Abstract
Here, we evaluated relationships between ABCB1 (P-glycoprotein, MDR1) polymorphisms and paclitaxel (Taxol)-induced toxicity and pharmacokinetics. Twenty-six patients were assessable for pharmacogenetics and pharmacokinetics, 22 for neurotoxicity and 18 for myelotoxicity. Patients carrying two reference alleles for the ABCB1 3435C>T polymorphism trended toward a reduced risk to develop neuropathy as compared to patients carrying at least one variant allele (P=0.09). Additionally, patients that were homozygous variant at the 2677 and 3435 loci had a significantly greater percent decrease in absolute neutrophil count at nadir (P=0.02). Neither polymorphism correlated with paclitaxel pharmacokinetics. This pilot study suggests that paclitaxel-induced neuropathy and neutropenia might be linked to inherited variants of ABCB1 through a mechanism that is unrelated to altered plasma pharmacokinetics.
Keywords: Paclitaxel, polymorphisms, ABCB1, neutropenia, neuropathy
1. Introduction
One of the primary proteins involved in paclitaxel elimination and distribution is ABCB1 (P-glycoprotein; MDR1).1,2 ABCB1 is expressed in several tissues, including the blood-brain barrier,3 and hematopoietic precursor cells.4 Although ABCB1 has not been detected in peripheral nerve cells, the cells that make up the blood-nerve barrier express ABCB1 and are thought to protect the peripheral nervous tissue by transporting toxic substances from the nervous system back into the systemic circulation.5
There are three common single nucleotide polymorphisms (SNPs) in ABCB1 that have been associated with altered ABCB1 expression, and that have profound implications in the pharmacokinetics and pharmacodynamics of various drugs.6 These include the synonymous 1236C>T, the non-synonymous 2677G>T/A (Ala893Ser/Thr), and the synonymous 3435C>T SNPs. Animal models have indicated that transduction of haematopoietic cells with human ABCB1 could protect from severe paclitaxel-induced myelotoxicity.7 Based on differential expression, those patients with low-expressing ABCB1 genetic variants could be more likely to experience paclitaxel-induced peripheral neuropathy and neutropenia. Therefore, we investigated the association of ABCB1 genetic variants with the incidence and severity of these side effects in patients treated with paclitaxel.
2. Patients and methods
2.1 Study design and patients
Patients with advanced solid tumours were treated with weekly, single-agent paclitaxel (Taxol), administered as a 1- or 3-hour infusion. Eligibility criteria, treatment schedules, and assessment of pharmacokinetic and toxicity profiles have been described previously.8,9 The current analysis was approved by the review board of the National Cancer Institute and all patients provided informed consent.
2.2 ABCB1 genotype analysis
Polymerase chain reaction (PCR) was performed using the Platinum Taq PCR Kit from Invitrogen (Carlsbad, CA, USA). Direct nucleotide sequencing PCR was conducted using the Big Dye Terminator Cycle Sequencing Ready Reaction kit V3.1 (Applied Biosystems, Foster City, CA, USA) and an ABI Prism 310 Genetic Analyzer.
2.3 Statistical considerations
Associations of variant genotypes with neutropenia and individual pharmacokinetic parameters were evaluated statistically with a Kruskal-Wallis test or an exact Wilcoxon rank sum test after a Bonferroni adjustment. The probability of the development of peripheral neuropathy was analyzed using the Kaplan-Meier method,10 with an exact log-rank test.11 All P values are two-tailed, and P<0.05 was considered to reflect statistical significance.
3. Results
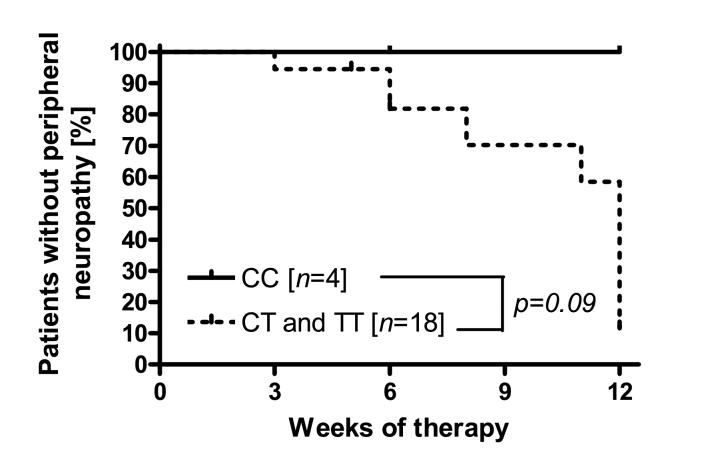
None of the patients carrying the reference allele for the ABCB1 3435C>T transition developed peripheral neuropathy during their observation period, whereas patients carrying at least one variant allele trended toward an increased risk for developing peripheral neuropathy (P=0.09; Fig 1).
