Abstract
Longitudinal bone growth results from endochondral ossification, a process that requires proliferation and differentiation of chondrocytes. It has been shown that proper endochondral bone formation is critically dependent on the retinoblastoma family members p107 and p130. However, the precise functional roles played by individual E2F proteins remain poorly understood. Using both constitutive and conditional E2F1 transgenic mice, we show that ubiquitous transgene-driven expression of E2F1 during embryonic development results in a dwarf phenotype and significantly reduced postnatal viability. Overexpression of E2F1 disturbs chondrocyte maturation, resulting in delayed endochondral ossification, which is characterized by reduced hypertrophic zones and disorganized growth plates. Employing the chondrogenic cell line ATDC5, we investigated the effects of enforced E2F expression on the different phases of chondrocyte maturation that are normally required for endochondral ossification. Ectopic E2F1 expression strongly inhibits early- and late-phase differentiation of ATDC5 cells, accompanied by diminished cartilage nodule formation as well as decreased type II collagen, type X collagen, and aggrecan gene expression. In contrast, overexpression of E2F2 or E2F3a results in only a marginal delay of chondrocyte maturation, and increased E2F4 levels have no effect. These data are consistent with the notion that E2F1 is a regulator of chondrocyte differentiation.
The vertebrate skeleton is formed by two distinct mechanisms of bone formation, intramembranous and endochondral ossification (23, 52). In the former, bone is generated directly from mesenchymal progenitor cells, whereas in the latter, a cartilage matrix precedes the formation of bone structures. The axial and appendicular skeletons develop through endochondral bone formation, while intramembranous ossification is mostly restricted to the skull and part of the clavicle. Endochondral ossification is especially important for longitudinal growth of the limbs, which requires the establishment of growth plates at the extreme ends of the long bones, and spatial and temporal control of chondrocyte differentiation (80). Growth plate chondrocytes form regular columns that sequentially and synchronously progress through proliferative, prehypertrophic, and terminally differentiating hypertrophic stages (13). Hypertrophic chondrocytes play a pivotal role in coordinating endochondral ossification, since they deposit the cartilage matrix that provides a template for invading osteoblasts to form trabecular bone at the site of the bone collar.
The first step in the process of endochondral bone formation involves the chondrogenic differentiation of mesenchymal cells, and this is mainly regulated by transcription factors of the Sox family (Sox5, Sox6, and Sox9) (11, 67), which control the expression of cartilage-specific marker genes, including type II collagen [α1 (II) collagen] and aggrecan genes (10, 44, 61). Sox Sox6 double-null mice develop severe chondrodysplasia characterized by the virtual absence of cartilage, implying that Sox5 and Sox6 are redundant regulators of chondroblast differentiation (67). Similarly, Sox9 is largely essential for the formation of chondrogenic condensations and cartilage (11) and regulates Sox5 and Sox6 expression (1). During the later phases of the chondrocyte differentiation program, Sox9 expression is required for overt chondrocyte proliferation and prevents conversion of proliferating chondrocytes into hypertrophic chondrocytes (1). In contrast, the transcriptional regulator Cbfa1 stimulates hypertrophic chondrocyte differentiation (72, 75), which is independent from its essential role in controlling osteoblast differentiation necessary for skeletal mineralization.
Furthermore, endochondral ossification is controlled by specific local growth factors, including fibroblast growth factors (FGFs), members of the transforming growth factor-beta (TGF-β) superfamily, parathyroid hormone-related peptide (PTHrP), and Indian hedgehog (Ihh) (17). FGF signaling inhibits longitudinal bone growth, and gain-of-function mutations in FGFR3 are associated with several forms of dwarfism in humans (18, 50). TGF-β represses chondrocyte hypertrophic differentiation (25, 85) and induces the expression of PTHrP (54, 62). Enforced expression of PTHrP in cartilage results in delayed endochondral ossification (6, 79), while mice with a targeted deletion of the PTHrP gene demonstrate increased bone formation (5, 38). Ihh, which is mainly expressed in prehypertrophic chondrocytes, is required for chondrocyte proliferation and the establishment of orderly arranged columns of maturating chondrocytes in the growth plates (39). Ihh stimulates the production of PTHrP, and activation of the PTHrP receptor delays the conversion of proliferative chondrocytes to hypertrophic chondrocytes (15, 69).
There is also an important contribution of cell cycle proteins in the regulation of chondrogenic and hypertrophic differentiation. TGF-β and PTHrP signaling stimulate chondrocyte proliferation with concomitant induction of cyclin D1 transcription (8, 36). Mice deficient in cyclin D1 gene expression show diminished postnatal growth (24, 64) and reduced growth plate proliferation (8). In the absence of cyclin-dependent kinase (Cdk) inhibitor p57KIP2, there is a marked delay in cell cycle exit during chondrocyte differentiation and reduced type X collagen [α1 (X) collagen gene] expression, a marker for hypertrophic chondrocytes (84, 87). Deficiency for the pRb family members p107 and p130 results in severe skeletal defects associated with perinatal lethality, attributed to delayed chondrocyte hypertrophy (16), while Rb+/− p107−/− animals show a milder form of growth retardation (43). FGFs seem to exhibit an exclusive growth inhibitory effect on chondrocytes that is largely dependent on the action of the pRb family members p107 and p130 (40). In addition, pRb family members can alter the expression and function of Cbfa1 (58, 73), a critical regulator of hypertrophic chondrocyte differentiation.
In many cell types, pRb family members play an important role in regulating terminal differentiation by exerting direct control on cell division through regulation of E2F-dependent promoters (21, 31). E2F transcription factors (E2F1 to E2F5) bind DNA in complex with DP proteins (DP1 and DP2), but their transcriptional activity is repressed upon interaction with pocket proteins. Although pRb may interact with most E2F species, p107 and p130 bind predominantly to the E2F family proteins E2F4 and E2F5. Studies of mice deficient for each of the individual E2F gene products (26, 33, 34, 45, 49, 57, 83), as well as E2f1−/− E2f2−/− mice (88), have not yet revealed any role for E2F transcription factors in the regulation of chondrocyte maturation. In the present study, we show that constitutive E2F1 overexpression in transgenic mice and in the chondrogenic cell line ATDC5 inhibits chondrocyte differentiation, which results in delayed endochondral ossification in vivo. In contrast, E2F4 overexpression has no effect on the differentiation program of chondrocytes.
MATERIALS AND METHODS
Eμ-pp-E2F1 transgenic mice.
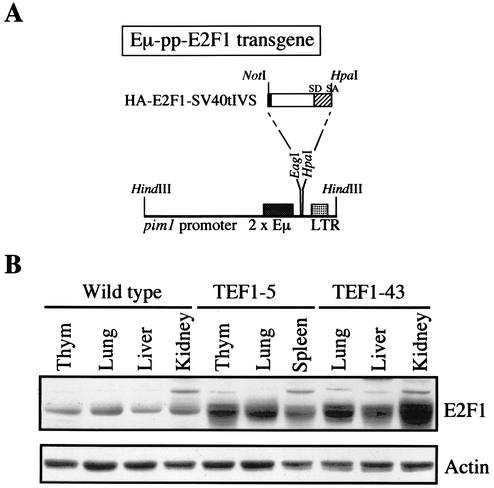
For the generation of Eμ-pp-HA-E2F1 mice, a 1.5-kbp hemagglutinin (HA)-tagged human E2F1 cDNA was inserted into the SacI-KpnI site of pJ3Ω. The NotI-HpaI fragment containing HA-tagged E2F1 (HA-E2F1), followed by splice donor and acceptor (SA) elements derived from simian virus 40 (SV40) small t antigen intervening sequences (IVS) present in pJ3Ω, was cloned in the EagI-HpaI site of the Eμ-pim1 promoter-MoMLVLTR transgenic vector (2). The transgene was isolated from the vector backbone with HindIII, microinjected into the pronuclei of FVB zygotes, and subsequently transferred to (B6 × DBA)F1 foster mice. Eμ-pp-E2F1 founder mice (in which expression of the human E2F1 cDNA was driven by the mouse pim1 promoter [pp] linked to a duplicate version of the immunoglobulin heavy-chain enhancer [Eμ]) and subsequent transgenic progeny were identified with PCR analysis on genomic DNA isolated from tail biopsies with the transgene-specific primers TDK5′ 5′-CGGCCTTTGATGGCTTTG-3′ and EMU3′ 5′-AGGGTATGAGAGAGCCTC-3′.
Targeting of the R26 locus with conditional E2F1 transgene in embryonic stem (ES) cells.
A 1.5-kbp HA-tagged human E2F1 was inserted into the R26-en2SA-loxP-βgeo-loxP-PLAP targeting vector. The targeting vector consisted of the IRES-βgeo-SV40polyA gene, with partial intron and exon sequences of mouse engrailed-2 (en2) (65), upstream of the internal ribosomal entry site (IRES)-βgeo. loxP sites were inserted between the en2SA and βgeo gene and after the SV40 polyadenylation site. The IRES-human placental alkaline phosphatase (PLAP) gene (19) was cloned into the targeting vector downstream of the floxed βgeo gene. The 3′ end of the PLAP gene was fused to the polyadenylation signal from the rabbit β-globulin gene (51). The en2SA-loxP-βgeo-loxP-PLAP fragment was inserted in the NheI site of a 13-kbp genomic XhoI-EcoRI fragment from the ROSA26 (R26) locus between exons 1 and 2 (86). Triple NotI sites surrounding exon 1 of R26 were inactivated by digestion with NotI; Klenow end filling was followed by self-ligation of the modified pBR322 vector. The 1.5-kbp HA-E2F1 cDNA was ligated in the remaining unique NotI site between the floxed βgeo and PLAP genes.
