benzyl alcohol was subjected to enzymatic oxidation separately from its unlabelled counterpart. The kinetic mechanism for this enzyme is a preferentially ordered random one, but at high NAD+ and limiting alcohol, it can be regarded as ordered, as illustrated in the reaction line diagram of scheme 2.
Scheme 1.
Scheme 2.
The conversion of E-NAD+ to E*-NAD+ represents a conformational change common to all pyridine nucleotide dehydrogenases, and will be important in the analysis below. Concentrations of both the pyridine nucleotide and the alcohol were varied, but the data analysis focused on the kinetic parameter called substrate capture (Northrop 1998), which stands for V/K or Vmax/KM or, if one divides by the enzyme concentration, kcat/KM, of the benzyl alcohol. It includes the availability of the enzyme to the alcohol plus all rate constants for binding the benzyl alcohol, S, up to and including the first irreversible step, which in this case is the release of the first product, P, benzaldehyde.
The rate expression for substrate capture as a function of pressure is given below (Cho & Northrop 1999) and illustrated in figure 5,
| (3.1) |
Figure 5.
A generalized free energy diagram for the kinetics of capture.
The components have been arranged as two parenthetical functions; the one on the left is insensitive to isotopic substitution, whereas the one on the right is sensitive. Therein lies the resolving power of combining pressure effects and isotope effects that allows significant deconstruction of kinetic functions. Beginning with the left function: k1 is the rate constant for coming together of substrate and enzyme. As such, it is dependent upon the viscosity of the medium, which follows complex functions of pressure and temperature. Bett & Cappi (1965) measured the effects of pressure and temperature on the viscosity of water and found that the change was negligible between 25 and 30 °C for up to nearly 3 kbar of pressure. KG/E represents the equilibrium between an enzyme form, E, which can react with the substrate and other form(s), G, which cannot; for YADH, these are E*-NAD+ and E-NAD+, respectively, in scheme 2.
Turning now to the function on the right side of equation (3.1), R0 is a ratio of rate constants between free substrate and the isotopically sensitive TS, equal to k3k5/k2k4 in figure 5. ΔV‡ is again the activation volume as described earlier, only at this stage in figure 5 it can be seen as it really is, a thermodynamic function of state (Northrop 2002). This is an important feature in which the volume changes associated with substrate binding to form ES, or of conformational changes to form EX, are encompassed by the activation volume and need not be addressed separately. C stands for the sum of the commitments to catalysis, Cf+Cr, equal to k5/(k4(1+k3/k2)) and k6/(k7(1+k8/k9)), respectively, in figure 5. The commitments are a measure of the tendencies of intermediate complexes, e.g. ES or FY, to proceed in the direction of the isotopically sensitive step, indicated by the double dagger, as opposed to being converted to a form of free enzyme, e.g. E and F, respectively.
In order to accommodate isotopic substitutions, equation (3.1) must be modified by the inclusion of the term Ci[Dk−1] in the denominator of the second parenthetical expression:
| (3.2) |
Ci is the fraction of isotopic labelling and varies from zero (no label) to 1.0 (100% isotopic substitution). It adjusts well for less than complete substitution, as for example, a 97% labelled compound would have a Ci of 0.97 (Cleland 1977). Thus, equation (3.2) provides a global expression for fitting isotopic data, in contrast to the less precise approach often used within the non-competitive method of fitting isotopic and non-isotopic data separately, and then comparing the fitted parameters. Performing regression analyses on the global expression provides more appropriate weighting and returns more precise error estimates than the separate approach.
