Abstract
We recently demonstrated that the product of the HERV-W env gene, a retroviral envelope protein also dubbed syncytin, is a highly fusogenic membrane glycoprotein inducing the formation of syncytia on interaction with the type D mammalian retrovirus receptor. In addition, the detection of HERV-W Env protein (Env-W) expression in placental tissue sections led us to propose a role for this fusogenic glycoprotein in placenta formation. To evaluate this hypothesis, we analyzed the involvement of Env-W in the differentiation of primary cultures of human villous cytotrophoblasts that spontaneously differentiate by cell fusion into syncytiotrophoblasts in vitro. First, we observed that HERV-W env mRNA and glycoprotein expression are colinear with primary cytotrophoblast differentiation and with expression of human chorionic gonadotropin (hCG), a marker of syncytiotrophoblast formation. Second, we observed that in vitro stimulation of trophoblast cell fusion and differentiation by cyclic AMP is also associated with a concomitant increase in HERV-W env and hCG mRNA and protein expression. Finally, by using specific antisense oligonucleotides, we demonstrated that inhibition of Env-W protein expression leads to a decrease of trophoblast fusion and differentiation, with the secretion of hCG in culture medium of antisense oligonucleotide-treated cells being decreased by fivefold. Taken together, these results strongly support a direct role for Env-W in human trophoblast cell fusion and differentiation.
In humans, fetal cytotrophoblasts play a key role both in the embryo implantation process and in placental development. In early pregnancy, mononuclear cytotrophoblasts proliferate and invade the maternal endometrium to form the anchoring villi (4, 14). Cytotrophoblasts also fuse and differentiate into a continuous layer of multinucleated syncytiotrophoblast. This cell layer, which covers the chorionic villi, is bathed by maternal blood in the intervillous spaces from early gestation (31, 34). The syncytiotrophoblast layer plays a major role throughout pregnancy, since it is the site of numerous placental functions including ion and nutrient exchange and the synthesis of steroid and peptide hormones required for fetal growth and development (13, 33). Some of these hormones, such as human chorionic gonadotropin (hCG) and human placental lactogen, are specific to pregnancy and can be used as markers of syncytium formation (20, 22).
It has been established both in vivo and in vitro that the syncytiotrophoblast layer arises from the differentiation and fusion of mononuclear cytotrophoblasts. Isolated mononuclear cytotrophoblasts aggregate and fuse to form a nonproliferative multinucleated syncytiotrophoblast which synthesizes and secretes specific hormones required for fetal development (2, 25). This cytotrophoblast differentiation is stimulated in vitro by a number of factors such as growth factors (epidermal growth factor, granulocyte-macrophage colony-stimulating factor) and hormones (hCG, estradiol, and dexamethasone). We recently demonstrated the direct involvement of cyclic AMP (cAMP)-dependent protein kinase (24) and oxidative stress related to the overexpression of copper zinc superoxide dismutase (15, 16) in the modulation of trophoblast cell fusion and differentiation.
Evidence has recently accumulated suggesting that endogenous retroviral gene expression may be involved in mediating the cell fusion observed in the placenta. Indeed, high expression of retroviruses is one of the characteristics of the human syncytiotrophoblast (21, 23). The observation of retroviral particles in the placenta, along with the presence of fused placental cells morphologically reminiscent of virally induced syncytia, led to the proposal that an ancient retroviral infection may have been a pivotal event in mammalian evolution (21). In connection with this, we previously characterized the HERV-W family by screening a placental cDNA library (5). Surprisingly, this multicopy family contains a unique locus, termed ERVWE1, coding for a full-length envelope (43). The presence of inactivating mutations in the other viral genes borne by the ERVWE1 provirus led us to propose that Env function has been selectively preserved (5). We recently demonstrated that the product of the HERV-W env gene (Env-W) is a highly fusogenic membrane glycoprotein that induces the formation of syncytia on interaction with the type D mammalian retrovirus receptor (7).
This property and the detection of Env-W in placental tissue sections (6, 7) led us to further investigate the role of HERV-W Env in placental development. Such a role was indeed supported by the observation that a rabbit polyclonal antibody raised against a mixture of Env-W peptides was able to partially inhibit heterologous fusion between the choriocarcinoma BeWo cell line and COS reporter cells (30). Nevertheless, the relevance of the BeWo choriocarcinoma model of human placental trophoblasts may be questioned. Reports suggesting a role for the ERV-3 envelope protein (produced by the single-copy human endogenous retrovirus ERV-3) in proliferation and differentiation of BeWo cells (27, 28) were weakened by the observation of the physiological knockout of the ERV-3 envelope (lacking both the fusion peptide and the immunosuppressive domain) in 1% of the Caucasian population (11). This strongly suggests that no essential function in placentation is associated with the expression of the ERV-3 envelope protein (37) as was previously suggested (40). In this study, we have analyzed the involvement of Env-W in the differentiation of primary cultures of human villous cytotrophoblasts that spontaneously differentiate in vitro by cell fusion into syncytiotrophoblasts. We found that expression of HERV-W env mRNA and glycoprotein is colinear with primary cytotrophoblast differentiation and that a factor regulating the primary trophoblast differentiation also regulates HERV-W env mRNA and protein expression. Moreover, by using specific antisense oligonucleotides, we demonstrated that inhibition of Env-W protein expression leads to a decrease of trophoblast fusion and differentiation.
MATERIALS AND METHODS
Immunohistochemistry.
Normal human adult placental tissue sections were deparaffinized using standard procedures. Briefly, paraffin sections were immersed three times (5 min each) in toluene baths and three times (5 min each) in 100, 95, and 70% ethanol and then hydrated in distilled water before being subjected to tissue treatment. Antigens were retrieved by treatment in a microwave oven after immersion in MS unmasker buffer (Microm, Francheville, France) for three cycles (5 min each) at a setting of 800 W. The slides were then cooled to room temperature and stained with anti-Env HERV-W 6A2B2 monoclonal antibody (diluted 1/100) and/or anti-desmoplakin antibody (Progen) by a streptavidin-biotin staining procedure, using the ChemMate kit (DAKO).
Cell cultures.