For targeting, 5 × 106 ES cells (129/OLA) at embryonic day 14 (E14) were electroporated (1 μF/800 V) with 25 μg of SfiI-linearized R26-βgeo-E2F1 and cultured on gelatin-coated dishes in conditioned medium (Gibco BRL) in the presence of 250 μg of G418 per ml for 1 week. Genomic Southern blot hybridization was performed on DNA from G418-resistant ES cell clones digested with EcoRI using the pHA607 probe (0.8-kb PstI-SalI) located outside the 5′ targeting region. Of the 35 clones analyzed, 27 had a novel 6-kb band besides the 18-kb wild-type band, due to the presence of a EcoRI site within the polylinker region in front of the HA-E2F1 cDNA sequence.
In vitro excision of the floxed βgeo gene in ES cells.
ES cells from two G418-resistant clones containing one targeted R26-βgeo-E2F1 allele were electroporated (1 μF/800 V) with 20 μg of plasmid containing the cytomegalovirus (CMV) promoter/enhancer-driven Cre gene (pOG231) and 2 μg of plasmid harboring the phosphoglycerate kinase 1-driven puromycin gene. ES cell clones were selected with puromycin (2 μg/ml) for 5 days. Cre-mediated excision of βgeo was demonstrated by the absence of β-galactosidase enzyme activity of puromycin-resistant ES cell clones and confirmed by Southern blot analysis on BglII-digested genomic DNA, probed with pHA607. The R26 germ line band of 17 kb remained present after Cre-mediated recombination, whereas the 24-kb targeted allele was replaced by a 20-kb recombined allele, due to the loss of the floxed βgeo sequence in the targeting vector.
Generation of Actin-Cre/R26E2F1 mice.
Diploid targeted R26-βgeo-E2F1/+ ES cells were injected into E3.5 C67BL/6 blastocysts and transferred to foster mice. The resulting chimeric mice were mated to FVB females. Progeny were intercrossed to generate homozygous R26-βgeo-E2F1/R26-βgeo-E2F1 mice or mated to (129/OLA × FVB) β-Actin-Cre mice (generously provided by M. Vooys and A. Berns, The Netherlands Cancer Institute, Amsterdam, The Netherlands). The presence and recombination status of the transgenic R26-(βgeo)-E2F1 allele was detected by Southern blotting of BglII-digested tail DNA, using a PstI-SalI fragment constituting probe pHA607. The Cre transgene was detected with PCR using primers Cre1 (5′-GCACGTTCACCGGCATCAAC-3′) and Cre2 (5′-CGATGCAACGAGTGATGAGGTTC-3′), which generates a fragment of 375 bp.
RNA extraction and Northern blot analyses.
Total RNA was isolated from frozen tissues or cell pellets harvested from 150-cm2 confluent dishes of ATDC5 cells using TRIzol (Gibco BRL). Poly(A)+ mRNA was isolated from total RNA by PolyATtract isolation system (Promega). Samples of either 20 μg of total RNA or 2 μg of poly(A)+ mRNA were separated on a 1% paraformaldehyde-containing agarose gel, transferred to Protran nitrocellulose filter (Schleicher & Schuell), and hybridized to a randomly primed, [α-32P]dATP-labeled 1.5-kb human E2F1 cDNA, 0.8-kb β-actin cDNA, 0.4-kb α1 (II) collagen cDNA, 0.7-kb α1 (X) collagen cDNA, or 0.5-kb aggrecan cDNA probe using conditions as described elsewhere (60).
Immunoblotting and electrophoretic mobility shift analyses.
Total cell extracts were generated by lysis of frozen tissues, ES cell clones, or ATDC5 cells in ice-cold ELB buffer (250 mM NaCl, 0.1% NP-40, 50 mM HEPES [pH 7.0], 5 mM EDTA) supplemented with protease inhibitors (Complete; Boehringer Mannheim). Samples were cleared by centrifugation for 10 min at 20,000 × g, and equivalent samples of 40 μg of protein were separated on a sodium dodecyl sulfate-polyacrylamide gel and transferred to Immobilon-P membranes (Millipore). Polyclonal antibodies against E2F1 (C-20), E2F2 (C-20), E2F3 (C-18), E2F4 (C-20), and actin (C-11) (Santa Cruz) were used to detect protein expression.
For electrophoretic mobility shift analysis, total cell lysates were obtained by incubating cells for 20 min in ice-cold lysis buffer (20 mM HEPES [pH 7.9], 400 mM NaCl, 1 mM EDTA, 20% glycerol, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride) and then two rounds of freeze-thawing. Cell lysates were cleared by centrifugation, and 10 μg of extract was used in a 20-μl band shift assay containing 0.1 ng of [γ-32P]ATP end-labeled E2F-specific double-stranded oligonucleotide (sense [5′-AATTTAAGTTTCGCGCCCTTTCTCAA-3′]) in band shift buffer (10 mM HEPES [pH 7.9], 100 mM KCl, 1 mM EDTA, 4% Ficoll) in the presence of 1 μg of sonicated salmon sperm DNA. Supershift reactions were performed with antibodies directed against pRb (21C9) (gift from S. Mittnacht, Chester Beatty Laboratories, London, United Kingdom), p107 (C-18,) and p130 (C-20) (Santa Cruz). Binding reaction mixtures were separated by electrophoresis on a 4.5% nondenaturing polyacrylamide gel in 0.25× Tris-borate-EDTA (TBE) buffer. Gels were dried and exposed for autoradiography.
Skeletal analysis, histology, and immunohistochemistry.
For skeletal staining, E17.5 embryos were isolated, skinned, eviscerated, and fixed in 95% ethanol for 24 h. The skeletons were then stained for 24 h with 0.015% Alcian blue dissolved in 75% ethanol-20% glacial acetic acid for 24 h. Thereafter, the skeletons were rinsed with ethanol and fixed for another 24 h. Samples were cleared in 1% KOH for 6 h and stained with 0.005% Alizarin red in 2% KOH for 3 h. The skeletons were subsequently transferred to decreasing concentrations of KOH (1.6, 1.2, 0.8, and 0.4%) mixed with increasing amounts of glycerol (20, 40, 60, and 80%) at 1-day intervals. For histology and immunohistochemistry, limbs from 1-week-old pups were fixed in formalin, decalcified, and embedded in paraffin. Sections were cut every 4 μm and either stained with hematoxylin and eosin or processed using a standard protocol for diaminobenzidine immunostaining incorporating an antigen retrieval step (citrate buffer microwave method) and using a 1:2,000 dilution of the mouse monoclonal HA.11 antibody (Covance) raised against the HA epitope tag. Diaminobenzidine-stained sections were counterstained with hematoxylin.
ATDC5 cell culture and E2F retroviral expression.
The chrondogenic cell line ATDC5 (7, 63) was cultured in Dulbecco modified Eagle medium-Ham's F12 (1:1) medium (Gibco BRL) supplemented with 5% fetal calf serum, 10 μg of bovine insulin per ml, 10 μg of human transferrin per ml and 3 × 10−8 M sodium selenite (ITS) (Sigma), 100 U of penicillin per ml, and 100 μg of streptomycin (Gibco BRL) per ml. During differentiation assays, standard media were replaced every 2 or 3 days. For long-term culture after 21 days, medium was changed to minimal essential medium with the same additives to facilitate mineral deposition. HA-tagged human E2F1, E2F2, E2F3a (82), and E2F4 cDNAs cloned in pBabepuro were transfected by the calcium phosphate method in φX ecotropic packaging cell line. Viral supernatants harvested 48 h after transfection were added to exponentially growing ATDC5 cells in the presence of 8 μg of Polybrene per ml. Infected ATDC5 cells were selected for 5 days in the presence of 2 μg of puromycin per ml, and polyclonal pools were used in cell proliferation and chondrocyte differentiation assays. For cartilage differentiation assay, cells were fixed in 4% paraformaldehyde-phosphate-buffered saline and stained with 0.25% Alcian blue at pH 0.75.
RESULTS
Eμ-pp-E2F1 transgenic mice.
Recent studies in mice and mouse embryonic fibroblasts (MEFs) deficient for more than one E2F gene product have revealed considerable redundancy between the various E2F family members (29, 81, 88). To obtain additional information about the distinct biological activities of E2F transcription factors in vivo, we have pursued an alternative approach by creating different transgenic gain-of-function mouse mutants for each of the E2F family members. In this study, we report on our analysis of E2F1 transgenic mice.
To investigate the effects of E2F1 overexpression during embryonic and early postnatal development, we generated Eμ-pp-E2F1 mice. Expression of the human E2F1 cDNA was driven by the mouse pim1 promoter (pp) linked to a duplicate version of the immunoglobulin heavy-chain enhancer (Eμ), which has been shown to result in broad constitutive transgenic expression during embryonic development (2) (Fig. 1A). The efficiency of generating Eμ-pp-E2F1 founders was rather low. Four transgenic E2F1 (TEF1) founder mice were obtained (TEF1-5, TEF1-21, TEF1-43, and TEF1-62), which each produced transgenic offspring. Immunoblotting indicated that Eμ-pp-E2F1 mice showed increased E2F1 expression levels in all tissues analyzed, including kidney, lung, liver, thymus, and spleen (Fig. 1B). Furthermore, the level of transgene expression was comparable in the offspring of all four TEF1 founders included in our analysis (Fig. 1B and data not shown). On average, a six- to eightfold increase in total amount of E2F1 protein levels was observed in the various tissues of the Eμ-pp-E2F1 mice examined.
FIG. 1.
Generation of Eμ-pp-E2F1 transgenic mice. (A) Transgene expression vector contains a duplicate version of the immunoglobulin heavy-chain enhancer (Eμ) inserted in the mouse pim1 promoter region (pp), followed by the Moloney murine leukemia virus long terminal repeat (LTR), which provides transcription termination sequences. The 3′ end of the human HA-E2F1 cDNA is fused to the SV40 small t antigen intervening sequence (IVS) of the pJ3Ω vector and provides an extra splice donor (SD) and splice acceptor (SA) site. (B) Immunoblotting shows E2F1 expression levels in total cell extracts from different tissues of 4-day-old Eμ-pp-E2F1 transgenic mice of two independent founder lines (TEF1-5 and TEF1-43) compared to control littermates. Actin serves as a loading control. Thym, thymus.
