Abstract
Human cytomegalovirus (CMV) infection initiates in mucosal epithelia and disseminates via leukocytes throughout the body. Langerhans cells (LCs), the immature dendritic cells (DCs) that reside in epithelial tissues, are among the first cells to encounter virus and may play important roles in the immune response, as well as in pathogenesis as hosts for viral replication and as vehicles for dissemination. Here, we demonstrate that CD34+ progenitor cell-derived LC-type DCs exhibit a differentiation state-dependent susceptibility to CMV infection. In contrast to the small percentage (3 to 4%) of the immature LCs that supported infection, a high percentage (48 to 74%) of mature, LC-derived DCs were susceptible to infection with endotheliotropic strains (TB40/E or VHL/E) of CMV. These cells were much less susceptible to viral strains AD169varATCC, TownevarRIT3, and Toledo. When exposed to endotheliotropic strains, viral gene expression (IE1/IE2 and other viral gene products) and viral replication proceeded efficiently in LC-derived mature DCs (mDCs). Productive infection was associated with downmodulation of cell surface CD83, CD1a, CD80, CD86, ICAM-1, major histocompatibility complex (MHC) class I, and MHC class II on these cells. In addition, the T-cell proliferative response to allogeneic LC-derived mDCs was attenuated when CMV-infected cultures were used as stimulators. This investigation revealed important characteristics of the interaction between CMV and the LC lineage of DCs, suggesting that LC-derived mDCs are important to viral pathogenesis and immunity through their increased susceptibility to virus replication and virus-mediated immune escape.
Dendritic cells (DCs) play a central role in priming CD4+- and CD8+-T-cell responses (4, 5, 76) to generate effective cell-mediated immunity against viruses and other infectious agents (46). Bone marrow-derived CD34+ progenitor cells differentiate into at least two types of immature DCs that distribute to tissues and participate in antigen uptake: interstitial DCs and Langerhans cells (LCs) (4, 11). LCs residing in squamous epithelia are likely to be among the first cells to encounter viruses such as cytomegalovirus (CMV), whereas interstitial DCs residing in the dermis and deeper tissues would only encounter pathogens that breach the epithelium. The generation of these two immature DC types from CD34+ progenitors in vitro depends upon the cytokine conditions (8). After an encounter with antigen, both DC types mature and migrate to secondary lymphoid organs, where they present antigen to T lymphocytes via major histocompatibility complex (MHC) class I and class II cell surface proteins, influencing both the type and the quality of that response (18). Although DC lineages may be distinguished by cellular markers, differentiation pathways, and tissue localization, differences in function are just beginning to unfold (34).
Virus infection generally stimulates DC maturation and antigen presentation and helps initiate the adaptive immune response. Human CMV (1, 43, 51), as well as other human viruses such as herpes simplex virus type 1 (30), human immunodeficiency virus (16), and measles virus (35), encode gene products that interfere with antigen-presenting cell (APC) functions. Such impairment may alter the initiation of the adaptive immune response or slow viral clearance and cause a generalized suppression of the immune response to other pathogens (44). Acute CMV infection in immunocompromised patients has long been associated with generalized immunosuppression (47), which has been demonstrated even in healthy individuals (33).
CMV is a ubiquitous, species-specific betaherpesvirus that interacts with leukocytes in the myeloid lineage during acute and latent infection (40). CMV continues to be an important pathogen in immunocompromised hosts, even though it typically causes subclinical infections in the general population (47). Despite the fact that infection with CMV is common and active replication is controlled by the cellular immune response (55), the virus remains latent in myeloid progenitors for the life of the host (6, 23, 28, 29, 36, 65, 66). Monocytes, macrophages, DCs, and their progenitors may be reservoirs from which virus reactivates. Viral disease in solid organ or bone marrow transplant recipients typically originates from reactivation.