In vitro studies were performed on cytotrophoblast cells isolated from term placentas, which represent an easily accessible and relevant source of material since term trophoblasts conserve their capacity to differentiate in vitro (2, 25). Term placentas were obtained after elective cesarean section from healthy mothers near term following uncomplicated pregnancies. Villous tissue was dissected free of membranes, rinsed, and minced in Ca2+-, Mg2+-free Hanks' balanced salt solution. Cytotrophoblast cells were isolated after trypsin-DNase digestion and discontinuous Percoll gradient fractionation, using a slight modification of the method of Kliman et al. and Alsat et al. (2, 25) as previously described (15). Briefly, the villous sample was subjected to sequential enzymatic digestions in a solution which contains 0.5% (wt/vol) trypsin powder (Difco), 5 IU of DNase I per ml, 25 mM HEPES, 4.2 mM MgSO4, and 1% (wt/vol) penicillin-streptomycin (Biochemical Industry) in Hanks' balanced salt solution and monitored under light microscopy. The first and/or second digestion was discarded after light microscopy analysis to eliminate syncytiotrophoblast fragments, and the following four or five sequential digestions were kept. The cells collected during these last digestions were purified on a discontinuous Percoll gradient (5 to 70% in 5% steps). The cells which had migrated in the middle layer (density, 1.048 to 1.062 g/ml) were plated onto culture dishes (106 cells/cm2) and 3 h later were carefully washed three times with culture medium. Following this procedure, we determined that at 3 h of culture, 95% of the cells isolated from term placentas are cytotrophoblasts as determined by a cytokeratin 7-positive staining, using a specific monoclonal antibody (dilution, 1:200 [DAKO]). The cells were plated in triplicate either on glass slides for immunocytochemistry studies or onto 60-mm culture dishes (106 cells/cm2). They were cultured in 3 ml of Dulbecco's modified Eagle's medium supplemented with 25 mM HEPES, 2 mM glutamine, 20% heat-inactivated fetal calf serum, and antibiotics (100 IU of penicillin per ml and 100 mg of streptomycin per ml). The dishes were maintained at 37°C in humidified 5% CO2.
Cell staining.
To detect desmoplakin, cultured cells were rinsed with phosphate-buffered saline (PBS), fixed, and permeabilized in methanol at −20°C for 25 min. A monoclonal anti-desmoplakin antibody (1/400; Sigma) was then applied, followed by fluorescein isothiocyanate-labeled goat anti-mouse immunoglobulin (Sigma), as previously described (3, 17).
Syncytium formation assay.
Syncytium formation was monitored by observing the distribution of desmoplakin and nuclei in cells after fixation and immunostaining as previously described (24). The staining of desmoplakin present at the intercellular boundaries in aggregated cells progressively disappears with syncytium formation (3, 12). Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI)-containing mounting medium (Vectashield). From a random point near the middle of the coverslips, the nuclei contained in 100 desmoplakin-delimited syncytia were counted. Three coverslips were examined for each experimental condition. The results are expressed as a function of nuclei number per syncytium.
Hormone assay.
The hCG concentration was determined in culture media by using the chemiluminescent immunoassay analyzer ACS-180SE system (Bayer Diagnostics). The assay sensitivity was 2 mU/ml. All values are given as means ± standard error of the mean (SEM) of triplicate determinations.
RNA isolation and analysis.
Total RNA was extracted from cultured cells by the procedure of Qiagen (Courtabeuf, France). The total RNA concentration was determined at 260 nm, and its integrity was monitored by agarose gel electrophoresis (1% agarose). Relative mRNA levels of the different genes of interest were measured by a quantitative reverse transcription PCR assay, essentially as previously described (15), using the ABI Prism 7700 sequence detection system (Perkin-Elmer Applied Biosystems) and the SYBR Green PCR core reagents kit (Perkin-Elmer Applied Biosystems). The nucleotide sequences of the primers are listed in Table 1. Each sample was analyzed in duplicate, and a calibration curve was run in parallel for each analysis. The level of transcripts was normalized using the RPLP0 gene (also known as 36B4), encoding human acidic ribosomal phosphoprotein P0, as an endogenous RNA control, and each sample was normalized on the basis of its RPLP0 content.
TABLE 1.
RT-PCR primers and antisense oligonucleotides used in this study
| Target mRNA | Oligonucleotide(s) |
|---|---|
| Env-Wa | (+) CGGACATCCAAAGTGATACATCCT, (−) TGATGTATCCAAGACTCCACTCCA |
| RPLP0a | (+) GGCGACCTGGAAGTCCAACT, (−) CCATCAGCACCACAGCCTTC |
| hCGβa | (+) GCTACTGCCCCACCATGACC, (−) ATGGACTCGAAGCGCACATC |
| Env-W 744-766b | GCCATGGGGATTTATGATTTTAG |
| Env-W 750-770b | GAGGGCCATGGGGATTTATGA |
| Env-W 758-777b | GATAAGGGAGGGCCATGGGG |
| Scrambledb | GNNNNNNNNNNNNNNNNNNG |
Upper (+) and lower (−) primers used in RT-PCR assays.
Phosphorothio (underlined) antisense oligonucleotides used to modulate gene expression. The Env CAT initiation anticodon is indicated in boldface; N, fully degenerated base.
Immunoblot analysis.
Cells were lysed in an ice-cold PBS containing 0.5% Triton X-100 supplemented with protease inhibitors. Incubation of the lysates at 4°C for 10 min was followed by centrifugation at 10,000 × g for 10 min to pellet the nuclei. The protein concentration was determined by the method of Bradford (Bio-Rad) using bovine serum albumin as the standard. Supernatants were then frozen at −70°C until required for further analysis. Immunoblotting was performed in accordance with standard procedures. A 30-μg portion of cell lysate was mixed 3:1 (vol/vol) in a 100 mM Tris HCl (pH 6.8) buffer containing 1% sodium dodecyl sulfate, 10% glycerol, and 5% β mercaptoethanol, heated at 95°C for 15 min, and then loaded onto sodium dodecyl sulfate-10% acrylamide gels. After transfer, the nitrocellulose membrane was saturated overnight at 4°C in Tris-buffered saline plus 5% milk powder and 0.05% Tween 20. Immunostaining was performed in the same buffer with 1% milk powder. The blots were respectively probed with the following antibodies: a rabbit polyclonal anti-SU antibody (rp69) at 1/2,000 dilution or a mouse monoclonal anti-actin antibody (Immunotech, Marseilles, France) at 1/200 dilution. Rabbit anti-SU was raised against two keyhole limpet hemocyanin-conjugated peptides (unpublished data). Finally, blots were developed by using horseradish peroxidase-conjugated antibodies (Jackson) and an enhanced chemiluminescence kit (Amersham Pharmacia).
Heterologous cell-cell fusion assay.
Effector cells used in the cell-cell fusion assay corresponded to XC cells, which are rat sarcoma cells (ATCC CCL-165). XC-RDR cells were derived from XC cells by transfection of the pcD3.1VHR16/4 expression plasmid (38) carrying a human cDNA encoding the RDR type D mammalian retrovirus receptor and the neomycin-selectable marker. Stable transfectants were recovered after G418 selection, and one clone was selected. XC and XC-RDR cell lines were then transfected with an expression plasmid carrying a nuclear localization signal-lacZ gene (nls-lacZ) encoding a nuclear galactosidase and the hygromycin-selectable marker. Stable transfectants were recovered after hygromycin B selection and pooled. Primary cytotrophoblasts were plated as described above. After overnight culture (3 × 106 cells/dish), indicator XC or XC-RDR+ cells (1 × 106/dish) were overlaid. The cells were cocultured for 72 h and then fixed and stained with anti-desmoplakin and anti-galactosidase (1/100) monoclonal antibodies as described above. Cell nuclei were counterstained with DAPI-containing mounting medium (Vectashield).