Several problems were encountered in the attempts to expand each of the transgenic lines to obtain transgenic progeny, which was successful only for the TEF1-5 founder line. First, the frequency of viable Eμ-pp-E2F1 transgenic offspring in the first generation (F1), as well as the second and third generations (F2 and F3), was much lower than the expected Mendelian segregation, i.e., only 40% of the expected TEF1-5 transgenic mice were alive at the age of three weeks (Table 1). This was not observed at E17.5 or earlier, where Eμ-pp-E2F1 embryos of the TEF1-5 line arose at the expected frequency (Table 1). Quantitative analysis of the amount of apoptotic cells in different tissues of E12.5 and E14.5 Eμ-pp-E2F1 transgenic embryos did not reveal any significant increase in programmed cell death as a result of E2F1 overexpression (data not shown). Second, both male and female Eμ-pp-E2F1 mice displayed reduced fertility. In fact, we were not able to obtain any offspring from female Eμ-pp-E2F1 animals. The exact reason for this defect is not yet clear and will be studied further. Finally, most of the Eμ-pp-E2F1 mice that survived beyond the age of one week were considerably smaller than their control littermates, although we observed quite a variation in penetration of the phenotype (see below). These data show that Eμ-pp-E2F1 transgene expression results in increased neonatal lethality in independent transgenic founder lines.
TABLE 1.
Viability data of constitutive and inducible E2F1 transgenic animalsa
| E2F1 transgenic line | 3-week-old mice
|
Embryos at E17.5
|
||
|---|---|---|---|---|
| Viabilityb | Observed/expected ratioc | Viability | Observed/expected ratio | |
| TEF1-5 (Eμ-pp-E2F1) | 32/151 (F2 and F3) | 32/75 = 0.43 | 16/35 | 16/17 = 0.94 |
| TEF1-21 (Eμ-pp-E2F1) | 2/57 (F1) | 2/28 = 0.07 | NDd | |
| TEF1-43 (Eμ-pp-E2F1) | 1/26 (F1) | 1/13 = 0.08 | ND | |
| TEF1-62 (Eμ-pp-E2F1) | 2/10 (F1) | 2/5 = 0.40 | ND | |
| Actin-Cre;+/R26E2F1;R26E2F1;+/Actin-Cre | 30/181 | 30/45 = 0.67 | ND | |
The mice were genotyped by PCR or Southern blot analysis of tail DNA. Viability of 3-week-old mice and of embryos at E17.5 were determined. Embryonic analysis on founder lines TEF1-21, TEF1-43, and TEF1-62 were not determined, since these transgenic lines could not be maintained. For heterozygous Actin-Cre; R26E2F1;+ crosses, embryonic survival data were not determined.
Number of mice that survived to 3 weeks of age (or E17.5)/total number of mice (or embryos).
Observed/expected ratio is the ratio of the observed number of mice (or embryos) that survived to the number of mice (or embryos) expected to survive.
ND, not determined.
Generation of Cre-loxP conditional R26-E2F1 allele.
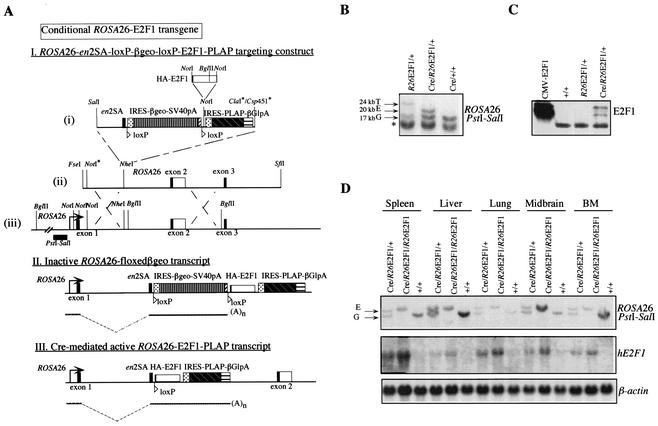
To circumvent the compromised viability of a conventional expressed E2F1 transgene during embryonic development, the binary Cre-loxP system (59) was employed to generate a conditional E2F1 transgene. To this end, we targeted a conditional E2F1 allele to the ubiquitously expressed R26 (68) gene locus, originally defined by a gene trap mouse line (27). By using the endogenous R26 promoter to drive expression of E2F1, several problems associated with conventional transgenic constructs are alleviated, including integration site- and copy number-dependent expression, repeat-induced silencing, and mosaic gene expression (28, 32). The R26 targeting vector was constructed as described in Materials and Methods. The resulting R26-en2SA-loxP-βgeo-loxP-E2F1-IRES-PLAP targeting construct was electroporated in ES cells and selected for homologous recombination at the R26 locus, using G418 selection (Fig. 2A). Homologous recombination at the R26 locus was very efficient, as 27 of 35 clones (77%) showed successful targeting of the R26-en2SA-loxP-βgeo-loxP-E2F1-IRES-PLAP construct. Two independent clones were used for subsequent analysis.
FIG. 2.
Conditional ROSA26-E2F1 transgenic mice. (A) (I) Structure of the ROSA26 (R26) locus, the R26-en2SA-loxP-E2F1-loxP-E2F1-PLAP targeting construct, and targeted locus. Restriction sites that have been inactivated are indicated with an asterisk. The position and orientation of the loxP sites are represented by triangles. The location of probe pHA607 (PstI-SalI fragment) and the BglII restriction sites used for genotyping the mice are depicted. (II and III) Schematic representation of the genomic structure and mRNA transcripts generated from the ROSA26 promoter before (II) and after (III) Cre-mediated excision. The HA-E2F1 cDNA is transcribed together with the PLAP cDNA, because of their linkage through the internal ribosomal entry site (IRES). (B) Southern blot analysis of BglII-digested DNA from ES cell clones containing one targeted R26-βgeo-E2F1 allele before and after Cre-mediated excision, compared to Cre-expressing control cells. Germ line (G), targeted (T), and excised (E) R26 alleles are indicated, as well as a nonspecific band recognized by probe pHA607 (asterisk). (C) Immunoblotting shows E2F1 expression levels in control, R26-βgeo-E2F1, and stable Cre-expressing R26E2F1 ES cells. Total cell lysate from CMV-HA-E2F1-transfected U2OS cells serves as a positive control. (D) Southern and Northern blot analyses on tissues isolated from Actin-Cre/R26E2F1;+ heterozygous, Actin-Cre/R26E2F1;R26E2F1 homozygous, and control (+/+) mice at the age of 5 weeks. The positions of the germ line (G) and excised (E) R26 alleles when probe pHA607 was used on BglII-digested genomic DNA are indicated to the left of the top blot. Northern blots demonstrate transgenic human E2F1 mRNA levels in several tissues, including spleen, liver, lung, midbrain, and bone marrow (BM), compared to β-actin loading control.
Correctly targeted ES cells were G418 resistant and showed β-galactosidase activity but did not express the HA-E2F1-IRES-PLAP transcript, due to the SV40 polyadenylation and transcription termination sequences at the 3′ end of the βgeo gene (Fig. 2A). Only the βgeo gene flanked by loxP (floxed) was transcribed (data not shown). After electroporation, the targeted allele was tested for Cre-mediated excision by transient cotransfection of ES cells with the phosphoglycerate kinase-driven puromycin gene and CMV-Cre. Southern blot analysis on puromycin-resistant ES cell clones indicated the appearance of a 20-kb excised band in Cre-expressing R26-E2F1 cell clone, replacing the 24-kb targeted allele in R26-βgeo-E2F1/+ ES cells (Fig. 2B). Expression of HA-E2F1 on the excised allele was confirmed by immunoblotting (Fig. 2C). These results show that the conditional R26-E2F1 transgene can be expressed upon Cre-mediated excision in ES cells.
Next, correctly targeted R26-βgeo-E2F1 ES cells were used to generate chimeric animals. A new transgenic founder line was established by crossing one R26-βgeo-E2F1 chimera to FVB females, producing offspring of mixed (FVB × 129/OLA) genetic background. To confirm the phenotype we had observed for Eμ-pp-E2F1 mice and to analyze the excision efficiency and transgenic expression levels in vivo, R26-βgeo-E2F1 mice were crossed with ubiquitous Cre-expressing (β-)Actin-Cre mice. Heterozygous Actin-Cre/R26E2F1;+ and homozygous Actin-Cre/R26E2F1;R26E2F1 mice were obtained and analyzed for recombination at the R26 locus as well as for expression of the 4.5-kb HA-E2F1-IRES-PLAP mRNA transcript. Southern blotting indicated 100% efficiency of Cre-mediated excision in heterozygous and homozygous R26-E2F1 mice as tested in spleen, liver, lung, midbrain, bone marrow, thymus, kidney, testes, and heart (Fig. 2D and data not shown). Northern blot analysis showed dose-dependent transgene expression, although absolute human E2F1 mRNA levels varied between different tissues (Fig. 2D), reflecting distinct tissue-specific R26 promoter activity, as has been reported in R26cre-ERT mice (76). In conclusion, these analyses demonstrate that ectopic E2F1 can be expressed in a broad range of tissues and cell lineages upon Cre-mediated recombination.
Ubiquitous E2F1 overexpression results in dwarf mice and delayed endochondral ossification.