Fig 1.
Association between risk of peripheral neuropathy and ABCB1 3435C>T genotype status in 22 patients. CC - ABCB1 3435-CC genotype; CT - ABCB1 3435-CT genotype; TT - ABCB1 3435- TT genotype. The P value was obtained from an exact two-tailed log rank test.
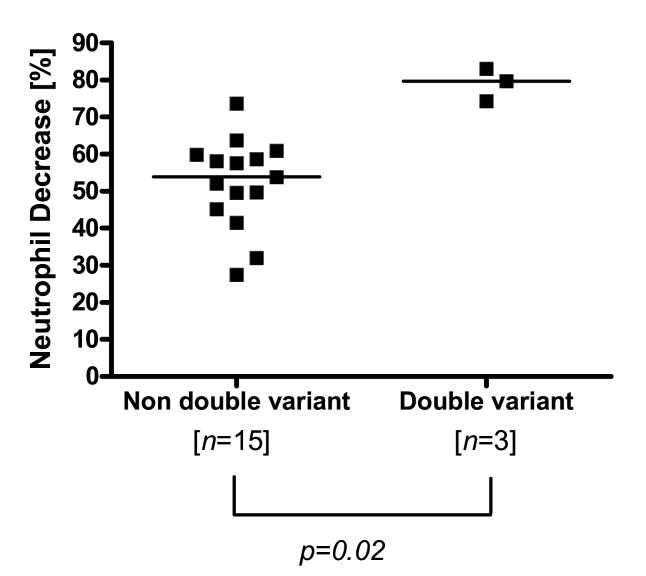
Patients carrying variants at both the 2677 and 3435 loci demonstrated a 1.5-fold greater percent decrease (P=0.02) in absolute neutrophil count at nadir (median, 79.7%; range, 74.2-83.1%) as compared to the rest of the population (median, 53.8%; range, 27.5-73.7%) (Fig 2).
Fig 2.
Association between ANC and ABCB1 2677G>T and 3435C>T genotype status in 18 patients. Non double variants - for both ABCB1 2677G>T and ABCB1 3435C>T alleles; Double variants - for both ABCB1 2677G>T and ABCB1 3435C>T alleles. The unadjusted P value was 0.0025.
None of the studied ABCB1 genotypes was associated with interindividual differences in plasma pharmacokinetic parameters of paclitaxel (Table 1).
Table 1.
Association between ABCB1 genotype status and paclitaxel pharmacokinetics
| Genotype | T<0.05 μM (hours) | AUCp [(ng/mL) x hours] | AUCu [(ng/mL x hours] | |||
|---|---|---|---|---|---|---|
| Median (95%CI) | P | Median (95%CI) | P | Median (95%CI) | P | |
| ABCB1 1236C<T | ||||||
| Wild-type (N=5)† | 20.3 (8.8-34.2) | 0.67 | 4657 (2909-9916) | 0.34 | 507 (371-641) | 0.39 |
| Heterozygous (N=17) | 15.6 (9.3-19.3)* | 5264 (3879-6235) | 470 (445-519) | |||
| Variant (N=4)† | 15.6 (8.8-24.9) | 3547 (2309-5600) | 407 (362-522) | |||
| ABCB1 2677G<T | ||||||
| Wild-type (N=2)† | 15.1 (8.8-21.3) | 0.97 | 3348 (2909-3787) | 0.18 | 540 (507-572) | 0.26 |
| Heterozygous (N=13) | 17.3 (9.6-20.7)* | 5146 (3776-7762) | 482 (371-641) | |||
| Variant (N=11) | 12.6 (8.8-19.8) | 4203 (2309-5600) | 445 (362-516) | |||
| ABCB1 3435C<T | ||||||
| Wild-type (N=4)† | 9.7 (8.8-19.3) | 0.23 | 4534 (2909-5146) | 0.18 | 420 (313-572) | 0.31 |
| Heterozygous (N=14) | 19.8 (10.5-21.3)* | 5485 (3787-6344) | 512 (432-523) | |||
| Variant (N=8) | 13.7 (8.7-18.9) | 3656 (2259-5600) | 448 (362-522) | |||
Indicates the genotype of the patient with unavailable T>0.05 μM data;
Indicates that 95% confidence intervals are unavailable and the range is quoted instead.
Abbreviations: T>0.05 μM, duration of total plasma concentration of paclitaxel exceeding 0.05 μM; AUCp, area under the curve of total paclitaxel; AUCu, area under the curve of unbound paclitaxel; 95%CI, 95% confidence interval; P, Kruskal-Wallis test.