Modulation of gene expression by antisense oligonucleotides.
The nucleotide sequences of the phosphorothio-oligonucleotides purchased from Eurogentec (Belgium) are listed in Table 1. Synthetic antisense oligonucleotides targetting the HERV-W Env translation start site were designed. Analysis of the ATG region in the context of the whole 2.7-kb env mRNA previously identified (5) was performed with mfold 3.1 (29). Analyses of duplex and hairpin formations and of the absence of cross-reactivities with related sequences were performed using oligo 6.2 (Molecular Biology Insights, Inc.). Considering the described sequence-independent non-antisense effect of phosphorothio-oligonucleotides (18), we designed a scrambled 20-mer oligonucleotide containing 18 fully degenerated bases. Normal cytotrophoblastic cells in culture were incubated for 48 h either with 10 μM HERV-W Env antisense oligonucleotide or with 10 μM scrambled antisense oligonucleotide used as control.
Statistical tests.
Statistical analysis was performed using the StatView F-4.5 software package (Abacus Concepts, Inc.). Values are presented as mean ± SEM. Differences in the level of detection of hormonal secretions and Env-W and hCGβ mRNA and in the mononuclear versus fused phenotype distribution (percentage of of mononuclear cells, fusion indices) were analyzed using the Mann-Whitney U test for the treated cells (8-bromo-cAMP- or env-specific antisense oligonucleotide) versus the control cells (untreated or scrambled antisense oligonucleotide); P < 0.05 was considered significant.
RESULTS
In vivo expression of Env-W.
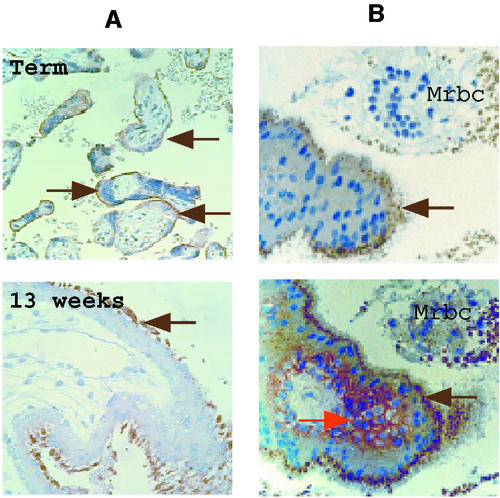
As illustrated in Fig. 1A, Env-W glycoprotein is expressed at the apical membrane of the syncytiotrophoblast layer of term and first-trimester placentas. In vitro, the differentiation-fusion of isolated human cytotrophoblast cells is currently monitored by staining cells with anti-desmoplakin antibodies to reveal cell boundaries (3, 12). In vivo, from the second trimester of pregnancy, the density of villous cytototrophoblasts decreases. Conversely, the staining of desmoplakin present at the intercellular boundaries of aggregated cytotrophoblasts was readily detectable on the plasmatic membranes of the cytotrophoblasts from a first-trimester placenta (Fig. 1B). The fused tissue can be evidenced by the absence of a desmoplakin signal on the syncytiotrophoblast (Fig. 1B).
FIG. 1.
Expression of the HERV-W Env glycoprotein at the apical syncytiotrophoblast microvillus membrane. (A) Detection of the Env glycoprotein with the 6A2B2 monoclonal antibody on the external face of the syncytiotrophoblast (brown arrows) on term and 13-week normal placenta sections. (B) Double labeling on a serial section of a 10-week-gestation normal placenta with anti-Env (upper and lower image, brown arrows) and antidesmoplakin (lower image, red arrow) antibodies. Desmoplakin, a protein of the desmosomiale plaque involved in intercellular junctions, is absent from the syncytiotrophoblast fused tissue and lines the plasmatic membranes of the cytotrophoblasts. Note the presence of maternal red blood cells (Mrbc) in the intervillous space.
In vitro cytotrophoblast differentiation and Env-W expression.
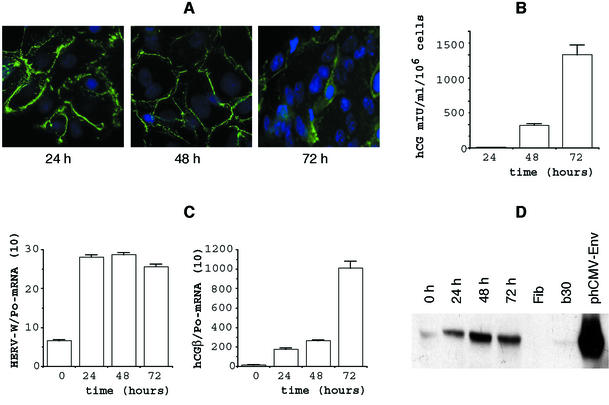
As illustrated in Fig. 2A and previously shown (2, 25), purified mononuclear cytotrophoblasts isolated from normal human term placenta aggregate, fuse, and form a large multinucleated syncytiotrophoblast. The staining of desmoplakin present at the intercellular boundaries in aggregated cells progressively disappears with syncytiotrophoblast formation (3, 12). At 72 h of culture, the mononuclear cytotrophoblasts are mostly differentiated into syncytiotrophoblasts. This is indicated by a gathering of numerous multinucleated cells in a large cytoplasmic mass, as revealed by cell staining. This in vitro syncytiotrophoblast formation is associated with a significant increase in hCG levels (Fig. 2B) in conditioned media of these cultured cells (15, 25).
FIG. 2.
Expression of HERV-W env mRNA and glycoprotein is colinear with primary cytotrophoblast differentiation. (A) Desmoplakin immunodetection after 24, 48, and 72 h of culture of trophoblast cells isolated from a term placenta. Positive immunofluorescent staining is observed only in aggregated cytotrophoblasts (24 h) or in cytotrophoblasts in contact with the syncytiotrophoblast (48 h); staining has disappeared in the fused syncytiotrophoblast characterized by multiple nuclei (72 h). Nuclei were labeled with DAPI (blue fluorescence). (B) Levels of hCG (expressed in milli-international units per milliliter per 106 cellules) secreted into the culture medium at the indicated times and characterizing the observed differentiation. Since cells were plated in triplicate (see Materials and Methods), hCG levels were determined for each plate. Results are expressed as the mean and SEM of the three culture dishes. (C) Env-W and hCG-mRNA expression in cytotrophoblasts (CT0) and during their in vitro differentiation for 3 days. mRNA data are expressed as the level of each marker mRNA normalized by RPLPo mRNA levels (Po). Two culture dishes were pooled for each determination, and transcripts were assayed in duplicate. (D) Western blot analysis of Env glycoprotein detected by the anti-SU polyclonal antibody during trophoblast differentiation (0 to 72 h). Fibroblast protein extract was used as a negative control (Fib), and a BeWo cell line (B30) and env-transfected TE 671 cells (phCMV-Env) were used as positive controls. The whole figure (A through D) illustrates an experiment which is representative of four.