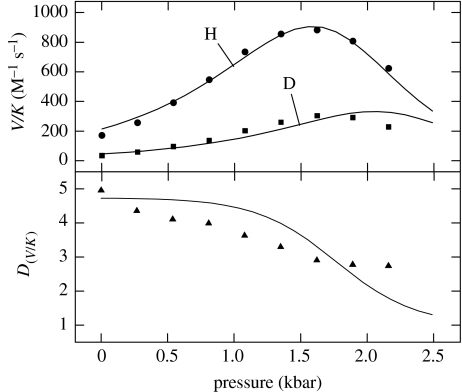
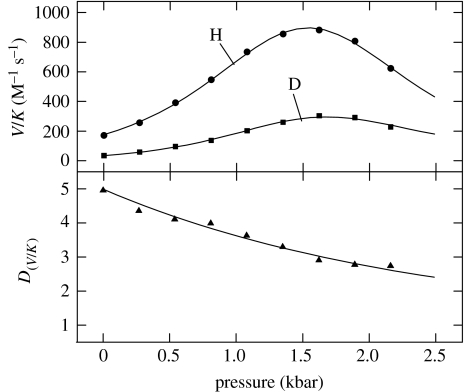
Figure 6 illustrates the results of the effects of pressure on the oxidation of deuterated and non-deuterated benzyl alcohol by YADH, and fitted to equation (3.2) (Northrop & Cho 2000a). In the upper panel are the V/K data, which at first increase with increasing pressure up to ca 1.5 kbar, after which they decrease. The ratio of the two curves and sets of data points are shown in the lower panel, depicting isotope effects. Clearly, there is a poor agreement between point and line in both the panels, but to a greater extent in the lower panel. The results show that a pressure-induced change in rate constants leading to a change in the expression of a pressure-insensitive intrinsic isotope effect will not account for the data. Or, to state it differently a pressure-dependent increase in the commitments to catalysis will not by themselves account for the decrease in the isotope effect. Instead, it is apparent that pressure must be having some direct effect on the intrinsic isotope effect itself, as one might expect hydrogen tunnelling to be involved.
Figure 6.
Effect of pressure on the capture of benzyl alcohol and deutero-benzyl alcohol by YADH, fitted to equation (3.2).
Incorporating the expression for the effect of pressure on hydrogen tunnelling, shown in equation (2.5), into equation (3.2) yields the following rate equation (Northrop & Cho 2000a):
| (3.3) |
A fit of the data in figure 6 to equation (3.3) is shown in figure 7. Clearly, agreements between points and lines are now apparent in both panels. The fitted parameters are shown in the second column of table 2, the most striking of which is the null value for Dk, which originates in differences in zero point energies. Surprisingly, the entire kinetic isotope effect (KIE) seems to reside in the transition-state phenomenon represented by DQ.
Figure 7.
Effect of pressure on the capture of benzyl alcohol and deutero-benzyl alcohol by YADH, fitted to equation (3.3).
Table 2.
Kinetic and volume parameters of alcohol capture by YADH.
| benzyl alcohol | isopropanol | ethanol | |
|---|---|---|---|
| D k | 0.99±0.03 | 0.97±0.04 | 0.74±0.29 |
| D Q | 4.99±0.37 | 4.0±0.3 | 3.0±0.8 |
| ΔVH‡ (ml mol−1) | −38±1 | −29.6±2.0 | −84±61 |
| ΔVQ (ml mol−1) | 10.4±1.5 | 7.7±2.1 | 27±54 |
| K G/E | 0.012±0.002 | 0.011±0.003 | 0.37±0.55 |
| ΔVG/E (ml mol−1) | −72.5±1.4 | −74±2 | −97±31 |
| C | [0] | [0] | 0.12±0.78 |
Given that this finding is unusual and unexpected, a crucial aspect of this analysis is a rigorous elimination of a change in the commitments to catalysis as a contributing factor to the measured decrease in the KIE. The data shown in table 2 were obtained from a regression analysis in which C was masked at a value of zero. This was, in fact, the last regression run in a long series of analyses, wherein a large variety of initial estimates were employed in order to ensure that we had not fallen into a false minimum. When C was not masked, the regression converged, but with large standard errors, i.e. C<0.001±10, with similar lack of precision in other parameters. When one of the other parameters was masked, C<0.001±0.0012. In separate experiments of the same design in which the effect of pressure was measured on solvent isotope effects, the regression converged without masking and returned a value of C<0.001±0.0014 (Northrop & Cho 2000b).