Myeloid progenitors of monocyte/macrophages and DCs are not susceptible to active viral replication. These cells provide sites of viral latency from which reactivation occurs after proinflammatory cytokine or allogeneic stimulation (23, 28, 36, 65, 66). The first evidence of a maturation-dependent increased susceptibility to CMV infection in a primary cell type was obtained by using activated peripheral blood (PB) monocyte-derived macrophages in comparison to monocytes (24, 31). Studies in CMV-infected patients identified tissue-resident macrophages as sites of viral replication (61). Cytokine-driven differentiation of latently infected bone marrow- or PB-derived mononuclear leukocytes leads to active viral replication (23, 64), and these conditions can drive reactivation from naturally infected cells (65, 66). Progenitors of DCs become latently infected (23) and immature PB monocyte-derived interstitial DCs support viral replication so long as so-called endotheliotropic virus strains, such as TB40/E and VHL/E, are used (25, 56). Most laboratory-propagated strains of CMV fail to replicate in this cell type. Immature interstitial DCs are susceptible to direct infection with endotheliotropic strains of virus, and this alters the level of cell surface MHC class I and class II proteins, as well as certain costimulatory markers (43, 51). As yet there is no consensus on susceptibility of mature monocyte-derived interstitial DCs or the impact of virus on the levels of cell surface proteins.
Little is known about the interaction of CMV with the LC-type DCs that reside in squamous epithelia. This distinct DC subset arises from CD34+ myeloid precursors and homes to epithelial sites, including mucosal sites (22), where CMV typically gains entry and initiates infection. Due to their particular anatomical location, LCs are likely to encounter CMV early during a natural infection, probably in advance of virus spread to deeper body sites where interstitial DCs predominate. Indeed, maturation of LCs and migration to lymph nodes may provide the initial step in viral dissemination and may serve to bring CMV into contact with interstitial DCs and many other cell types.
To study the interaction of CMV with LCs, we utilized a well-defined system for in vitro culture and differentiation of LCs (69, 71). Here, we report that LC-derived mature DCs (mDCs) are highly permissive to CMV infection while immature LCs are less susceptible to this virus. Productive CMV infection of LC-derived mDCs results in the downregulation of cell surface markers important in immune function and in attenuation of the ability to stimulate alloreactive T-cell responses. Thus, active replication in mDCs may provide CMV with a means of subverting the host immune response while serving as a vehicle for dissemination and a site of persistent infection.
MATERIALS AND METHODS
Generation of CD34+ progenitor-derived cell populations.
Granulocyte-colony-stimulating factor (G-CSF) mobilized PB or cord blood CD34+ progenitors were purchased from BioWhittaker (Walkersville, Md.) and DC populations were cultured as described previously (20, 69). Briefly, LCs were generated in serum-free X-VIVO 15 medium (BioWhittaker, Walkersville, Md.) containing 1,500 IU of granulocyte macrophage-colony stimulating factor (GM-CSF)/ml, 2.5 ng of tumor necrosis factor alpha (TNF-α)/ml, 20 ng of stem cell factor (SCF)/ml, 100 ng of Flt3 ligand/ml, and 0.5 ng of transforming growth factor β1 (TGF-β1)/ml (LC growth medium) after CD34+ progenitors were plated at a density of 104 cells/ml in six-well tissue culture plates (5 ml/well). GM-CSF (Leukine Sargramostim; Immunex, Seattle, Wash.) was obtained from the inpatient pharmacy at Stanford University Hospital, and all other cytokines were purchased from Peprotech (Rocky Hill, N.J.). Clusters of immature LCs that developed after 10 days in culture were gently harvested by using a 25-ml pipette and purified by 30 min of 1 × g sedimentation through X-VIVO 15 containing 7.5% lipopolysaccharide (LPS)-free bovine serum albumin (BSA; Sigma, St. Louis, Mo.). The clusters were concentrated by centrifugation at 300 × g for 5 min at room temperature, suspended in medium, and seeded at a density of 2 × 105 cells/well in 48-well tissue culture plates. To generate mDCs, purified LC clusters were disaggregated by using a pipette and seeded at the same density in X-VIVO 15 containing 10% fetal bovine serum (FBS), 200 ng of CD40 ligand (CD40L; Immunex, Seattle, Wash.)/ml, and 1,500 IU of GM-CSF/ml for 2 days. To generate monocytes, CD34+ progenitor cells were cultured for 10 days in LC growth medium without TGF-β1 (69). The CD34+ cells from mobilized PB used here were CMV DNA negative when assayed by sensitive, nested PCR methods (63).