A Northern blot qualitative analysis of HERV-W mRNA expression, using an env probe, showed an 8-kb transcript and an abundant 3.1-kb transcript which exhibited the characteristic features of a singly spliced subgenomic retroviral env mRNA derived from the ERVWE1 locus, as previously observed in total placenta extract (data not shown). To quantitate specific env mRNA by real-time reverse transcription-PCR, a set of primers was further designed, one of which targets the splice junction. As shown in Fig. 2C, an mRNA quantitative analysis showed that induction of HERV-W mRNA is a rapid phenomenon compared with hCG mRNA synthesis. The HERV-W mRNA signal reached a plateau as early as 24 h of culture. Conversely, the hCG mRNA induction curve showed an exponential shape from 0 to 72 h. In addition, the range of induction between the two genes is dramatically different, since an HERV-W mRNA increase of about 4 was observed at 24 h of culture, compared to increases of 20 and 100 for the hCG mRNA at 24 and 72 h, respectively. Globally, Env-W glycoprotein (Fig. 2D) and hCG hormone synthesis exhibited kinetics similar to their mRNA counterparts. Although these features were observed for all tested cultures (n = 4), the levels of the transcripts for Env-W and hCGβ varied greatly from one term trophoblast primary culture to another: Env-W/Po mRNA ranged from 1.1 to 6.4 at 0 h of culture and from 4 to 26.1 at 72 h of culture and hCGβ/Po mRNA ranged from 1 to 10.1 at 0 h of culture and 55.7 to 1,150 at 72 h of culture. Similarly, hCG secretion varied from one culture to another (n = 14; range, 24 to 2,100 mIU/ml/106 cells). The constant increase in Env-W mRNA after 72 h of culture was also found to occur during the in vitro fusion and differentiation of cytotrophoblasts isolated from early placentas. Thus, the syncytiotrophoblast formation obtained with purified mononuclear cytotrophoblasts isolated from such early placentas (39) was associated with a 3.9 ± 2.9 (n = 7 different cultures) increase in Env-W mRNA after 72 h of culture, similar to the 4.5 ± 1.8 (n = 4 different cultures) increase observed using term cytotrophoblasts (P = 0.2568, Mann-Whitney U test).
In vitro modulation of cytotrophoblast differentiation and Env-W expression.
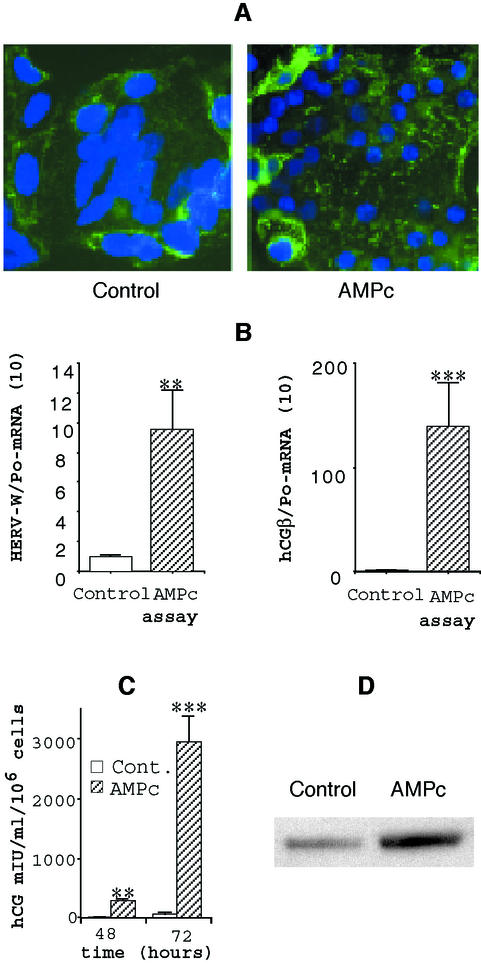
We previously showed that cAMP-dependent protein kinase plays a role in human trophoblast cell fusion and differentiation (24). As illustrated in Fig. 3A, treatment of trophoblast cells with 0.1 mM 8-bromo-cAMP led to an increase in the number and size of syncytia by fusion between cytotrophoblasts or between newly formed syncytia. This modulation of cell fusion and differentiation was associated with changes in hCG secretion (Fig. 3B) and transcript levels (Fig. 3C), used as markers of cell differentiation; hCG secretion and expression were significantly increased in 8-bromo-cAMP-treated cells compared to controls. Interestingly, we observed that Env-W transcript levels (Fig. 3C) or protein expression as detected by Western blot analysis (Fig. 3D) was similarly clearly increased in 8-bromo-cAMP-treated cells. Therefore, in vitro stimulation of trophoblast cell fusion and differentiation by cAMP was associated with a concomitant increase in Env-W expression.
FIG. 3.
A factor regulating primary trophoblast differentiation also regulates HERV-W env mRNA and protein expression. (A) After 24 h of culture, the primary cytotrophoblasts were incubated for 48 h in the presence of 0.1 mM 8-bromo-cAMP. At 72 h, the cells were fixed and immunostained with anti-desmoplakin monoclonal antibody (green fluorescence). Nuclei were labeled with DAPI (blue fluorescence). Large syncytia were observed in the control and with the cAMP treatment. (B) HERV-W and hCGβ mRNA expression after 72 h of cytotrophoblast culture. mRNA data are expressed as the level of each marker mRNA normalized by Po mRNA levels. Three culture dishes were pooled for each determination, and transcripts were assayed in duplicate. (C) Levels of hCG (expressed in milli-international units per milliliter) secreted at the indicated time into the culture medium of control cells or cells treated with 0.1 mM 8-bromo-cAMP. (D) Western blot analysis of HERV-W Env expression in cell lysates of cells treated with cAMP or left untreated (detection by the anti-SU polyclonal antibody). **, P < 0.01; ***, P < 0.001.
Expression of functional Env-W by human trophoblast cells.
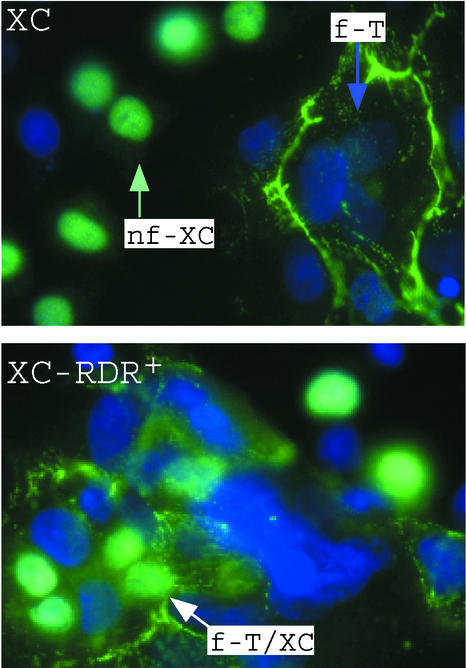
To confirm that Env-W glycoprotein is functionally expressed in primary trophoblast cells, a heterotypic fusion assay was developed based on the observation than Env-W fuses cells expressing the type D mammalian retrovirus receptor (RDR) (7). Since no syncytium formation was detected with XC rat cells cocultured with HERV-W Env-transfected TE671 cells (7), XC cells were used as indicator cells in the heterotypic cell-cell fusion assay. Compared to that in parental XC cells, formation of syncytia containing both cytotrophoblast and XC nuclei was readily detected in RDR-transfected cells (Fig. 4). This showed that the RDR-dependent fusogenic HERV-W Env glycoprotein was expressed at the surface membrane of primary cytotrophoblasts.