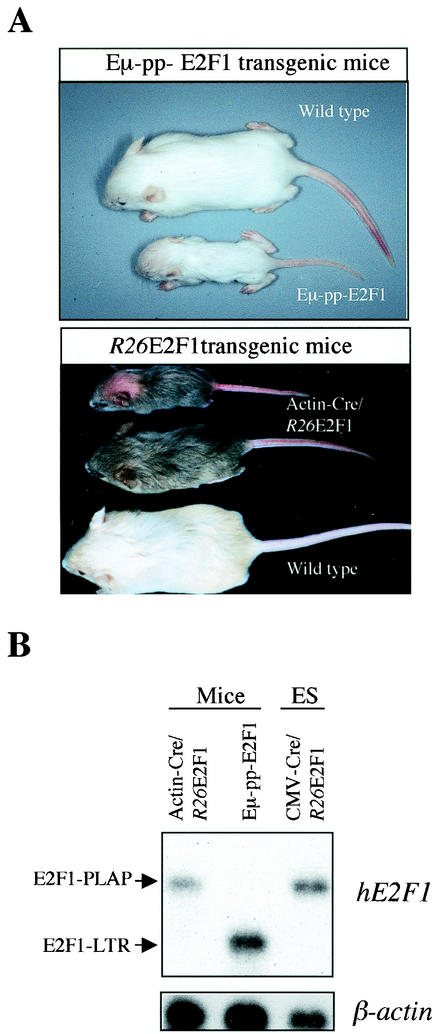
Approximately one-third (10 of 32) of viable Eμ-pp-E2F1 mice (line TEF1-5) exhibited severely decreased body size compared to wild-type control littermates (Fig. 3A). This obvious dwarf phenotype was evident from birth, and pups continued to exhibit strongly retarded growth. Often these animals had to be euthanized at the age of 4 weeks because of inappropriate food intake. For the other two-thirds of viable TEF1-5 animals, the extent of dwarfism was less significant, with only a marginal decrease in body size. Actin-Cre/R26E2F1 mice were also examined for potential growth abnormalities. There was only a marginal increase in neonatal lethality among heterozygous Actin-Cre/R26E2F1;+ mice (Table 1), and only 2 of 30 (7%) of Actin-Cre/R26E2F1;+ newborn mice displayed significant growth retardation (Fig. 3A). These observations indicate that the severity and penetration of skeletal defects are stronger in Eμ-pp-E2F1 mice than in Actin-Cre/R26E2F1 animals.
FIG. 3.
Phenotypes of E2F1 transgenic mice. (A) Gross physical appearance of 3-week-old Eμ-pp-E2F1 and Actin-Cre/R26E2F1/+ mice. Variability in the dwarf phenotype is demonstrated for the Actin-Cre/R26E2F1;+ mice. The bald spot on the smallest Actin-Cre/R26-E2F1;+ mouse was the result of excessive nurturing by the mother. (B) Northern blot analysis on poly(A)+ mRNA isolated from neck and trunk shows transgenic E2F1 gene expression in newborn Actin-Cre/R26E2F1;+ and Eμ-pp-E2F1 mice. RNA sample of CMV-Cre/R26E2F1;+ ES cells serves as a positive control for the 4.5-kb E2F1-IRES-PLAP transcript.
To assess whether the difference in phenotype reflected E2F1 expression levels, Northern blot analysis was performed on newborn TEF1-5 and Actin-Cre/R26E2F1;+ mice. Using a human E2F1 cDNA probe, we could detect the 4.5-kb E2F1-IRES-PLAP and 1.8-kb E2F1-LTR transcripts, respectively. The expression level of E2F1-IRES-PLAP mRNA directed from the R26 promoter was on average threefold lower than E2F1-LTR transcript levels, indicating that the severity of skeletal phenotype correlated with E2F1 transgenic expression levels (Fig. 3B).
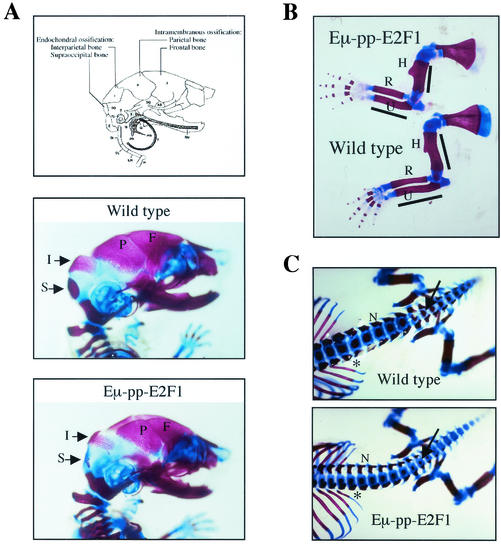
To study bone development in Eμ-pp-E2F1 transgenic animals in more detail, whole-mount Alizarin red and Alcian blue skeletal preparations of E17.5 embryos of the TEF1-5 transgenic line were examined for the presence of primary skeletal abnormalities. Alcian blue stains unmineralized cartilaginous matrices, whereas Alizarin red stains mineralized cartilaginous and bony matrices. In comparison to wild-type littermates, Eμ-pp-E2F1 transgenic skeletons showed defined skeletal changes, involving reduced or absent mineralization of skeletal elements that develop by endochondral bone formation, i.e., interparietal and supraoccipital bones (Fig. 4A). In contrast, frontal and parietal bones that are formed through intramembranous ossification were not affected in TEF1-5 transgenic embryos (Fig. 4A). Similar changes were observed in some of the heterozygous and homozygous Actin-Cre/R26E2F1mice, although to a lesser extent (data not shown). At postnatal day 3, TEF1-5 transgenic mice demonstrated restored mineralization of the interparietal and supraoccipital skull bones, indicating that the primary defect relates to a delayed onset of endochondral ossification.
FIG. 4.
Skeletal analysis on E17.5 Eμ-pp-E2F1 transgenic mice. (A) Schematic representation of skull indicates distinct forms of ossification in interparietal (I) and supraoccipital (S) bones versus parietal (P) and frontal (F) bones. Lateral views of wild-type and Eμ-pp-E2F1 skulls stained with Alizarin red (calcified tissues) and Alcian blue (cartilage) are shown. (B) Lateral view of Eμ-pp-E2F1 and wild-type forelimbs. The humerus (H), radius (R), and ulna (U) are indicated. (C) Dorsoventral view of lumbosacral and tail vertebra showing reduced width of neural arches (N) as well as diminished degrees of ossification in distal vertebral bodies (arrow) and rib shaft regions (asterisk) of E2F1 transgenic mice compared to that of wild-type control mice.
The long bones of the forelimbs and hind limbs were smaller in Eμ-pp-E2F1 embryos and exhibited shorter regions of mineralization (Fig. 4B). The absolute and relative sizes of the mineralized rib segments were smaller in the TEF1-5 transgenic mice at E17.5 (Fig. 4C). This resulted in a reduced rib cage circumference that may underlie the partial perinatal lethality observed in the Eμ-pp-E2F1 mice. The overall shapes of the different skeletal elements in the E2F1 transgenic animals were similar to those of the wild-type controls, with no evidence of increased thickness or bending of the long bones. We found no abnormalities in knees, vertebral bones, or sternum joints, indicating that articular cartilage differentiated normally. Thus, E2F1 overexpression specifically alters the onset of endochondral bone formation, not bone differentiation itself.
Abnormal cartilage differentiation in growth plates of E2F1 transgenic mice.
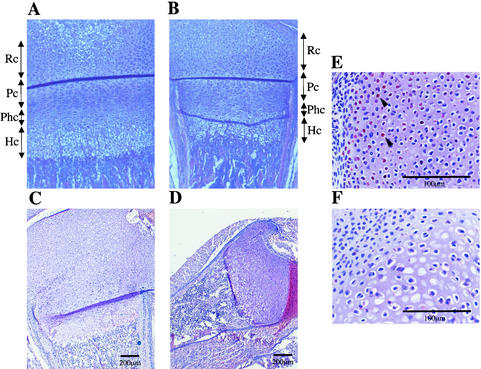
The extent and rate of skeletal growth are determined by the highly ordered progression of chondrocytes residing within the growth plate through the distinct stages of proliferation and differentiation. Since we observed dwarfism and delayed onset of endochondral ossification in E2F1 transgenic mice, we decided to study the composition of the growth plates at the hind limbs (femur and tibia) in more detail at postnatal day 7. The growth plate in early postnatal wild-type mice consists of four major layers of chondrocytes: the resting zone close to the articular surface, followed by the regular columns containing proliferating, prehypertrophic, and hypertrophic layers (Fig. 5A). The most differentiated, hypertrophic, chondrocytes can easily be distinguished from proliferating chondrocytes by their enlarged cytoplasm.
FIG. 5.
Histological analysis of epiphyseal growth plates of E2F1 transgenic mice. (A and B) Sections of the growth plates of the femurs of 7-day-old wild-type mice (A) and Actin-Cre/R26-E2F1;+ mice (B) were stained with hematoxylin and eosin (H&E). Chondrocytes can be divided in four distinct zones: resting (Rc), proliferating (Pc), maturing prehypertrophic (Phc), and hypertrophic (Hc). The growth plates of Actin-Cre/R26-E2F1;+ mice display a relative larger zone of columnar proliferating chondrocytes and a shorter zone of prehypertrophic and hypertrophic chondrocytes. (C and D) H&E-stained sections of epiphyseal growth plates of wild-type (C) and Eμ-pp-E2F1 (D) newborn mice. Note the disorganization of hypertrophic zones within the smaller epiphysis of E2F1 transgenic mice. (E and F) Immunostaining with HA antibody on section of epiphysis of newborn Eμ-pp-E2F1 (E) and control wild-type (F) mice. Arrowheads indicate positively stained nuclei for HA-E2F1.