Preparation of virus and infection of CD34+ progenitor-derived cells.
Strain AD169varATCC was obtained from the American Type Culture Collection (ATCC), and a green fluorescent protein (GFP)-tagged virus, RC2940, was prepared from a plaque-purified derivative of TownevarRIT3 (J. Xu, D. Formankova, and E. S. Mocarski, unpublished results). Non-plaque-purified stocks of Toledo (passage 8), TB40/E, and VHL/E were kind gifts of S. Plotkin (Philadelphia, Pa.), C. Sinzger, (Tübingen, Germany), and W. J. Waldman (Columbus, Ohio), respectively. Virus strains were propagated at a multiplicity of infection (MOI) of 0.01 in confluent human foreskin fibroblasts (HFs), purified as described previously (17), and briefly sonicated and stored at −80°C in 200-μl aliquots. Virus titers were determined by plaque assay on monolayers of HFs in six-well tissue culture plates. For infections of LCs, mDCs, or monocytes, 2 × 105 cells/well in 48-well plates were infected at an MOI of 100 PFU/cell. The virus stock was diluted in X-VIVO 15 medium and filtered through a 0.45-μm (pore-size) filter immediately prior to use (a step that did not reduce infectivity). The culture medium from each well was removed and replaced with 250 μl of virus inoculum. After adsorption for 2 h at 37°C, the virus-containing medium was removed and replaced with 300 μl of medium/well supplemented with the specific cytokine cocktail for each cell type. Mock-infected cells were treated in the same manner without adding virus. For the single-step growth curve, mDCs were washed 10 times with culture medium to remove residual input virus, and titers in supernatants and cells from two separate wells of mDCs were independently determined at days 1, 5, 9, and 15 postinfection (p.i.).
Flow cytometry and immunofluorescence analyses.
After incubation in blocking buffer (BB; 0.5% bovine serum albumin, 0.1% sodium azide, 5% pooled human AB serum, and 5% normal goat serum in phosphate-buffered saline [PBS]) for 30 min, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated antibodies in BB for 30 min, washed twice with wash buffer (WB; 0.5% bovine serum albumin and 0.1% sodium azide in PBS), and then resuspended in WB. Propidium iodide (PI; 5 μg/ml) was added to each sample prior to flow cytometry. Y analysis and gating (Fig. 1) was applied and PI-positive cells were eliminated from analysis. For simultaneous cell surface and intranuclear antigen staining, cells were blocked as described above, incubated with phycoerythrin (PE)-conjugated antibodies to cell markers for 30 min, fixed with 1% paraformaldehyde in PBS for 15 min, and permeabilized with 0.2% Triton X-100 for 15 min. After being washed, cells were incubated with FITC-conjugated anti-immediate-early 1 (IE1)/IE2 antibody (1:800 dilution of MAB810; Chemicon, Temecula, Calif.) in BB for 30 min, washed once again, and resupended in WB. All procedures were carried out at 4°C. The cells were analyzed on a FACScan (BD Pharmingen, San Jose, Calif.) by using CellQuest 3.1 software. For immunofluorescence analysis, cystospin preparations were fixed with 1% paraformaldehyde in PBS for 30 min at 25°C, permeabilized, and stained for IE1/IE2 as described above. After three washes with 0.05% Tween 20 in PBS, cells were stained with PI (1 μg/ml) for 3 min, washed three times in PBS, and mounted with FluoroGuard Antifade Reagent (Bio-Rad, Hercules, Calif.). For IE1/IE2 and ppUL44 double staining, fixed cells were first incubated with a ppUL44 monoclonal