FIG. 4.
Expression of the HERV-W Env glycoprotein at the surface membrane of primary trophoblasts. The cocultivation of primary cytotrophoblasts (T) with XC (top) indicator cells induced the formation of homotypic syncytia (f-T) after 72 h of culture. These syncytia contained only cytotrophoblast nuclei; no XC nuclei were associated with the fusion event (nf-XC). Conversely, the cocultivation of primary cytotrophoblasts with XC-RDR+ (bottom) indicator cells induced the formation of heterotypic syncytia (f-T/XC) containing both cytotrophoblast and XC nuclei, showing that the RDR-dependent fusogenic HERV-W Env glycoprotein was expressed at the surface membrane of primary cytotrophoblasts. Anti-β-galactosidase antibody specifically indicated the XC cell nuclei and antidesmoplakin antibody specifically indicated the syncytiotrophoblasts. Nuclei of both cell types were labeled by DAPI (blue immunofluorescence).
Modulation of Env-W expression by antisense oligonucleotide and trophoblast cell fusion.
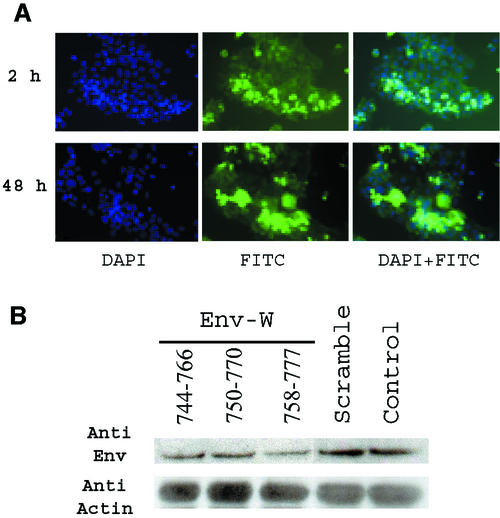
Three different antisense oligonucleotides were designed on the basis of the following criteria: they overlapped the ATG codon (nucleotides 762 to 764 of Env mRNA; accession number AF072506), with the chloramphenical acetyltransferase anticodon being located near the 5′, central, or 3′ part of the oligonucleotide, and they included a free 3′ end to favor initiation of duplex formation and at least one G at both ends to stabilize DNA-RNA duplexes (18, 35). Analysis of the ATG region in the context of the whole 2.7-kb env mRNA (mfold 3.1) allowed precise design of the oligonucleotide borders on the basis of the above rules, notably the definition of the free 3′ end entering a stem-loop structure. A search for false priming sites (oligo 6.2) showed that these designed HERV-W antisense oligonucleotides are almost totally unrelated to other HERV families that are highly transcribed in placenta, such as HERV-E and ERV-3 (reviewed in reference 6). An almost fully degenerated oligonucleotide was used as a control (except for one flanking G at both ends to stabilize DNA-RNA duplexes). This represents 7 × 1010 molecular species, so that its concentration at the site of action is totally insufficient to achieve any antisense or generally sequence-dependent cellular effect. Since a single base shift could induce huge differences in inhibition efficiency, antisense oligonucleotides were first evaluated in an in vitro fusion test based on cocultivation of phCMV-env-transfected TELac2 cells cocultured with indicator TE671 cells as previously described (7). When the env-specific oligonucleotides (4.3 × 10−2 μM) were simultaneously cotransfected with the phCMV-env plasmid (3.5 × 10−5 μM), more than 95% inhibition of syncytium formation was observed after 30 h of coculture (data not shown); conversely, the formation of numerous syncytia containing many nuclei, similar to results of the positive-control test, was observed with the cotransfected scrambled oligonucleotide. No inhibition of syncytium formation was observed when the oligonucleotides (HERV-W specific or scrambled) were directly added to the culture medium in the absence of calcium phosphate used for transfection (4.3 × 10−2 μM), showing that none of the oligonucleotides interfered with the fusion process at the cell membrane and that cellular uptake was required for efficient inhibition (data not shown). The inefficient oligonucleotide uptake by TE-rhabdomysarcoma cell lines was confirmed by fluorescence-activated cell sorter analysis by adding fluorescein isothiocyanate-labeled oligonucleotide to the cell culture. The percentage of labeled cells after 24 h was 72 and 0.3% in the presence and absence of calcium phosphate, respectively. Note that similar percentages were observed in b30 BeWo carcinoma cells (70 and 5%, respectively). Conversely, even after extensive PBS washings (consisting of pipetting up and down 15 times, repeated three times), a significant uptake of oligonucleotides was observed after 1 h with primary cells (20% of labeled cells), increasing progressively with time (26% at 2 h, 41% at 48 h) (Fig. 5A). A preliminary experiment using all three specific env antisense oligonucleotides was then performed with primary cells in the context of the natural full-length Env transcript induced during mononuclear cytotrophoblast differentiation into syncytiotrophoblasts, as described above. The most efficient reduction of Env expression, analyzed by Western blotting, was obtained with the Env-W-758-777 oligonucleotide characterized by the longer free 3′ end, the presence of a chloramphenicol acetyltransferase anticodon located toward the 3′ end, and absence of a hairpin (Fig. 5B).
FIG. 5.
(A) Oligonucleotide uptake by primary cytotrophoblasts. After 5 h of culture, cytotrophoblasts were incubated with FITC-labeled scrambled oligonucleotide for 1, 2, 24, and 48 h. For each time point, cells were washed three times in PBS, fixed, and analyzed by fluorescence microscopy. Nuclei were stained in blue by DAPI. Intensely fluorescent green cells have internalized the scrambled antisense oligonucleotide (FITC). (B) Evaluation of Env-W antisense oligonucleotide. After 5 h of culture, cytotrophoblasts were incubated with three specific env antisense oligonucleotides (Env-W 744-766, Env-W 750-770, and Env-W 758-777) or a random oligonucleotide (scrambled) or without oligonucleotide (control). After 48 h of culture, proteins were extracted and Env glycoprotein expression was analyzed by Western blotting using an anti-SU polyclonal antibody and standardized using an antiactin monoclonal antibody. The most efficient reduction of Env expression was obtained with the Env-W 758-777 antisense oligonucleotide.