As a bonus, we obtain additional new information about the enzyme, YADH. The volume of activation for hydride transfer is the first of its kind to be measured, equivalent to the space occupied by approximately two water molecules, and has a negative sign meaning that the TS occupies a smaller volume than the reactant state. The difference for deuteride transfer is 10 ml less, or ΔVD‡=−48 ml mol−1. Obviously, as stated earlier, this number cannot represent real space as the volume difference between a hydride ion and a deuteride ion is only a few per cent. As a further bonus, this exercise returns a rather precise measure of the equilibrium constant for the conformational change associated with the binding of NAD+ to the free enzyme, which has not been defined before, despite numerous examples of its detection in a variety of dehydrogenases. Finally, the analysis returns a precise measure of the volume change associated with the change in enzyme–nucleotide conformation. Upon binding of the nucleotide, YADH appears to ‘puff up’ to a larger volume, which probably reflects the exclusion of a number of water molecules from the interior of the protein.
These experiments have recently been repeated with other alcohols, isopropanol and ethanol (Park et al. 2005), and the results of data fittings to equation (3.3) are also listed in table 2. In both cases, graphical analysis resembled figure 7 (not shown). More importantly, in both cases, regressions again returned null values for Dk, the portion of the isotope effect that originates in differences in zero point energies, leaving the entire isotope effect as originating from a transition-state phenomenon. However, the volumes of activation for hydride and deuteride transfer were different for the different alcohols, making this the first of its kind as a new type of structure/activity analysis reflecting a physical property of a TS. The data for isopropanol, obtained with a regression run with the commitments masked at a value of zero, returned equivalent parameters regarding the conformational change of the enzyme–nucleotide complex, which is reassuring as these should not be different since the conformational change occurs in the absence of the alcohol.
Given that equivalency, the regression was re-run with KG/E masked at (0.012) and ΔVG/E masked at (−72.5). This regression returned identical values for the other parameters plus a value of C<0.001±0.3, adding additional evidence that the commitments were not significant, but simply buried under extensive covariance. The data for ethanol, whose oxidation is known to proceed with YADH without completely expressing the intrinsic isotope effect, converged with poorly defined kinetic parameters including commitments that seem to approach some significance. These data were disappointing because we had entertained the hope that such a dataset might provide a new method for extracting intrinsic isotope effects that were not completely expressed. Unfortunately, the extensive covariance would appear to make it impossible. Curiously, however, the equilibrium constant for the conformational change seemed now to be different, but buried under extensive standard errors. To bring out this apparent difference, the regression was repeated with the commitments masked at a zero value, which returned a value for KG/E=0.43±0.23. While quantitatively not determined with much precision, the equilibrium constant is clearly different from that obtained with benzyl alcohol or isopropanol. It turns out that the difference can be traced to the presence of isozymes of YADH in yeast, and only the second isozyme acts on benzyl alcohol and isopropanol. Both isozymes act on ethanol and the first is more prevalent in the yeast than the second. Hence, a change in the enzyme is reflected in a change in this parameter, which suggests that this new method of analysis is sensitive to such changes; it does not gravitate to some sort of generic minimum.
These high-pressure experiments have also been repeated with another enzyme, formate dehydrogenase, with similar results (Quirk & Northrop 2001). Most importantly, regression analysis again returned a null value for Dk of 1.02±0.03, together with the entire isotope effect residing in a transition-state phenomenon, namely DQ=2.73±0.20. The pressure experiments have also been repeated with other pyridine nucleotides with similar findings (H. Park & D. B. Northrop 2005, unpublished results).