antibody (1:500 dilution; Goodwin Institute, Plantation, Fla.) and a Texas red-conjugated goat anti-mouse antibody (1:100; Vector Laboratories, San Bruno, Calif.), blocked with normal mouse immunoglobulin G (1:100; Caltag, Burlingame, Calif.), and then stained with FITC-conjugated anti-IE1/IE2 monoclonal antibody prior to being viewed on an Olympus BX60 epifluorescence microscope equipped with ×40 or ×60 phase-contrast objectives. Images were collected by using a Hamamatsu ORCA-100 digital camera with Image Pro Plus 4.0 software (MediaCybernetics, Silver Spring, Md.). Digitized images were stored, electronically colorized, and then overlaid for evaluation of two-color experiments. Phase-contrast micrographs of live cells were obtained with a Nikon Eclipse TE300/200 inverted microscope equipped with Hoffman modulation contrast optics. Antibodies to CD1a (FITC, clone HI149), CD80 (FITC, clone BB1), CD83 (FITC or PE, clone HB15e), CD40 (PE, clone 5C3), and CCR7 (FITC, clone 2H4) were obtained from BD Pharmingen. CD86 (FITC, clone BU63), HLA-DR (FITC or PE, clone TU36), HLA class I (FITC or PE, clone TU149), CD54 (FITC, clone MEM111), and CD14 (FITC, clone TUK4) antibodies were purchased from Caltag.
FIG. 1.
Morphological appearance and flow cytometry profiles of cell surface CD1a, CD14, CD83, and MHC class II on LCs, LC-derived mDCs, and monocytes. (A) Morphology of CD34+ progenitor-derived LCs (left), LC-derived mDCs (middle), and monocytes (right). Representative low-magnification phase-contrast photomicrographs of cultures are shown. Single cells at high magnification are displayed as insets. (B) SSC versus FSC dot plots of LC cultures (left), LC-derived mDC cultures (middle), and monocyte cultures (right). Ovals depict gates used for flow cytometry analyses. (C) Cell surface staining of CD1a, CD14, CD83, and HLA DR (MHC II) on LCs (left), LC-derived mDCs (middle), and monocytes (right) as determined by flow cytometry analysis (black lines). Staining with an isotype control antibody is shown in each panel (gray lines).
Immunoblot analysis.
Cells were suspended in lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, and 1% NP-40), rotated for 30 min at 4°C, and centrifuged at 14,000 × g for 30 min at 4°C. Clarified lysates were loaded at 100 μg of protein/lane and then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis through a 12% gel, transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.), incubated with the primary antibody for 3 h at room temperature, and developed by using enhanced chemiluminescence (Pierce, Rockford, Ill.). Rabbit antisera recognizing US2N (75) and US3N were kindly provided by D. C. Johnson (Oregon Health Sciences University, Portland, Oreg.).
Mixed leukocyte reaction.
Mixed leukocyte reaction analysis was performed in triplicate in 96-well round bottom plates. Mock-infected, TB40/E-infected, and LPS-treated (10 μg/ml for 2 days) mDCs were gamma-irradiated (3 mR) and then added to a fixed number of purified allogeneic T cells (8 × 104) that had been purified from PB mononuclear cells by using the Rosette Sep Procedure (StemCell Technologies, Vancouver, British Columbia, Canada). T-cell proliferation was assessed by the incorporation of [3H]thymidine (1 μCi/well; Perkin-Elmer, Foster City, Calif.) added 18 h prior to harvest. On day 3 of the coculture, the cells were harvested with a 96-well harvester (Tomtec, Hamden, Conn.) and counted on a Microbeta scintillation counter (Perkin-Elmer, Boston, Mass.).