The organization of the different layers was clearly perturbed in the growth plates of Actin-Cre/R26E2F1;+ transgenic mice (Fig. 5B). First, the relative size of the proliferating zone was increased in the growth plate compared to the wild-type control. Second, the maturing prehypertrophic and hypertrophic layers were significantly reduced in size. The reduced amount of hypertrophic chondrocytes was also evident in the growth plates of the humerus, ulna, and radius (data not shown). Third, the growth plates of Actin-Cre/R26E2F1;+ transgenic mice displayed irregular hypertrophic chondrocyte columns. There was no evidence of increased bone width or positioning of the growth plate at another distance from the articular surface. A similar defect in bone formation was observed in Eμ-pp-E2F1 animals (Fig. 5C and D). Epiphyseal chondrocytes of Eμ-pp-E2F1 mice, but not control wild-type chondrocytes, stained positive for transgene-specific HA-E2F1 protein (Fig. 5E and F), consistent with the notion that E2F1 transgene expression is responsible for the defect in endochondral bone formation. Thus, longitudinal growth in the long bones initiated from the epiphyseal growth plates is disturbed in E2F1 transgenic animals and correlates with disorganized chondrocyte hypertrophy.
Effects of ectopic E2F expression on chondrogenic differentiation of ATDC5 cells.
The delayed onset of endochondral ossification and the disorganization of the growth plates suggest that enforced E2F1 expression primarily perturbs chondrocyte maturation. To investigate whether these biological effects resulted from a cell autonomous E2F1-mediated effect on chondrocyte differentiation, we used the chondrogenic cell line ATDC5, which provides an excellent in vitro model for the process of endochondral ossification (7, 63). In the presence of insulin and confluent culture conditions, ATDC5 cells differentiate from prechondrogenic cells (week 0) into proliferating chondrocytes expressing the aggrecan or α1 (II) collagen gene, which form cartilage nodules in culture (week 1). Thereafter, hypertrophic chondrocytes expressing the α1 (X) collagen gene gradually appear (week 2 and 3), followed by matrix mineralization (week 4).
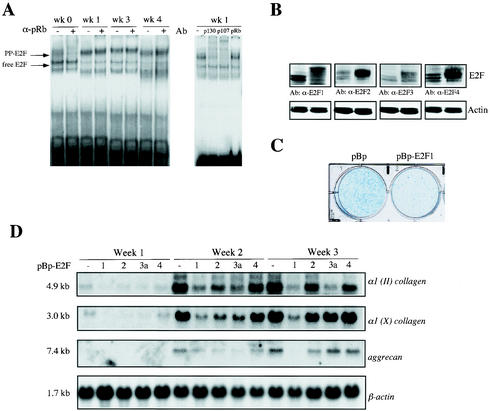
To monitor composition of the different E2F-DNA complexes during the distinct phases of chondrocyte differentiation, electrophoretic mobility shift analysis was performed on ATDC5 cell extracts of proliferating undifferentiated prechondrogenic ATDC5 cells (week 0) and early-phase (week 1) and late-phase (week 3 and 4) differentiating chondrocytes (Fig. 6A). Antibodies directed against murine pRb (21C9), p107 (C-18), and p130 (C-20) were used to analyze the exact composition of the slower-migrating pocket protein (pp)-E2F complexes. In undifferentiated exponentially growing ATDC5 cells, E2F-pRb complexes were the most abundant higher-order E2F species, since pRb antibody interfered with the formation of this E2F-DNA complex, whereas p107 and p130 antibodies showed no significant activity (data not shown). As cells grew to confluency and differentiated, E2F-pRb complexes disappeared and were replaced by an accumulation of E2F-p107 and E2F-p130 complexes.
FIG. 6.
Ectopic expression of E2F transcription factors alters differentiation of chrondogenic ATDC5 cells. (A) Electrophoretic mobility shift analysis on extracts from prechondrogenic cells (week [wk] 0) and early (week 1) and late differentiating chondrocytes (week 3 and 4). The positions of the free E2F-DNA and pocket protein (PP)-E2F-DNA complexes are indicated by the arrows to the left of the gel. Supershift analyses with specific antibodies (Ab) against pRb (α-pRb) (21C9), p107 (C-18), and p130 (C-20) indicate the presence of distinct pocket protein complexes. (B) Western blot analysis shows expression of different E2F species in mock-infected pBabepuro ATDC5 cells (left lane of each blot) or polyclonal populations of ATDC5 cells infected with pBp-HA-E2F1, pBp-HA-E2F2, pBp-HA-E2F3a, or pBp-HA-E2F4 (right lane of each blot). Antibodies (Ab) against E2F1 (α-E2F1), E2F2, E2F3a, and E2F4 were used. (C) Cultures of polyclonal cells infected with pBabepuro (pBp) and pBp-HA-E2F1 were stained with Alcian blue after differentiation for 15 days, which detects differentiated cartilage nodules. (D) Expression of cartilage markers at different phases of chondrocyte differentiation in ATDC5 cells, which stably overexpress pBabepuro (pBp), pBp-HA-E2F1, pBp-HA-E2F2, pBp-HA-E2F3a, and pBp-HA-E2F4 (−, 1, 2, 3a, and 4, respectively). Total RNA was isolated at the indicated times and subjected to Northern blot analysis. Filters were serially hybridized with cDNA probes for type II collagen [α1 (II) collagen]), type X collagen [α1 (X) collagen], and aggrecan genes and β-actin, which serves as a loading control. Transcript sizes are indicated to the left of the blots.
To examine the role of each individual E2F family member in chondrocyte differentiation, we decided to overexpress E2F1, E2F2, E2F3a, or E2F4 in ATDC5 cells by means of retroviral transduction. Western blot analyses indicated that each of the polyclonal infected-cell populations displayed at least a threefold increase in the individual E2F protein levels compared to pBabepuro-mock-infected cells, although E2F2 and E2F4 retroviral expression levels were relatively higher than E2F1 and E2F3a (Fig. 6B). To prevent the problem of clonal variation, we decided to perform all subsequent studies with polyclonal infected pools of stable transduced ATDC5 cells. We first analyzed E2F1-overexpressing ATDC5 cells, which were allowed to differentiate for a period of 2 weeks. Control ATDC5 cells showed the development of many multilayered nodules, which had deposited Alcian blue-reactive cartilage matrix, whereas nodule formation and Alcian blue staining were severely reduced in E2F1-overexpressing chondrogenic cells (Fig. 6C). These results confirm our observations in vivo, indicating that increased E2F1 levels delay the onset of chondrogenic differentiation.
To compare the individual E2F family members in their ability to interfere with proper chondrocyte maturation, all four E2F-overexpressing ATDC5 polyclonal cell populations were cultured for a period of 21 days at confluency in the presence of insulin. After each week, cells were harvested, RNA was isolated, and subjected to Northern blot analysis using the cartilage-specific marker genes, the α1 (II) collagen, aggrecan, and α1 (X) collagen genes. Ectopic expression of E2F1, as well as E2F2 and E2F3a, resulted in a delay of early-phase chondrocyte differentiation, as expression of the α1 (II) collagen and aggrecan genes in week 2 was reduced compared to mock-infected control cells (Fig. 6D). In contrast, overexpression of E2F4 had no significant effect on chondrocyte maturation and expression of differentiation markers. However, at later stages of chondrocyte maturation with progression to terminally differentiated hypertrophic chondrocytes (week 3), only ectopic E2F1 expression resulted in a continued and pronounced inhibition of chondrocyte differentiation, where expression of the α1 (II) collagen and aggrecan genes as well as the hypertrophic marker gene, the α1 (X) collagen gene, was strongly inhibited.
In conclusion, these results show that early-phase differentiation of chondrocytes is partially delayed by enforced expression of E2F1, E2F2, and E2F3a, whereas ectopic E2F4 expression has no effect. However, E2F1 has the unique property among the E2F family members to inhibit both early- and late-phase chondrocyte differentiation, especially the process of chondrocyte hypertrophy.
DISCUSSION
E2F1 shares several features with E2F2 and E2F3a, but it also has unique properties that relate to its capacity to induce apoptosis and its ability to regulate differentiation. Although loss of E2F1 does not appear to inhibit either proliferation or cellular differentiation during embryonic development (26, 83), overexpression has been shown to prevent terminal differentiation in different cell lineages. These include myocytes (78), epidermal keratinocytes (20, 53), granulocytes (70), megakaryocytes (30), macrophages (4), and preadipocytes (55).
In this study, for the first time, we provide evidence for the involvement of E2F1 in controlling chondrogenic differentiation and endochondral bone formation in mice. We have generated and analyzed the phenotype of Eμ-pp-E2F1 mice, which express the E2F1 transgene under control of the pim1 promoter or enhancer sequences (pp) and duplicate versions of the immunoglobulin heavy-chain gene enhancer (Eμ). The combination of these two transcriptional regulatory elements has been shown to produce ubiquitous transgene expression at early stages of embryonic development (2), mainly due to the upstream sequences within the pim1 promoter element, reflecting the expression pattern of the mouse pim1 gene during embryogenesis (22). Furthermore, we employed the binary Cre-loxP system and created an E2F1 knock-in transgene in the ubiquitously expressed R26 locus (68), resulting in a conditional R26-loxP-βgeo-loxP-E2F1 allele. By intercrossing the Actin-Cre transgene, this binary system enabled the induction of ubiquitous E2F1 expression without the possibility of negative selection of deleterious E2F1 transgene levels, which may occur by generating constitutive transgenic mice.
Similar skeletal defects were observed in Eμ-pp-E2F1 and Actin-Cre/R26E2F1 mice, although E2F1 transgenic expression levels were significantly lower in Actin-Cre/R26E2F1 animals, which correlated with a less severe dwarf phenotype in these mice. Accordingly, only Eμ-pp-E2F1 mice displayed significantly increased perinatal lethality, which most likely occurred as a consequence of the abnormal skeletal development associated with E2F1 overexpression. Within each transgenic strain, there was a notable variation in penetration of the phenotype, but growth retardation and reduced limb size could be observed in both Eμ-pp-E2F1 and Actin-Cre/R26E2F1 mice. Detailed skeletal analysis at E17.5 indicated that most bones that develop by endochondral ossification showed delayed mineralization in E2F1 transgenic animals, including the interparietal and supraoccipital bones in the skull. Besides the delayed onset of endochondral bone formation during embryonic stages of development, we also found evidence that overexpression of E2F1 affected the course of terminal chondrocyte differentiation in the growth plates of young adult E2F1 transgenic mice. Zones of proliferation in growth plates of 1-week-old Actin-Cre/R26E2F1 and Eμ-pp-E2F1 mice were increased, whereas zones of prehypertrophy and hypertrophy were severely diminished in size. Furthermore, the normal orderly arranged columns present in the hypertrophic layer were significantly perturbed in E2F1 transgenic animals.