4. The effect of pressure on characteristics of nucleotide binding to YADH
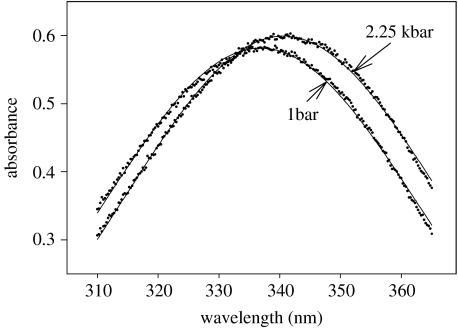
To validate these kinetic assignments in part, thermodynamic experiments were undertaken to investigate the effects of pressure on the enzyme–nucleotide conformational change directly. Sytkowski (1977) had reported that binding of NADH to YADH was accompanied by a hypsochromic shift of its absorbance maximum from 340 to 335 nm and a small attenuation of maximal absorbance. Figure 8 shows an example of the spectrum of NADH complexed with YADH at atmospheric pressure, plus the effect of 2.25 kbar of pressure on the spectrum (Kidman & Northrop 2005). The application of pressure causes a bathochromic shift of the absorbance maximum, returning it to the 340 nm region, plus a reversal of the attenuation. The bathochromic shift is consistent with pressure favouring the collision complex between the free enzyme and reduced pyridine nucleotide and disfavouring the tightened complex with its associated conformational and spectral change. If pressure favours the conversion of E*-NADH to E-NADH, then it would seem reasonable to conclude that it favours the parallel conversion of E*-NAD+ to E-NAD+ as well, thus providing a confirmation of the kinetic assignment. Such independent controls and confirmations are important in all studies, but particularly so in this pressure study because this represents the first time any such assignments have been made in an enzyme-catalysed reaction.
Figure 8.
The effect of pressure on the absorbance spectrum of NADH complexed with YADH.
5. The effect of pressure on a heavy atom isotope effect of YADH
While hydrogen tunnelling may be partially responsible for the pressure sensitivity of the primary DIE of YADH, it is difficult to understand how it alone can account for these results. In the Bell formulation of equation (2.1), reactant-state and transition-state effects are multiplied together, so the zero point vibrational differences will not simply go away when tunnelling becomes more significant. Some other transition-state phenomenon must certainly be contributing to some, if not all, of the isotope effect. In an attempt to distinguish between tunnelling and some other transition-state phenomenon, a determination of the effect of pressure on a 13C isotope effect on the same bond cleavage (see the position of isotopic label in scheme 1) was initiated because tunnelling does not contribute to heavy atom isotope effects, at least not to any significant degree at ambient temperatures (Zuev et al. 2003). Being much smaller than DIEs, heavy atom isotope effects are most often determined by a competitive experimental design in which both isotopic and non-isotopic substrates compete for the enzyme. The isotopic discrimination that ensues is governed by the equation of Bigeleisen & Wolfsberg (1958):
| (5.1) |
where f is the fractional conversion of the reaction, Rp the 13C/12C isotopic ratio of the product at fractional conversion f and R0 is the 13C/12C isotopic ratio of the initial substrate, or as measured here, the final product at f=1.0. For an enzymatic reaction, isotopic discrimination is dependent solely upon substrate capture; it is independent of the availability of enzyme, e.g. E-NAD+ versus E*-NAD+, as the discrimination only reports from the active enzyme, and is independent of events following the first irreversible step. Enzymatic isotopic discrimination obeys the equation:
| (5.2) |
where 13k is the intrinsic 13C isotope effect, and Cf and Cr are again forward and reverse commitments to catalysis, respectively (Northrop 1977). If the sum of the commitments changes as a function of pressure, equation (5.2) becomes:
| (5.3) |
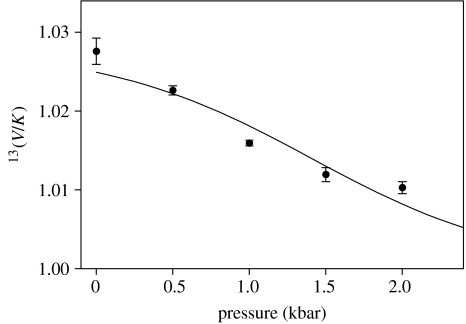
Figure 9 shows the results of isotopic discrimination of 13C-benzyl alcohol by YADH as a function of increasing pressure (Park et al. 2006). The data were fitted to equation (5.3), with the intrinsic isotope effect masked at 13k=1.028, the value observed at atmospheric pressure (when not so masked, an unrealistic value for the intrinsic isotope effect was obtained). The regression returned a value for the commitments of C=0.13±0.22. This poorly determined parameter plus the lack of agreement between point and line show that the apparent decrease in the heavy atom isotope effect at high pressures cannot be accounted for by a change in commitments by themselves. Therefore, despite the expectation that carbon is a too large particle to undergo quantum mechanical tunnelling, some sort of mass-dependent transition-state phenomenon must be present.