RESULTS
Human CMV infection of CD34+ progenitor-derived immature LCs, LC-derived mature DCs, and monocytes.
Immature DCs can be identified by their surface dendrites and by cell surface markers involved in antigen presentation to T cells, including MHC class I, MHC class II, adhesion (e.g., ICAM-1), and costimulatory proteins (e.g., CD40, CD80, and CD86). After antigen uptake, maturing DCs migrate to lymph nodes, acquire more pronounced dendrites, develop higher cell surface levels of both MHC and costimulatory markers, and initiate expression of CD83, a marker of mature DCs whose role in immune function is still under evaluation (19, 32). Separate populations of cells representing monocytes, epidermal LCs, and LC-derived mDCs were grown from cord blood or G-CSF-mobilized PB CD34+ progenitor-enriched leukocytes and evaluated for susceptibility to human CMV. Immature LCs and monocytes were cultured under serum-free conditions in the presence of GM-CSF, TNF-α, SCF, and Flt3 ligand, together with TGF-β1 (LC growth medium) or lacking TGF-β1 (monocyte growth medium). As expected (69), over a period of 10 days, LCs expanded 10- to 40-fold and acquired dendrites typical of DCs (Fig. 1A, left panel, and data not shown). Phenotypically uniform LCs aggregated in clusters, which were separated from single cells (20) and cultured either in fresh LC growth medium (to enrich for LCs) or in maturation medium with GM-CSF and CD40L to induce differentiation and enrich for LC-derived mDCs. In the latter case, cells with long dendrites were found in large clusters after 2 days, but the total cell number remained unchanged (Fig. 1A, middle panel, and data not shown). In parallel with the LC and mDC populations, monocytes were separately derived from CD34+ progenitors (71). These cells exhibited a rounded morphology and did not cluster (Fig. 1A, right panel). Flow cytometry was used to assess forward scatter (FSC) and side scatter (SSC) and set gates for analysis of cell surface markers on each cell type (Fig. 1B). All cell populations had the expected (70) large size (high FSC) and both LCs and mDCs exhibited a greater level of granularity (SSC) than monocytes. A majority of cells cultured in LC growth medium had the expected phenotype and expressed high levels of CD1a, E-cadherin and the unique LC marker, langerin (71). LCs had no detectable CD83 and only low levels of CD14, CD80, CD86, and MHC class II, a finding consistent with their immature phenotype (Fig. 1C, left panels, and data not shown). Differentiation of LCs into mDCs was associated with an increase in cell surface CD83, MHC class II, MHC class I, CD80, and CD86 (Fig. 1C, middle panels, and data not shown). Levels of CCR7 and CD40 transiently increased in response to CD40L (data not shown). As expected, CD14 was absent and CD1a was present at a lower intensity on LC-derived mDCs than on LCs. In contrast, most of the monocytes expressed cell surface CD14 and low levels of both MHC class II and MHC class I but had no detectable DC markers, CD1a, or CD83 (Fig. 1C, right panels, and data not shown).