The observed defects in chondrocyte maturation in E2F1 transgenic mice were further corroborated by experiments we performed in the chondrogenic cell line ATDC5, which in vitro follows the multistep chondrocyte differentiation process required for endochondral ossification (7, 63). Our studies indicated that ectopic expression of E2F1, E2F2, and E2F3a inhibited early-phase differentiation as demonstrated by the decreased α1 (II) collagen and aggrecan mRNA levels. At this moment, it is unclear whether this observation is directly linked to the ability of E2F1, E2F2, and E2F3a to delay cell cycle exit and entry into G0 of differentiating chondrocytes. Overexpression of the AP-1 transcription factor c-Fos, a positive regulator of cell proliferation, also inhibits chondrocyte differentiation in ATDC5 cells with markedly reduced α1 (II) collagen and aggrecan gene expression (74). Therefore, the timing of cell cycle exit in ATDC5 cells could be directly linked to early-phase chondrocyte differentiation and expression of these marker genes. Importantly, only E2F1 overexpression prevented late-phase differentiation and chondrocyte hypertrophy, demonstrated by the fact that Alcian blue-reactive cartilage nodules were significantly diminished, and expression of the hypertrophic differentiation marker α1 (X) collagen and the general chondrocyte differentiation marker aggrecan exhibited strong reduction.
These data suggest that E2F1 may directly control the expression of one or more genes that are critical for chondrocyte differentiation, while these transcriptional targets are much less affected by E2F2 or E2F3a overexpression. Indeed, microarray analysis on U2OS cells has revealed the identification of various transcriptional target genes that are more sensitive to E2F1 activation (48), of which several have been implicated in chondrocyte differentiation. These include the transcription factor gene Sox9, which acts as an inhibitor of hypertrophic chondrocyte differentiation (1), and the local growth factor genes TGFβ2 and Bmp2. TGF-β2 is a key intermediate molecule in the inhibitory effect of Hedgehog on hypertrophic differentiation and PTHrP expression (3), while BMP signaling inhibits the onset and pace of hypertrophic differentiation (46, 47). Thus, these genes are potential candidates for mediating the inhibitory function of E2F1 on hypertrophic differentiation, and future studies will address these issues.
Alternatively, E2F1 could indirectly alter the functional activity of key regulator(s) of hypertrophic differentiation. pRb acts as a transcriptional coactivator of Cbfa1 (73), which has been shown to promote hypertrophic chondrocyte differentiation (72, 75). Cbfa1 interacts directly with pRb, and both the C terminus and the B pocket of pRb are required for binding to Cbfa1. Since the B pocket partially overlaps with the E2F-binding site of pRb, it is conceivable that E2F1 overexpression may compete with or alter binding of pRb to Cbfa1. Importantly, the observed defects in chondrocyte differentiation cannot be explained by the given ability of E2F1 to elicit apoptosis, since induction of programmed cell death in hypertrophic chondrocytes is associated with accelerated endochondral bone formation. This is evident in mice deficient for Bcl-2, which show accelerated maturation of chondrocytes and shortening of the long bones (6).
Interestingly, overexpression of E2F4 in ATDC5 cells neither increased nor decreased the chondrocyte differentiation rate. In agreement with these findings, neither Eμ-pp-E2F4 nor Eμ-pp-E2F4/p130−/− transgenic mice showed any defective endochondral bone formation or aberrant growth plate morphology, even with high levels of E2F4 overexpression (B. Scheijen and R. Bernards, unpublished observations). E2F4, like E2F1, E2F2, and E2F3a, contains a transcription activation domain but has no nuclear localization signal (9). However, binding to one of the pocket proteins (pRb, p107, or p130) enables nuclear localization of E2F4. E2F4/p107 and E2F4/p130 bound to histone deacetylases have been shown to act predominantly as repression complexes on E2F-responsive promoters at the G0/G1 phase of the cell cycle (56, 71). However, overexpression of E2F4 in stratified epithelium by a keratin 5 promoter can induce unscheduled cell division (77), and loss of E2F4 expression suppresses inappropriate cell proliferation and tumorigenesis in pRb-deficient cells (42). The absence of the E2F4-induced phenotype in differentiating chondrocytes may be related to a sufficient abundance of p107/p130 protein levels to control E2F4 function without evoking inappropriate gene regulation.
Our findings raise the issue of whether the delayed onset of endochondral ossification and chondrocyte maturation that is observed in p107−/− p130−/− mice (16) is mediated through altered E2F1 function or expression. Recent data indicate that a fraction of the cellular pool of p107 and p130 can directly interact with E2F1 and likely control its function (12, 42). Moreover, transcription of the E2F1 gene is under E2F-dependent negative control in G0 and early G1, coinciding with the presence of p130-E2F4 complexes (37, 66). Fibroblasts deficient for both p107 and p130 display derepression of several E2F target genes, including the E2F1 gene (35). Thus, it is conceivable that the defects in bone development present in p107−/− p130−/− animals result from relief of E2F4- and/or E2F5-mediated transcriptional repression and consequent enhanced E2F1 expression. However, mice deficient for either E2f4 (33, 57) or E2f5 (45) display no defect in endochondral ossification, while mice lacking both E2f4 and E2f5 expression die during later stages of embryonic development with no reported phenotype in bone development (29). Importantly, E2f4−/− E2f5−/− MEFs show no derepression of the E2F target genes B-myb, RRM2, and E2F1, as observed in p107−/− p130−/− MEFs. Furthermore, p107−/− p130−/− cells exit G0 and reenter the cell cycle earlier than control cells, while this is not the case in E2f4−/− E2f5−/− fibroblasts. Therefore, control of chondrogenic differentiation in p107−/− p130−/− chondrocytes could be independent of E2F4- or E2F5-mediated derepression.
Instead, the cellular defects in p107/p130-deficient cells may result from their ability to bind and regulate other transcriptional regulators, as has been reported for pRb, where the dominant-negative antagonist of basic helix-loop-helix transcription factors Id2 represents an essential target in vivo (41). Recently, it has been demonstrated that p107 binds Smad2 or Smad3 together with E2F4 or E2F5, and in response to TGF-β, this complex moves from the cytoplasm into the nucleus, where it acts as a transcriptional repressor complex on a composite Smad-E2F site (14). Smad2 and Smad3 are direct substrates of the TGF-β type I receptor kinase, and their phosphorylation enables association with Smad4 and translocation to the nucleus. Smad proteins can interact with transcriptional coactivators as well as corepressors, thereby directing TGF-β-dependent gene activation and repression responses. TGF-β has a well-established role in chondrocyte maturation and induces the expression of PTHrP, which inhibits the rate of chondrocyte hypertrophic differentiation (54, 62). Thus, p107/p130 may function as scavengers of Smad proteins, and in their absence, transcription regulation by Smad proteins upon TGF-β receptor signaling could be altered.
In conclusion, our data demonstrate that enforced expression of E2F1 during embryogenesis has a profound effect on endochondral ossification and growth plate morphogenesis. In contrast, intramembranous ossification is not altered, suggesting that there is no defect in osteoblast differentiation. Therefore, E2F1 seems to display a unique property among the E2F family members for its ability to inhibit hypertrophic chondrocyte differentiation.
Acknowledgments
We thank Paul Krimpenfort and Anton Berns for kindly providing the R26-en2SA-loxP-βgeo-loxP-PLAP targeting vector, Jos Jonkers for technical advice, Agamemnon Grigoriadis for the ATDC5 cells, Eero Vuorio for chondrocyte-specific marker genes, Sybille Mittnacht for pRb antibody, Wilhelm Krek for pBabepuro-HA-E2F plasmids, and Jurjen Bulthuis, Martin van der Valk, and Kees de Goeij for histopathological analyses.
This work was supported in part by a Biomed-2 grant and a grant from the Dutch Cancer Society (KWF).