Figure 9.
Effect of pressure on the isotopic discrimination of 13C-benzyl alcohol by YADH, fitted to equation (5.3).
By analogy to equation (2.5), the effect of pressure on such a transition-state component of a heavy atom isotope effect of carbon should obey the equation:
| (5.4) |
By substituting the right half of equation (5.4) for the intrinsic isotope effect in equation (5.3), a general expression for the effect of pressure on a 13C isotope effect on substrate capture by an enzymatic reaction can be written as:
| (5.5) |
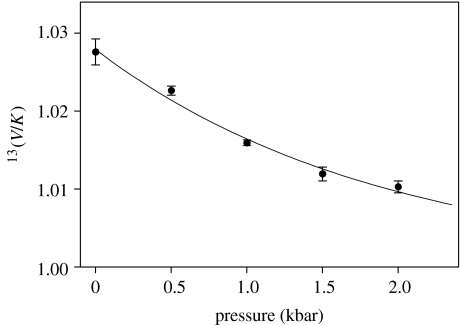
A fit of the data for isotopic discrimination to equation (5.5) is shown in figure 10, described by parameters of: 13k=1.00±0.01, 13Q=1.028±0.006, ΔVQ=13.1±4.5 ml mol−1, C<0.001±0.0013 (with ΔV‡=[−38 ml mol−1], taken from table 1).
Figure 10.
Effect of pressure on the isotopic discrimination of 13C-benzyl alcohol by YADH, fitted to equation (5.5).
This is a very good fit with close agreement between point and line, showing once again that the portion of the isotope effect arising from zero-point energy differences has a null value, and that the contribution of the commitments to suppress the expression of the isotope effect at high pressures is clearly negligible. Repeating the regression with 13k masked at a value of 1 and C masked at 0, parameters of 13Q=1.028±0.001 and ΔVQ=13.1±0.7 ml mol−1 were obtained. It is striking that the volume change between 12C and 13C discrimination of benzyl alcohol is virtually identical to that obtained for 1H versus 2H transfer from benzyl alcohol, i.e. ΔVQ=10.4±1.5 ml mol−1 in table 2. The equivalent decrease in activation volumes for hydride transfer, when one mass unit is added to the carbon end of a scissile C–H bond as when one mass unit is added to the hydrogen end, suggests a common origin.
6. Conclusions
These results are a puzzlement. While it is clear that the intrinsic isotope effects are completely expressed during the oxidation of isopropanol and benzyl alcohol by YADH, it is also clear that differences in zero point energies make no contribution to the intrinsic effects. Since carbon tunnelling is unlikely to make any contribution to heavy atom isotope effects, and because quantum mechanical contributions are multiplied times this missing contribution in DIEs (see equation (2.1)), it then follows that hydrogen tunnelling also makes no contribution to DIEs. Yet it is obvious that mass must be of fundamental importance to the TS, as both types of isotope effects are clearly sensitive to high pressure—and by the same degree.