4. Discussion
The current data suggest a possible genetic predisposition to the occurrence of paclitaxel-induced neuropathy and neutropenia regulated by the ABCB1 transporter gene. The finding that patients homozygous wild-type for the ABCB1 3435C>T transition are less likely to develop clinically-significant peripheral neuropathy indicates that ABCB1 may be differentially expressed in the blood nerve barrier in a genotype-dependent manner. Likewise, the notion that patients carrying variants at both the ABCB1 2677G>T/A and 3435C>T loci have a significantly more pronounced relative decrease in their absolute neutrophil count at nadir is consistent with an inherited factor regulating ABCB1 expression in repopulating hematopoietic cells.
Interestingly, we found that the plasma pharmacokinetics of paclitaxel were not correated with any of the evaluated ABCB1 SNPs, suggesting that a compensatory efflux mechanism, perhaps through ABCC2 (MRP2), may regulate paclitaxel elimination from the liver in patients with impaired ABCB1 function due to genetic variation. A more plausible explanation is the possibility that hepatocellular influx and subsequent metabolism of paclitaxel, rather than ABC transporter mediated biliary secretion, are the rate-limiting steps involved in systemic drug clearance.
The present data are in line with previous observations that the ABCB1 3435-T allele is associated with decreased expression of ABCB1 in the liver, and decreased activity in CD56+ cells,12 an effect that is likely due to decreased mRNA stability associated with this SNP.13 Recent studies have indicated that the ABCB1 2677G>T/A allele may also independently contribute to altered ABCB1 expression levels.14 Overall, our data suggest that the ABCB1 3435C>T transition might have predictive power in the assessment of ABCB1 expression and activity at the blood-nerve barrier, whereas the 2677G>T/A and 3435C>T alleles combined are important contributing factors to the expression and activity of ABCB1 within repopulating neutrophils.
It should be noted that the small sample size of this study clearly warrants independent confirmation in a larger patient population. It is hoped therefore that the pharmacologic results presented here will encourage investigators to perform similar exploratory genotype approaches to gain insight into the mechanisms underlying interindividual pharmacodynamic variability of taxanes and other ABCB1 substrate drugs.
Acknowledgements
We thank Dr. Sharon Marsh (Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA) for helpful suggestions and assistance.
Footnotes
This study was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Bethesda, MD, USA, and by investigator-initiated grants from Bristol-Myers Squibb, Munich, Germany.
Conflicts of interest statement
The authors have no conflict of interest.
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
References
- 1.Malingre MM, Beijnen JH, Rosing H, et al. Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br J Cancer. 2001;84:42–47. doi: 10.1054/bjoc.2000.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walle UK, Walle T. Taxol transport by human intestinal epithelial Caco-2 cells. Drug Metab Dispos. 1998;26:343–346. [PubMed] [Google Scholar]
- 3.Cordon-Cardo C, O’Brien JP, Casals D, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drach D, Zhao S, Drach J, et al. Subpopulations of normal peripheral blood and bone marrow cells express a functional multidrug resistant phenotype. Blood. 1992;80:2729–2734. [PubMed] [Google Scholar]
- 5.Schinkel AH, Smit JJ, van Tellingen O, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 6.Ieiri I, Takane H, Otsubo K. The MDR1 (ABCB1) gene polymorphism and its clinical implications. Clin Pharmacokinet. 2004;43:553–576. doi: 10.2165/00003088-200443090-00001. [DOI] [PubMed] [Google Scholar]
- 7.Fruehauf S, Wermann K, Buss EC, et al. Protection of hematopoietic stem cells from chemotherapy-induced toxicity by multidrug-resistance 1 gene transfer. Recent Results Cancer Res. 1998;144:93–115. doi: 10.1007/978-3-642-46836-0_12. [DOI] [PubMed] [Google Scholar]
- 8.Mielke S, Mross K, Gerds TA, et al. Comparative neurotoxicity of weekly non-break paclitaxel infusions over 1 versus 3 h. Anticancer Drugs. 2003;14:785–792. doi: 10.1097/00001813-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Gelderblom H, Mross K, ten Tije AJ, et al. Comparative pharmacokinetics of unbound paclitaxel during 1- and 3-hour infusions. J Clin Oncol. 2002;20:574–581. doi: 10.1200/JCO.2002.20.2.574. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EL. MP: Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 11.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chem Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 12.Hitzl M, Drescher S, van der Kuip H, et al. The C3435T mutation in the human MDR1 gene is associated with altered efflux of the P-glycoprotein substrate rhodamine 123 from CD56+ natural killer cells. Pharmacogenetics. 2001;11:293–298. doi: 10.1097/00008571-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Johnson AD, Papp AC, Kroetz DL, Sadee W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet Genomics. 2005;15:693–704. [PubMed] [Google Scholar]
- 14.Song P, Lamba JK, Zhang L, et al. G2677T and C3435T genotype and haplotype are associated with hepatic ABCB1 (MDR1) expression. J Clin Pharmacol. 2006;46:373–379. doi: 10.1177/0091270005284387. [DOI] [PubMed] [Google Scholar]