REFERENCES
- 1.Akiyama, H., M. C. Chaboissier, J. F. Martin, A. Schedl, and B. De Crombrugghe. 2002. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16:2813-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkema, M. J., N. M. van der Lugt, R. C. Bobeldijk, A. Berns, and M. van Lohuizen. 1995. Transformation of axial skeleton due to overexpression of bmi-1 in transgenic mice. Nature 374:724-727. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez, J., P. Sohn, X. Zeng, T. Doetschman, D. J. Robbins, and R. Serra. 2002. TGFbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development 129:1913-1924. [DOI] [PubMed] [Google Scholar]
- 4.Amanullah, A., B. Hoffman, and D. A. Liebermann. 2000. Deregulated E2F-1 blocks terminal differentiation and loss of leukemogenicity of M1 myeloblastic leukemia cells without abrogating induction of p15(INK4B) and p16(INK4A). Blood 96:475-482. [PubMed] [Google Scholar]
- 5.Amizuka, N., H. Warshawsky, J. E. Henderson, D. Goltzman, and A. C. Karaplis. 1994. Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J. Cell Biol. 126:1611-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amling, M., L. Neff, S. Tanaka, D. Inoue, K. Kuida, E. Weir, W. M. Philbrick, A. E. Broadus, and R. Baron. 1997. Bcl-2 lies downstream of parathyroid hormone-related peptide in a signaling pathway that regulates chondrocyte maturation during skeletal development. J. Cell Biol. 136:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atsumi, T., Y. Miwa, K. Kimata, and Y. Ikawa. 1990. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell Differ. Dev. 30:109-116. [DOI] [PubMed] [Google Scholar]
- 8.Beier, F., Z. Ali, D. Mok, A. C. Taylor, T. Leask, C. Albanese, R. G. Pestell, and P. LuValle. 2001. TGFbeta and PTHrP control chondrocyte proliferation by activating cyclin D1 expression. Mol. Biol. Cell 12:3852-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beijersbergen, R. L., R. M. Kerkhoven, L. Zhu, L. Carlee, P. M. Voorhoeve, and R. Bernards. 1994. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 8:2680-2690. [DOI] [PubMed] [Google Scholar]
- 10.Bell, D. M., K. K. Leung, S. C. Wheatley, L. J. Ng, S. Zhou, K. W. Ling, M. H. Sham, P. Koopman, P. P. Tam, and K. S. Cheah. 1997. SOX9 directly regulates the type-II collagen gene. Nat. Genet. 16:174-178. [DOI] [PubMed] [Google Scholar]
- 11.Bi, W., J. M. Deng, Z. Zhang, R. R. Behringer, and B. de Crombrugghe. 1999. Sox9 is required for cartilage formation. Nat. Genet. 22:85-89. [DOI] [PubMed] [Google Scholar]
- 12.Calbo, J., M. Parreno, E. Sotillo, T. Yong, A. Mazo, J. Garriga, and X. Grana. 2002. G1 cyclin/cyclin-dependent kinase-coordinated phosphorylation of endogenous pocket proteins differentially regulates their interactions with E2F4 and E2F1 and gene expression. J. Biol. Chem. 277:50263-50274. [DOI] [PubMed] [Google Scholar]
- 13.Cancedda, R., P. Castagnola, F. D. Cancedda, B. Dozin, and R. Quarto. 2000. Developmental control of chondrogenesis and osteogenesis. Int. J. Dev. Biol. 44:707-714. [PubMed] [Google Scholar]
- 14.Chen, C. R., Y. Kang, P. M. Siegel, and J. Massague. 2002. E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell 110:19-32. [DOI] [PubMed] [Google Scholar]
- 15.Chung, U. I., E. Schipani, A. P. McMahon, and H. M. Kronenberg. 2001. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J. Clin. Investig. 107:295-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobrinik, D., M. H. Lee, G. Hannon, G. Mulligan, R. T. Bronson, N. Dyson, E. Harlow, D. Beach, R. A. Weinberg, and T. Jacks. 1996. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev. 10:1633-1644. [DOI] [PubMed] [Google Scholar]
- 17.de Crombrugghe, B., V. Lefebvre, and K. Nakashima. 2001. Regulatory mechanisms in the pathways of cartilage and bone formation. Curr. Opin. Cell Biol. 13:721-727. [DOI] [PubMed] [Google Scholar]
- 18.Deng, C., A. Wynshaw-Boris, F. Zhou, A. Kuo, and P. Leder. 1996. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84:911-921. [DOI] [PubMed] [Google Scholar]
- 19.DePrimo, S. E., P. J. Stambrook, and J. R. Stringer. 1996. Human placental alkaline phosphatase as a histochemical marker of gene expression in transgenic mice. Transgenic Res. 5:459-466. [DOI] [PubMed] [Google Scholar]
- 20.Dicker, A. J., C. Popa, A. L. Dahler, M. M. Serewko, P. A. Hilditch-Maguire, I. H. Frazer, and N. A. Saunders. 2000. E2F-1 induces proliferation-specific genes and suppresses squamous differentiation-specific genes in human epidermal keratinocytes. Oncogene 19:2887-2894. [DOI] [PubMed] [Google Scholar]
- 21.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 22.Eichmann, A., L. Yuan, C. Breant, K. Alitalo, and P. J. Koskinen. 2000. Developmental expression of pim kinases suggests functions also outside of the hematopoietic system. Oncogene 19:1215-1224. [DOI] [PubMed] [Google Scholar]
- 23.Erlebacher, A., E. H. Filvaroff, S. E. Gitelman, and R. Derynck. 1995. Toward a molecular understanding of skeletal development. Cell 80:371-378. [DOI] [PubMed] [Google Scholar]
- 24.Fantl, V., G. Stamp, A. Andrews, I. Rosewell, and C. Dickson. 1995. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 9:2364-2372. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson, C. M., E. M. Schwarz, P. R. Reynolds, J. E. Puzas, R. N. Rosier, and R. J. O'Keefe. 2000. Smad2 and 3 mediate transforming growth factor-beta1-induced inhibition of chondrocyte maturation. Endocrinology 141:4728-4735. [DOI] [PubMed] [Google Scholar]
- 26.Field, S. J., F. Y. Tsai, F. Kuo, A. M. Zubiaga, W. G. Kaelin, Jr., D. M. Livingston, S. H. Orkin, and M. E. Greenberg. 1996. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell 85:549-561. [DOI] [PubMed] [Google Scholar]
- 27.Friedrich, G., and P. Soriano. 1991. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5:1513-1523. [DOI] [PubMed] [Google Scholar]
- 28.Garrick, D., S. Fiering, D. I. Martin, and E. Whitelaw. 1998. Repeat-induced gene silencing in mammals. Nat. Genet. 18:56-59. [DOI] [PubMed] [Google Scholar]
- 29.Gaubatz, S., G. J. Lindeman, S. Ishida, L. Jakoi, J. R. Nevins, D. M. Livingston, and R. E. Rempel. 2000. E2F4 and E2F5 play an essential role in pocket protein-mediated G1 control. Mol. Cell 6:729-735. [DOI] [PubMed] [Google Scholar]
- 30.Guy, C. T., W. Zhou, S. Kaufman, and M. O. Robinson. 1996. E2F-1 blocks terminal differentiation and causes proliferation in transgenic megakaryocytes. Mol. Cell. Biol. 16:685-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harbour, J. W., and D. C. Dean. 2000. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14:2393-2409. [DOI] [PubMed] [Google Scholar]
- 32.Henikoff, S. 1998. Conspiracy of silence among repeated transgenes. Bioessays 20:532-535. [DOI] [PubMed] [Google Scholar]
- 33.Humbert, P. O., C. Rogers, S. Ganiatsas, R. L. Landsberg, J. M. Trimarchi, S. Dandapani, C. Brugnara, S. Erdman, M. Schrenzel, R. T. Bronson, and J. A. Lees. 2000. E2F4 is essential for normal erythrocyte maturation and neonatal viability. Mol. Cell 6:281-291. [DOI] [PubMed] [Google Scholar]
- 34.Humbert, P. O., R. Verona, J. M. Trimarchi, C. Rogers, S. Dandapani, and J. A. Lees. 2000. E2f3 is critical for normal cellular proliferation. Genes Dev. 14:690-703. [PMC free article] [PubMed] [Google Scholar]
- 35.Hurford, R. K., Jr., D. Cobrinik, M. H. Lee, and N. Dyson. 1997. pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes Dev. 11:1447-1463. [DOI] [PubMed] [Google Scholar]
- 36.Ionescu, A. M., E. M. Schwarz, C. Vinson, J. E. Puzas, R. Rosier, P. R. Reynolds, and R. J. O'Keefe. 2001. PTHrP modulates chondrocyte differentiation through AP-1 and CREB signaling. J. Biol. Chem. 276:11639-11647. [DOI] [PubMed] [Google Scholar]
- 37.Johnson, D. G. 1995. Regulation of E2F-1 gene expression by p130 (Rb2) and D-type cyclin kinase activity. Oncogene 11:1685-1692. [PubMed] [Google Scholar]
- 38.Karaplis, A. C., A. Luz, J. Glowacki, R. T. Bronson, V. L. Tybulewicz, H. M. Kronenberg, and R. C. Mulligan. 1994. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 8:277-289. [DOI] [PubMed] [Google Scholar]
- 39.Karp, S. J., E. Schipani, B. St. Jacques, J. Hunzelman, H. Kronenberg, and A. P. McMahon. 2000. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development 127:543-548. [DOI] [PubMed] [Google Scholar]
- 40.Laplantine, E., F. Rossi, M. Sahni, C. Basilico, and D. Cobrinik. 2002. FGF signaling targets the pRb-related p107 and p130 proteins to induce chondrocyte growth arrest. J. Cell Biol. 158:741-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lasorella, A., M. Noseda, M. Beyna, Y. Yokota, and A. Iavarone. 2000. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature 407:592-598. [DOI] [PubMed] [Google Scholar]
- 42.Lee, E. Y., H. Cam, U. Ziebold, J. B. Rayman, J. A. Lees, and B. D. Dynlacht. 2002. E2F4 loss suppresses tumorigenesis in Rb mutant mice. Cancer Cell 2:463-472. [DOI] [PubMed] [Google Scholar]
- 43.Lee, M. H., B. O. Williams, G. Mulligan, S. Mukai, R. T. Bronson, N. Dyson, E. Harlow, and T. Jacks. 1996. Targeted disruption of p107: functional overlap between p107 and Rb. Genes Dev. 10:1621-1632. [DOI] [PubMed] [Google Scholar]
- 44.Lefebvre, V., P. Li, and B. de Crombrugghe. 1998. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 17:5718-5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindeman, G. J., L. Dagnino, S. Gaubatz, Y. Xu, R. T. Bronson, H. B. Warren, and D. M. Livingston. 1998. A specific, nonproliferative role for E2F-5 in choroid plexus function revealed by gene targeting. Genes Dev. 12:1092-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minina, E., C. Kreschel, M. Naski, D. Ornitz, and A. Vortkamp. 2002. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev. Cell 3:439-449. [DOI] [PubMed] [Google Scholar]