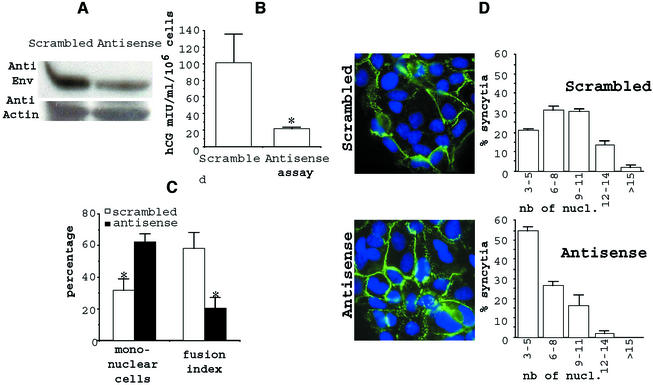
A quantitative analysis of the incidence of Env inhibition on trophoblast differentiation was then performed. As shown in Fig. 6A, a clear decrease of the Env-W protein band, as measured by Western blot analysis, was again observed in extracts of Env-W antisense oligonucleotide-treated cells compared to scrambled antisense oligonucleotide-treated control cells. Interestingly, as illustrated in Fig. 6B, secretion of hCG in the culture medium of antisense oligonucleotide-treated cells was decreased by fivefold compared to that for control cells. This was apparently not due to differential cellular viability, since the total protein amount was similar for both scrambled oligonucleotide- and antisense oligonucleotide-treated cells. Nevertheless, it is not known if the decrease in secreted hCG resulted from a lower transcription-translation level or from poorer export of the hormone. The extent of syncytium formation after 48 h of culture was further assessed by determining the number of DAPI-stained nuclei in mononuclear or multinuclear fused cells and the number of syncytia by immunostaining of desmoplakin (Fig. 6C and D). First, the enumeration of unfused cells showed that these mononuclear cells represented the majority of the cells when the culture was treated with the antisense oligonucleotide; conversely, there were twofold fewer mononuclear cells in the presence of the scrambled oligonucleotide, with most of the cells being part of the syncytia (Fig. 6C). Interestingly, calculation of the apparent fusion index showed a ratio of about 3 between antisense oligonucleotide- and scrambled oligonucleotide-treated cells (Fig. 6C), higher than the ratio for mononuclear cells. This difference indicated that the effect of the Env-W antisense oligonucleotide on syncytium formation led to a decrease in the number and size of syncytia formed by fusion between cytotrophoblasts or between newly formed syncytia (Fig. 6D). More precisely, contiguous syncytia observed in cells treated with the scrambled antisense oligonucleotide were characterized by the presence of 3 to 15 nuclei, following approximately a Gaussian distribution, with about 50% of the syncytia containing 6 to 9 nuclei. Conversely, in the presence of Env-W antisense, a skewed distribution was observed, reflecting the presence of smaller syncytia, the majority (more than 50%) of them containing only three nuclei.
FIG. 6.
Specific inhibition of HERV-W env expression reduces syncytium formation and hCG secretion. After 5 h of culture, the primary cytotrophoblasts were transfected with random (Scrambled) or HERV-W specific (Antisense) oligonucleotides. (A) Western blot analysis of Env glycoprotein in the cell lysates in the absence (Scrambled) or presence (Antisense) of HERV-W- specific oligonucleotides. Detection was done with the anti-SU polyclonal antibody and standardization using an anti-actin monoclonal antibody. (B) hCG titration in the absence (Scrambled) or presence (Antisense) of HERV-W-specific oligonucleotides. (C) After 48 h of culture, the fusion activity of the envelope glycoproteins was determined. The cells were fixed, immunostained with anti-desmoplakin monoclonal antibody, and counterstained with DAPI. Mononuclear cells were counted, and the fusion index (7) was determined as (N − S)/T, where N is the number of nuclei in the syncytia, S is the number of syncytia, and T is the total number of nuclei counted. Results are expressed as percentages of the fusion indices. (D) Large syncytia were observed using random oligonucleotide (scrambled), and smaller ones were observed using HERV-W-specific oligonucleotides (antisense). The nucleus distribution was evaluated as followed: 100 syncytia were scored after staining, and the nuclei were counted in each syncytium. Data from one representative experiment are expressed as the distribution of syncytia as a function of the number of nuclei per syncytium. Three coverslips incubated in separate wells of a six-well microplate were observed (means and SEM) for each incubation condition. *, P < 0.05.
DISCUSSION
Few human cell types can fuse and differentiate into multinucleated syncytia. This process is involved in the formation of myotubes (44), osteoclasts (45), and syncytiotrophoblast (31). The syncytiotrophoblast is the primary site of several placental functions, including nutrient exchange, metabolism, and steroid and peptide hormone synthesis, which are required for fetal growth and development. Despite a common morphological differentiation process, the three cell types which are able to differentiate into a syncytium differ notably. The syncytiotrophoblast in situ maintains a strong polarity, with microvilli on the apical membrane, whereas myotubes do not exhibit morphological polarity. The myoblast-myotube transition first requires the withdrawal of myoblasts from the cell cycle to G0 (25), while cytotrophoblasts which fuse to create the syncytiotrophoblast already are essentially in G0. In contrast to the villous syncytiotrophoblast, osteoclasts have major locomotor activity.
The cell-cell fusion process involved in syncytiotrophoblast formation is poorly understood. In vitro studies have established that soluble factors such as epidermal growth factor (1, 32), granulocyte-stimulating factor (19), glucocorticoids (8), estriol (10), and hCG (9, 36) activate different intracellular signaling pathways to stimulate the differentiation of villous cytotrophoblasts into the syncytiotrophoblast. In this study we have shown for the first time that (i) an envelope glycoprotein encoded by the ERVWE1 retroviral locus is highly expressed in normal human trophoblasts, since it is observed in more than 10 primary trophoblast cultures; and (ii) this glycoprotein is directly involved in human trophoblast cell fusion and differentiation. Indeed, direct inhibition of this retroviral envelope protein inhibited trophoblast cell fusion. Thus, the large amount of remaining unfused (mononuclear) cells in the presence of the specific Env-W antisense oligonucleotide may argue for involvement of the envelope glycoprotein in the initial steps of the trophoblast fusion process. However, due to the complex multifactorial mechanisms involved in syncytiotrophoblast formation, we cannot exclude that other, still unidentified factors might also be directly involved in trophoblast cell fusion.
The cellular processes leading to syncytium formation are associated with a concomitant increase in the intracellular level of cAMP (24). This elevation in cAMP levels is required for the synthesis of numerous specific trophoblast proteins and hormones. We also have reported a direct role for cAMP-dependent protein kinases in simulating cytotrophoblast fusion (24). Interestingly, in this study we observed that HERV-W mRNA and protein expression was also stimulated by cAMP. This could suggest a direct effect of this nucleotide on the promoter of the ENV-W gene.