How can atomic mass have kinetic importance in the TS of an enzymatic reaction? According to the Born–Oppenheimer approximation, isotopic and non-isotopic entities have the same chemistry, and isotopic differences reside solely in differences of vibrational states. Thus, some sort of vibrational mode or other motion is required for these atomic mass differences to be expressed. This importance of mass suggests that mechanical motion within the enzyme must be applied to achieve the TS. Such a mechanical component has been the overriding thesis of Rufus Lumry for over 50 years. In his view, it consists of protein domains that are paired ‘in a dyad arrangement’ connected by a ‘hinge’ and ‘dynamically matched to oscillate like a tuning fork so oriented as to raise by transient compression the potential energy’ of the enzyme–substrate reactant-state (Lumry 1991). What he means by this is, rather than lowering the energy of the TS by some sort of stabilization, i.e. the standard paradigm being challenged at this meeting, enzymes act as energy transducers to convert kinetic or mechanical energy to chemical potential, which raises the energy level of the reactant state. For many years, there was no evidence in support of such a motion contributing to rate acceleration by enzymes, but recently disulphide bridges were engineered between the two domains of a nucleotide phosphatase, rendering it almost inactive in comparison to the reduced form of the engineered enzyme (Schultz-Heienbrok et al. 2005). This report finally gives direct support to the hypothesis that domain motion can contribute to the rate enhancement of chemical steps of enzymatic catalysis. Finally, motion implies momentum, which in turn invalidates the quasi-equilibrium assumption represented by K‡ of absolute reaction rate theory, illustrated in figure 2 and equation (1.3).
This is a crucial assertion; buried in the assumptions of absolute rate theory, and often forgotten in later applications to enzymology, is a requirement that motion along the reaction coordinate is independent of all other motions. This may be true for reactions involving diatomic gases, but believing it to be true for enzymatic reactions is a bit of a stretch. Now, with the effect of pressure on a heavy atom isotope effect, the assumption fails, and with it the application of absolute rate theory to enzymatic reactions. We need a completely new paradigm for enzymatic rate theory, not just an adjustment to the old one.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Quantum catalysis in enzymes—beyond the transition state theory paradigm’.
References
- Bell R.P. Chapman and Hall; London, UK; New York, NY: 1980. The tunnel effect in chemistry. [Google Scholar]
- Bett K.E, Cappi J.B. Effect of pressure on the viscosity of water. Nature. 1965;207:620–621. [Google Scholar]
- Bigeleisen J, Wolfsberg M. Theoretical and experimental aspects of isotope effects in chemical kinetics. Adv. Phys. Chem. 1958;1:15–76. [Google Scholar]
- Cha Y, Murray C.J, Klinman J.P. Hydrogen tunneling in enzymic reactions. Science. 1989;243:1325–1330. doi: 10.1126/science.2646716. [DOI] [PubMed] [Google Scholar]
- Cho Y.-K, Northrop D.B. Effects of pressure on the kinetics of capture by yeast alcohol dehydrogenase. Biochemistry. 1999;38:7470–7475. doi: 10.1021/bi990625d. Correction: Biochemistry 38, 10 908 (1999) [DOI] [PubMed] [Google Scholar]
- Cleland W.W. Computer programs for determining deuterium isotope effects. In: Cleland W.W, O'Leary M.H, Northrop D.B, editors. Isotope effects on enzyme-catalyzed reactions. University Park Press; Baltimore, MD: 1977. pp. 261–270. Appendix B. [Google Scholar]