- 47.Minina, E., H. M. Wenzel, C. Kreschel, S. Karp, W. Gaffield, A. P. McMahon, and A. Vortkamp. 2001. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 128:4523-4534. [DOI] [PubMed] [Google Scholar]
- 48.Muller, H., A. P. Bracken, R. Vernell, M. C. Moroni, F. Christians, E. Grassilli, E. Prosperini, E. Vigo, J. D. Oliner, and K. Helin. 2001. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 15:267-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murga, M., O. Fernandez-Capetillo, S. J. Field, B. Moreno, L. R. Borlado, Y. Fujiwara, D. Balomenos, A. Vicario, A. C. Carrera, S. H. Orkin, M. E. Greenberg, and A. M. Zubiaga. 2001. Mutation of E2F2 in mice causes enhanced T lymphocyte proliferation, leading to the development of autoimmunity. Immunity 15:959-970. [DOI] [PubMed] [Google Scholar]
- 50.Naski, M. C., Q. Wang, J. Xu, and D. M. Ornitz. 1996. Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nat. Genet. 13:233-237. [DOI] [PubMed] [Google Scholar]
- 51.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 52.Olsen, B. R., A. M. Reginato, and W. Wang. 2000. Bone development. Annu. Rev. Cell Dev. Biol. 16:191-220. [DOI] [PubMed] [Google Scholar]
- 53.Paramio, J. M., C. Segrelles, M. L. Casanova, and J. L. Jorcano. 2000. Opposite functions for E2F1 and E2F4 in human epidermal keratinocyte differentiation. J. Biol. Chem. 275:41219-41226. [DOI] [PubMed] [Google Scholar]
- 54.Pateder, D. B., R. N. Rosier, E. M. Schwarz, P. R. Reynolds, J. E. Puzas, M. D'Souza, and R. J. O'Keefe. 2000. PTHrP expression in chondrocytes, regulation by TGF-beta, and interactions between epiphyseal and growth plate chondrocytes. Exp. Cell Res. 256:555-562. [DOI] [PubMed] [Google Scholar]
- 55.Porse, B. T., T. A. Pedersen, X. Xu, B. Lindberg, U. M. Wewer, L. Friis-Hansen, and C. Nerlov. 2001. E2F repression by C/EBPalpha is required for adipogenesis and granulopoiesis in vivo. Cell 107:247-258. [DOI] [PubMed] [Google Scholar]
- 56.Rayman, J. B., Y. Takahashi, V. B. Indjeian, J. H. Dannenberg, S. Catchpole, R. J. Watson, H. te Riele, and B. D. Dynlacht. 2002. E2F mediates cell cycle-dependent transcriptional repression in vivo by recruitment of an HDAC1/mSin3B corepressor complex. Genes Dev. 16:933-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rempel, R. E., M. T. Saenz-Robles, R. Storms, S. Morham, S. Ishida, A. Engel, L. Jakoi, M. F. Melhem, J. M. Pipas, C. Smith, and J. R. Nevins. 2000. Loss of E2F4 activity leads to abnormal development of multiple cellular lineages. Mol. Cell 6:293-306. [DOI] [PubMed] [Google Scholar]
- 58.Rossi, F., H. E. MacLean, W. Yuan, R. O. Francis, E. Semenova, C. S. Lin, H. M. Kronenberg, and D. Cobrinik. 2002. p107 and p130 coordinately regulate proliferation, Cbfa1 expression, and hypertrophic differentiation during endochondral bone development. Dev. Biol. 247:271-285. [DOI] [PubMed] [Google Scholar]
- 59.Sauer, B., and N. Henderson. 1988. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. USA 85:5166-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheijen, B., J. Jonkers, D. Acton, and A. Berns. 1997. Characterization of pal-1, a common proviral insertion site in murine leukemia virus-induced lymphomas of c-myc and Pim-1 transgenic mice. J. Virol. 71:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sekiya, I., K. Tsuji, P. Koopman, H. Watanabe, Y. Yamada, K. Shinomiya, A. Nifuji, and M. Noda. 2000. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J. Biol. Chem. 275:10738-10744. [DOI] [PubMed] [Google Scholar]
- 62.Serra, R., A. Karaplis, and P. Sohn. 1999. Parathyroid hormone-related peptide (PTHrP)-dependent and -independent effects of transforming growth factor beta (TGF-beta) on endochondral bone formation. J. Cell Biol. 145:783-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shukunami, C., K. Ishizeki, T. Atsumi, Y. Ohta, F. Suzuki, and Y. Hiraki. 1997. Cellular hypertrophy and calcification of embryonal carcinoma-derived chondrogenic cell line ATDC5 in vitro. J. Bone Miner. Res. 12:1174-1188. [DOI] [PubMed] [Google Scholar]
- 64.Sicinski, P., J. L. Donaher, S. B. Parker, T. Li, A. Fazeli, H. Gardner, S. Z. Haslam, R. T. Bronson, S. J. Elledge, and R. A. Weinberg. 1995. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82:621-630. [DOI] [PubMed] [Google Scholar]
- 65.Skarnes, W. C., J. E. Moss, S. M. Hurtley, and R. S. Beddington. 1995. Capturing genes encoding membrane and secreted proteins important for mouse development. Proc. Natl. Acad. Sci. USA 92:6592-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith, E. J., G. Leone, J. DeGregori, L. Jakoi, and J. R. Nevins. 1996. The accumulation of an E2F-p130 transcriptional repressor distinguishes a G0 cell state from a G1 cell state. Mol. Cell. Biol. 16:6965-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smits, P., P. Li, J. Mandel, Z. Zhang, J. M. Deng, R. R. Behringer, B. de Croumbrugghe, and V. Lefebvre. 2001. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev. Cell 1:277-290. [DOI] [PubMed] [Google Scholar]
- 68.Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21:70-71. [DOI] [PubMed] [Google Scholar]
- 69.St. Jacques, B., M. Hammerschmidt, and A. P. McMahon. 1999. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 13:2072-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strom, D. K., J. L. Cleveland, S. Chellappan, J. Nip, and S. W. Hiebert. 1998. E2F-1 and E2F-3 are functionally distinct in their ability to promote myeloid cell cycle progression and block granulocyte differentiation. Cell Growth Differ. 9:59-69. [PubMed] [Google Scholar]
- 71.Takahashi, Y., J. B. Rayman, and B. D. Dynlacht. 2000. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 14:804-816. [PMC free article] [PubMed] [Google Scholar]
- 72.Takeda, S., J. P. Bonnamy, M. J. Owen, P. Ducy, and G. Karsenty. 2001. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev. 15:467-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomas, D. M., S. A. Carty, D. M. Piscopo, J. S. Lee, W. F. Wang, W. C. Forrester, and P. W. Hinds. 2001. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol. Cell 8:303-316. [DOI] [PubMed] [Google Scholar]
- 74.Thomas, D. P., A. Sunters, A. Gentry, and A. E. Grigoriadis. 2000. Inhibition of chondrocyte differentiation in vitro by constitutive and inducible overexpression of the c-fos proto-oncogene. J. Cell Sci. 113:439-450. [DOI] [PubMed] [Google Scholar]
- 75.Ueta, C., M. Iwamoto, N. Kanatani, C. Yoshida, Y. Liu, M. Enomoto-Iwamoto, T. Ohmori, H. Enomoto, K. Nakata, K. Takada, K. Kurisu, and T. Komori. 2001. Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J. Cell Biol. 153:87-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vooijs, M., J. Jonkers, and A. Berns. 2001. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep. 2:292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang, D., J. L. Russell, and D. G. Johnson. 2000. E2F4 and E2F1 have similar proliferative properties but different apoptotic and oncogenic properties in vivo. Mol. Cell. Biol. 20:3417-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang, J., K. Helin, P. Jin, and B. Nadal-Ginard. 1995. Inhibition of in vitro myogenic differentiation by cellular transcription factor E2F1. Cell Growth Differ. 6:1299-1306. [PubMed] [Google Scholar]
- 79.Weir, E. C., W. M. Philbrick, M. Amling, L. A. Neff, R. Baron, and A. E. Broadus. 1996. Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc. Natl. Acad. Sci. USA 93:10240-10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White, A., and G. Wallis. 2001. Endochondral ossification: a delicate balance between growth and mineralisation. Curr. Biol. 11:R589-R591. [DOI] [PubMed]
- 81.Wu, L., C. Timmers, B. Maiti, H. I. Saavedra, L. Sang, G. T. Chong, F. Nuckolls, P. Giangrande, F. A. Wright, S. J. Field, M. E. Greenberg, S. Orkin, J. R. Nevins, M. L. Robinson, and G. Leone. 2001. The E2F1-3 transcription factors are essential for cellular proliferation. Nature 414:457-462. [DOI] [PubMed] [Google Scholar]
- 82.Xu, G., D. M. Livingston, and W. Krek. 1995. Multiple members of the E2F transcription factor family are the products of oncogenes. Proc. Natl. Acad. Sci. USA 92:1357-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamasaki, L., T. Jacks, R. Bronson, E. Goillot, E. Harlow, and N. J. Dyson. 1996. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell 85:537-548. [DOI] [PubMed] [Google Scholar]
- 84.Yan, Y., J. Frisen, M. H. Lee, J. Massague, and M. Barbacid. 1997. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 11:973-983. [DOI] [PubMed] [Google Scholar]
- 85.Yang, X., L. Chen, X. Xu, C. Li, C. Huang, and C. X. Deng. 2001. Tgf-beta/smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J. Cell Biol. 153:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zambrowicz, B. P., A. Imamoto, S. Fiering, L. A. Herzenberg, W. G. Kerr, and P. Soriano. 1997. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl. Acad. Sci. USA 94:3789-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang, P., N. J. Liegeois, C. Wong, M. Finegold, H. Hou, J. C. Thompson, A. Silverman, J. W. Harper, R. A. DePinho, and S. J. Elledge. 1997. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature 387:151-158. [DOI] [PubMed] [Google Scholar]
- 88.Zhu, J. W., S. J. Field, L. Gore, M. Thompson, H. Yang, Y. Fujiwara, R. D. Cardiff, M. Greenberg, S. H. Orkin, and J. DeGregori. 2001. E2F1 and E2F2 determine thresholds for antigen-induced T-cell proliferation and suppress tumorigenesis. Mol. Cell. Biol. 21:8547-8564. [DOI] [PMC free article] [PubMed] [Google Scholar]