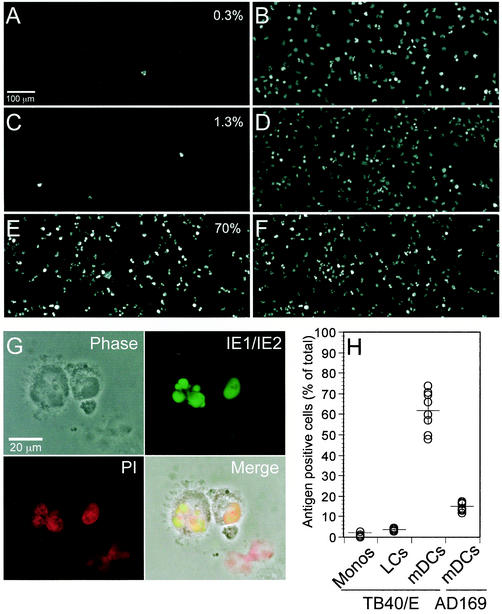
Populations of CD34+ progenitor-derived LCs, mDCs and monocytes were infected with one of two endothelial-cell-adapted strains of human CMV, either TB40/E (56) or VHL/E (77), as well as with other laboratory-adapted and low-passage level CMV strains. Preliminary evaluation was performed by using TB40/E at an MOI ranging from 10 to 200. A maximum percentage of cells showed evidence of infection based on the detection of the major viral immediate-early (IE) proteins IE1 and IE2 (48) at an MOI of 50 or greater (data not shown). All experiments described here were performed at an MOI of 100 with TB40/E or other strains of virus. At 2 days p.i. many mDCs in infected cultures appeared enlarged (Fig. 2), and some had multiple cytoplasmic vacuoles (data not shown). Cytospin preparations of several hundred infected cells were monitored by direct immunofluorescence analysis with a monoclonal antibody recognizing IE1 and IE2 antigens (48). Nuclear antigen was detected in only a small percentage of infected monocytes or LCs but in a large number of LC-derived mDCs. Signal was uniformly distributed in both polylobulated and nonlobulated nuclei of DCs but was not present in mock-infected cells (Fig. 2A to G and data not shown). Cytospin images such as those in Fig. 2A to F were collected and used to determine the percentage of antigen-positive nuclei from several different experiments. In four independent experiments, between 0 and 3% of monocytes (mean value, 1%) and between 3 and 4% of LCs (mean value, 3%) expressed IE1/IE2 antigens at day 2 p.i., the time of peak antigen detection. In contrast, the percentage of IE1/IE2-positive mDCs in eight independent experiments was consistently high, ranging from 48 to 74% (mean value, 62%) (Fig. 2H). A similarly high percentage of IE1/IE2 antigen-positive LC-derived mDCs was obtained after infection with CMV strain VHL/E (data not shown). These findings clearly demonstrate a differential susceptibility of CD34+ progenitor-derived mononuclear leukocyte types to CMV infection, with a proportionately higher number of mDCs showing evidence of efficient penetration and IE gene expression. For comparison, we exposed mDCs to other strains of human CMV, including a plaque-purified derivative of the laboratory-adapted strain AD169varATCC (15, 62), a GFP-tagged derivative of TownevarRIT3 (62), and a low-passage level preparation of the Toledo strain (14, 49). In seven independent experiments, between 12 and 17% (mean value, 15%) of AD169varATCC-infected mDCs but only 2 to 5% of TownevarRIT3-infected cells and none of the cells exposed to Toledo scored as IE1/IE2 antigen positive at day 2 p.i. (Fig. 2H and data not shown). These data show that two commonly used endotheliotropic strains of human CMV (TB40/E and VHL/E) can efficiently infect LC-derived mDCs and that at least one laboratory-propagated strain (AD169varATCC) exhibits the ability to enter and express IE proteins in a smaller proportion of these cells. Based on these results, CD34+ progenitor cell-derived LCs and monocytes appeared to be poorly susceptible to human CMV, whereas CD34+ progenitor, LC-derived mDCs were highly susceptible to CMV infection and different strains of CMV-infected mDCs with variable efficiency.
FIG. 2.