- Glastone S, Laidler K.J, Eyring H. McGraw-Hill; New York, NY: 1941. The theory of rate processes. pp. 472–473. [Google Scholar]
- Isaacs N.S. The effect of pressure on kinetic isotope effects. In: Buncel E, Lee C.C, editors. Isotope effects in organic chemistry. vol. 6. Elsevier; London, UK: 1984. pp. 67–105. [Google Scholar]
- Isaacs N.S, Javaid K, Rannala E. Reactions at high pressure. Part 5. The effect of pressure on some primary kinetic isotope effects. J. Chem. Soc. 1978;Perkin II:709–711. [Google Scholar]
- Kidman G, Northrop D.B. Effect of pressure on nucleotide binding to yeast alcohol dehydrogenase. Protein pept.e Lett. 2005;12:495–497. doi: 10.2174/0929866054395293. [DOI] [PubMed] [Google Scholar]
- Lumry R. Mechanical force, hydration, and conformational fluctuations in enzymic catalysis. In: Kuby S.A, editor. Mechanism of enzyme action. vol. 2. CRC Press; Boca Raton, FL: 1991. pp. 3–81. [Google Scholar]
- Northrop D.B. Determining the absolute magnitude of hydrogen isotope effects. In: Cleland W.W, O'Leary M.H, Northrop D.B, editors. Isotope effects on enzyme-catalyzed reactions. University Park Press; Baltimore MD: 1977. pp. 122–152. [Google Scholar]
- Northrop D.B. On the meaning of Km and Vmax/Km in enzyme kinetics. J. Chem. Educ. 1998;75:1153–1157. [Google Scholar]
- Northrop D.B. Effects of high pressure on isotope effects and hydrogen tunneling. J. Am. Chem. Soc. 1999;121:3521–3524. doi: 10.1021/ja984177t. [DOI] [Google Scholar]
- Northrop D.B. Effects of high pressure on enzymatic activity. Biochem. Biophys. Acta. 2002;1595:71–79. doi: 10.1016/s0167-4838(01)00335-1. [DOI] [PubMed] [Google Scholar]
- Northrop D.B, Cho Y.K. Effect of pressure on deuterium isotope effects of yeast alcohol dehydrogenase: evidence for mechanical models of catalysis. Biochemistry. 2000a;39:2406–2412. doi: 10.1021/bi992537z. [DOI] [PubMed] [Google Scholar]
- Northrop D.B, Cho Y.K. Effects of high pressure on solvent isotope effects of yeast alcohol dehydrogenase. Biophys. J. 2000b;79:1621–1628. doi: 10.1016/S0006-3495(00)76412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kidman G, Northrop D.B. Effects of pressure on deuterium isotope effects of yeast alcohol dehydrogenase using alternative substrates. Arch. Biochem. Biophys. 2005;433:335–340. doi: 10.1016/j.abb.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Park H, Girdaukas G.G, Northrop D.B. Effect of pressure on a heavy-atom isotope effect of yeast alcohol dehydrogenase. J. Am. Chem. Soc. 2006;128:1868–1872. doi: 10.1021/ja056525e. [DOI] [PubMed] [Google Scholar]
- Quirk D.J, Northrop D.B. Effect of pressure on deuterium isotope effects of yeast formate dehydrogenase. Biochemistry. 2001;40:847–851. doi: 10.1021/bi001991w. [DOI] [PubMed] [Google Scholar]
- Schultz-Heienbrok R, Maier T, Sträter N. A large hinge bending domain rotation is necessary for the catalytic function of Escherichia coli 5′-nucleotidase. Biochemistry. 2005;44:2244–2252. doi: 10.1021/bi047989c. [DOI] [PubMed] [Google Scholar]
- Sytkowski A.J. Metal stoichiometry, coenzyme binding, and zinc and cobalt exchange in highly purified yeast alcohol dehydrogenase. Arch. Biochem. Biophys. 1977;185:505–517. doi: 10.1016/0003-9861(77)90460-x. [DOI] [PubMed] [Google Scholar]
- Zuev P.S, Sheridan R.S, Albu T.V, Truhlar D.G, Hrovat D.A, Borden W.T. Carbon tunneling from a single quantum state. Science. 2003;299:867–870. doi: 10.1126/science.1079294. [DOI] [PubMed] [Google Scholar]