On the other hand, we and others have observed that cytotrophoblast fusion and differentiation are inhibited by hypoxia. Similarly, the histological abnormalities of term placentas in pregnancy associated with underperfusion and hypoxia, such as in preeclampsia, are characterized by cytotrophoblast prominence and abnormalities in syncytiotrophoblast differentiation. Interestingly, recent studies have shown that syncytin mRNA was poorly expressed in these pathological placentas (26), confirming that Env-W expression might reflect syncytiotrophoblast formation.
The results presented in this report strongly support a direct role for Env-W in syncytiotrophoblast formation. Nevertheless, the phylogenetic distribution of HERV-W sequences indicates that its ancestor entered the genomes of higher primates 25 million to 40 million years ago, after the divergence of Old World and New World monkeys (42). Thus, the ERVWE1 locus encoding the Env fusogenic glycoprotein is a young element in the genome. It is possible (and this is our working hypothesis) that this element has become a bona fide gene, i.e., has been positively selected during primate evolution to play a role in placentation. However, it is also possible that this locus is a still active pseudogene in the process of extinction and that its activity in the syncytiotrophoblast formation is simply a remnant of its original function for the propagation of retroviruses but that it has no real physiological role. To rule out this latter hypothesis, it is necessary to show the functional conservation of the ERVWE1 locus among the human species and during primate evolution. If this locus is indeed under selective pressure in human and other primates, it will be challenging to try to understand if this acquisition represents an additional adaptative or substitutive mechanism (21, 41), considering the extreme diversity of the placental structures observed in nature.
Acknowledgments
J.-L. Frendo and D. Olivier contributed equally to this work.
We thank Laurent Duret for helpful discussions and Robin Buckland for critical reading of the manuscript. We thank Fanny Lewin for her support and the staff of Saint Vincent de Paul Obstetrics Department for providing us with placentas. We thank Martine Olivi for her technical assistance. We thank Jerome Strauss III for providing the b30 BeWo cell line.
This work was supported by a grant from La Fondation pour la Recherche Medicale (ARS 2000) and the Association pour la Recherche dans la Trisomie 21.
REFERENCES
- 1.Alsat, E., J. Haziza, and D. Evain-Brion. 1993. Increase in epidermal growth factor receptor and its messenger ribonucleic acid levels with differentiation of human trophoblast cells in culture. J. Cell. Physiol. 154:122-128. [DOI] [PubMed] [Google Scholar]
- 2.Alsat, E., V. Mirlesse, C. Fondacci, M. Dodeur, and D. Evain-Brion. 1991. Parathyroid hormone increases epidermal growth factor receptors in cultured human trophoblastic cells from early and term placenta. J. Clin. Endocrinol. Metab. 73:288-294. [DOI] [PubMed] [Google Scholar]
- 3.Alsat, E., P. Wyplosz, A. Malassiné, J. Guibourdenche, D. Porquet, C. Nessmann, and D. Evain-Brion. 1996. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J. Cell. Physiol. 168:346-353. [DOI] [PubMed] [Google Scholar]
- 4.Aplin, J. 1991. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J. Cell Sci. 99:681-692. [DOI] [PubMed] [Google Scholar]
- 5.Blond, J.-L., F. Beseme, L. Duret, O. Bouton, F. Bedin, H. Perron, B. Mandrand, and F. Mallet. 1999. Molecular characterization and placental expression of HERV-W, a new human endogenous retrovirus family. J. Virol. 73:1175-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blond, J.-L., V. Cheynet, and F. Malet. 2001. Signification biologique des rétrovirus endogènes humains. Virologie 5:91-111. [Google Scholar]
- 7.Blond, J.-L., D. Lavillette, V. Cheynet, O. Bouton, G. Oriol, S. Chapel-Fernandes, B. Mandrand, F. Mallet, and F. L. Cosset. 2000. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 74:3321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cronier, L., E. Alsat, J.-C. Hervé, J. Delèze, and A. Malassiné. 1998. Dexamehasone stimulates Gap junctional communication peptide hormone production and differentiation in human term trophoblast. Trophoblast Res. 11:35-49. [Google Scholar]
- 9.Cronier, L., B. Bastide, J.-C. Hervé, J. Delèze, and A. Malassiné. 1994. Gap junctional communication during human trophoblast differentiation: influence of human chorionic gonadotropin. Endocrinology 135:402-408. [DOI] [PubMed] [Google Scholar]
- 10.Cronier, L., J. Guibourdenche, C. Niger, and A. Malassine. 1999. Oestradiol stimulates morphological and functional differentiation of human villous cytotrophoblast. Placenta 20:669-676. [DOI] [PubMed] [Google Scholar]
- 11.de Parseval, N., and T. Heidmann. 1998. Physiological knockout of the envelope gene of the single-copy ERV-3 human endogenous retrovirus in a fraction of the Caucasian population. J. Virol. 72:3442-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas, G., and B. King. 1990. Differentiation of human trophoblast cells in vitro as revealed by immunocytochemical staining of desmoplakin and nuclei. J. Cell Sci. 96:131-141. [DOI] [PubMed] [Google Scholar]
- 13.Eaton, B., and S. Contractor. 1993. In vitro assessment of trophoblast receptors and placental transport mechanisms, p. 471-503. In C. W. Redman, I. L. Sargent, and P. M. Starkey (ed.), The human placenta. Blackwell Scientific Publications, London, United Kingdom.
- 14.Fisher, S., T.-Y. Cui, L. Zhang, L. Hartman, K. Grahl, Z. Guo-Yang, J. Tarpey, and C. Damsky. 1989. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J. Cell Biol. 109:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frendo, J.-L., P. Thérond, T. Bird, N. Massin, F. Muller, J. Guibourdenche, D. Luton, M. Vidaud, W. Anderson, and D. Evain-Brion. 2001. Overexpression of copper zinc superoxide dismutase impairs human trophoblast cell fusion and differentiation. Endocrinology 142:3638-3648. [DOI] [PubMed] [Google Scholar]
- 16.Frendo, J.-L., P. Thérond, J. Guibourdenche, J.-M. Bidart, M. Vidaud, and D. Evain-Brion. 2000. Modulation of copper/zinc superoxide dismutase expression and activity with in vitro differentiation of human villous cytotrophoblast. Placenta 21:773-781. [DOI] [PubMed] [Google Scholar]
- 17.Frendo, J.-L., M. Vidaud, J. Guibourdenche, D. Luton, F. Muller, D. Bellet, Y. Giovangrandi, A. Tarrade, D. Porquet, P. Blot, and D. Evain-Brion. 2000. Defect of villous cytotrophoblast differentiation into syncytiotrophoblast in Down syndrome. J. Clin. Endocrinol. Metab. 85:3700-3707. [DOI] [PubMed] [Google Scholar]
- 18.Galeotti, N., C. Ghelardini, L. Papucci, S. Capaccioli, A. Quattrone, and A. Bartolini. 1997. An antisense oligonucleotide on the mouse Shaker-like potassium channel Kv1.1 gene prevents antinociception induced by morphine and baclofen. J. Pharmacol. Exp. Ther. 281:941-949. [PubMed] [Google Scholar]
- 19.Garcia-Lloret, M., D. Morrish, T. Wegmann, L. Honore, A. Turner, and L. Guilbert. 1994. Demonstration of functional cytokine-placental interactions: CSF-1 and GM-CSF stimulate human cytotrophoblast differentiation and peptide hormone secretion. Exp. Cell Res. 214:46-54. [DOI] [PubMed] [Google Scholar]
- 20.Handwerger, S. 1991. The physiology of placental lactogen in human pregnancy. Endocrinology 12:329-336. [DOI] [PubMed] [Google Scholar]
- 21.Harris, J. 1998. Placental endogenous retrovirus (ERV): structural, functional, and evolutionary significance. Bioessays 20:307-316. [DOI] [PubMed] [Google Scholar]
- 22.Jameson, J., and A. Hollenberg. 1993. Regulation of chorionic gonadotropin gene expression. Endocr. Rev. 14:203-221. [DOI] [PubMed] [Google Scholar]
- 23.Kalter, S., R. Heberling, R. Helmke, M. Panigel, G. Smith, D. Kraemer, A. Hellman, A. Fowler, and J. Strickland. 1975. A comparative study on the presence of C-type viral particles in placentas from primates and other animals. Bibl. Haematol. 40:391-401. [DOI] [PubMed] [Google Scholar]
- 24.Keryer, G., E. Alsat, K. Tasken, and D. Evain-Brion. 1998. Cyclic AMP-dependent protein kinases and human trophoblast cell differentiation in vitro. J. Cell Sci. 111:995-1004. [DOI] [PubMed] [Google Scholar]
- 25.Kliman, H., J. Nestler, E. Sermasi, J. Sanger, and J. Strauss III. 1986. Purification, characterization, and in vitro differenciation of cytotrophoblasts from human term placentae. Endocrinology 118:1567-1582. [DOI] [PubMed] [Google Scholar]
- 26.Knerr, I., E. Beinder, and W. Rascher. 2002. Syncytin, a novel human endogenous retroviral gene in human placenta: evidence for its dysregulation in preeclampsia and HELLP syndrome. Am. J. Obstet. Gynecol. 86:210-213. [DOI] [PubMed] [Google Scholar]
- 27.Lin, L., B. Xu, and N. Rote. 2000. The cellular mechanism by which the human endogenous retrovirus ERV-3 env gene affects proliferation and differentiation in a human placental trophoblast model, BeWo. Placenta 21:73-78. [DOI] [PubMed] [Google Scholar]
- 28.Lin, L., B. Xu, and N. Rote. 1999. Expression of endogenous retrovirus ERV-3 induces differentiation in BeWo, a choriocarcinoma model of human placental trophoblast. Placenta 20:109-118. [DOI] [PubMed] [Google Scholar]
- 29.Mathews, D., J. Sabina, M. Zuker, and D. Turner. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911-940. [DOI] [PubMed] [Google Scholar]
- 30.Mi, S., X. Lee, X.-P. Li, G. M. Veldman, H. Finnerty, L. Racie, E. LaVallie, X.-Y. Tang, P. Edouard, S. Howes, J. C. Keith, and J. M. McCoy. 2000. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403:785-789. [DOI] [PubMed] [Google Scholar]
- 31.Midgley, A., G. Pierce, G. Denau, and J. Gosling. 1963. Morphogenesis of syncytiotrophoblast in vivo: an autoradiographic demonstration. Science 141:350-351. [DOI] [PubMed] [Google Scholar]
- 32.Morrish, D., E. Linetsky, D. Bhardwaj, H. Li, J. Dakour, R. Marsh, M. Paterson, and R. Godbout. 1996. Identification by subtractive hybridization of a spectrum of novel and unexpected genes associated with in vitro differentiation of human cytotrophoblast cells. Placenta 17:431-441. [DOI] [PubMed] [Google Scholar]
- 33.Ogren, L., and F. Talamentes. 1994. The placenta as an endocrine organ: polypeptides, p. 875-945. In E. Knobil, and J. Neill (ed.), Physiology of reproduction. Raven Press, New York, N.Y.
- 34.Panigel, M. 1993. The origin and structure of extraembryonic tissues, p. 3-32. In C. Redman, I. Sargent, and P. Starkey (ed.), The human placenta. Blackwell Scientific publications, London, United Kingdom.
- 35.Patzel, V., U. Steidl, R. Kronenwett, R. Haas, and G. Sczakiel. 1999. A theoretical approach to select effective antisense oligodeoxyribonucleotides at high statistical probability. Nucleic Acids Res. 27:4328-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi, Q., Z. Lei, C. Rao, and J. Lin. 1993. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology 132:1387-1395. [DOI] [PubMed] [Google Scholar]
- 37.Stoye, J., and J. Coffin. 2000. A provirus put to work. Nature 403:715-717. [DOI] [PubMed] [Google Scholar]
- 38.Tailor, C., A. Nouri, C. Lee, C. Kozak, and D. Kabat. 1999. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc. Natl. Acad. Sci. USA 96:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarrade, A., R. Lai Kuen, A. Malassiné, V. Tricottet, P. Blain, M. Vidaud, and D. Evain-Brion. 2001. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab. Investig. 81:1199-1211. [DOI] [PubMed] [Google Scholar]
- 40.Venables, P., S. Brookes, D. Griffiths, R. Weiss, and B. M. T. Boyd. 1995. Abundance of an endogenous retroviral envelope protein in placental trophoblasts suggests a biological function. Virology 20:589-592. [DOI] [PubMed] [Google Scholar]
- 41.Villareal, L. 1997. On viruses, sex, and motherhood. J. Virol. 71:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voisset, C., A. Blancher, H. Perron, B. Mandrand, F. Mallet, and G. Paranhos-Baccala. 1999. Phylogeny of a novel family of human endogenous retrovirus sequences, HERV-W, in humans and other primates. AIDS Res. Hum. Retroviruses 15:1529-1533. [DOI] [PubMed] [Google Scholar]
- 43.Voisset, C., O. Bouton, F. Bedin, L. Duret, B. Mandrand, F. Mallet, and G. Paranhos-Baccala. 2000. Chromosomal distribution and coding capacity of the human endogenous retrovirus HERV-W family. AIDS Res. Hum. Retroviruses 16:731-740. [DOI] [PubMed] [Google Scholar]
- 44.Wakelam, M. 1985. The fusion of myoblasts. Biochem. J. 15:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zambonin Zallone, A., A. Teti, and M. Primavera. 1984. Monocytes from circulating blood fuse in vitro with purified osteoclasts in primary culture. J. Cell Sci. 66:335-342. [DOI] [PubMed] [Google Scholar]