Human CMV antigen expression in CD34+ progenitor-derived monocytes, LCs, and LC-derived mDCs. Cytospin preparations were made at day 2 p.i. with CMV strain TB40/E at an MOI of 100 and stained with an FITC-conjugated monoclonal antibody to the viral IE proteins IE1 and IE2 and with PI to detect cellular nuclei. Representative images of IE1/IE2 (left)- and PI (right)-stained infected monocytes (A and B), LCs (C and D), or LC-derived mDCs (E and F) are shown. The percentage of antigen-positive cells is shown in the upper right of panels A, C,and E. (G) High-magnification images of two infected LC-derived mDCs. The superimposition (Merge) of the phase-contrast micrograph (Phase), the IE1/IE2 staining (green), and the PI staining (red) shows the localization of IE1 and IE2 in both polylobulated and nonlobulated nuclei. (H) Percentage of IE1/IE2 antigen-positive nuclei in independent experiments with different cell types. Monocytes, LCs, or LC-derived mDCs infected with CMV strain TB40/E and mDCs infected with CMV strain AD169varATCC at MOIs of 100 were analyzed at day 2 p.i. The mean and standard deviation are shown for each series.
Monocytes were prepared by using CD34+ progenitors from one cord blood and two G-CSF-mobilized PB donors, LCs were prepared by using CD34+ cells from one cord blood and three G-CSF-mobilized PB donors and mDCs were prepared by using CD34+ cells from four different cord blood and three different G-CSF-mobilized PB donors. Two sources of CD34+ progenitor cells, i.e., from G-CSF mobilized PB and from cord blood, that were studied most extensively showed comparable behavior, suggesting that the source of CD34+ progenitors did not influence the intrinsic susceptibility to viral infection (data not shown).
CMV replication in LC-derived mature DCs.
The expression of additional viral gene products, including the IE protein, gpUS3 (74), and two delayed early proteins, gpUS2 (26) and ppUL44 (41, 79), was monitored in mDCs either by indirect immunofluorescence or immunoblot analyses. After infection with TB40/E (day 3 p.i.), 70 to 75% of IE1/IE2 antigen positive mDC also expressed ppUL44, and these antigens colocalized within nuclei (Fig. 3A to D). ppUL44 was not detected in IE1/IE2-negative cells exposed to virus or in mock-infected mDCs (data not shown). Although fewer cells were positive, ca. 70% of the IE1/IE2-positive AD169varATCC-infected mDCs were also ppUL44 positive (data not shown). These data suggest that some of the cells in the LC-derived mDC population supporting IE1/IE2 antigen expression do not proceed beyond this stage of infection. Both gpUS3 and gpUS2 were detected by immunoblot analysis in TB40/E-infected mDC extracts. As expected based on the level of IE1/IE2 expression, these proteins were detected at levels comparable to similar numbers of productively infected HFs (Fig. 3E). To determine whether infected mDCs supported a complete replication cycle and produced viral progeny, we completed a single-step growth curve analysis. As shown in Fig. 3F, virus titers reached a maximum at day 5 p.i., peaking at levels 10-fold higher than day 1 residual input virus. Altogether, these findings provide evidence that LC-derived mDCs become permissive as they differentiate and support the complete productive replication cycle of human CMV.
FIG. 3.
LC-derived mDCs support the complete replication cycle of human CMV strain TB40/E. (A to D) Colocalization of ppUL44 and IE1/IE2 by immunofluorescence analysis on cytospin preparations of LC-derived mDCs at day 3 p.i. (A) Phase-contrast photomicrograph of field; (B) IE1/IE2-positive nuclei (green); (C) ppUL44-positive nuclei (red); (D) merged image (panel B plus panel C). (E) The expression of the viral IE protein gpUS3 and the viral delayed early protein gpUS2 was monitored by immunoblot analysis of mock- or virus-infected HFs and LC-derived mDCs. Equivalent amounts of extracts from cells at day 2 p.i. were applied in each lane, and the presence of the two viral immunomodulatory proteins was detected with the polyclonal rabbit antisera US2N and US3N. (F) Single-step virus growth curve. At days 1, 5, 9, and 15 p.i., supernatant and cells from two separate wells were collected and sonicated and the titers were determined by plaque assay. Symbols represent virus titer values obtained from each of two separate wells at each time point.
Downmodulation of cell surface markers on LC-derived mature DCs.
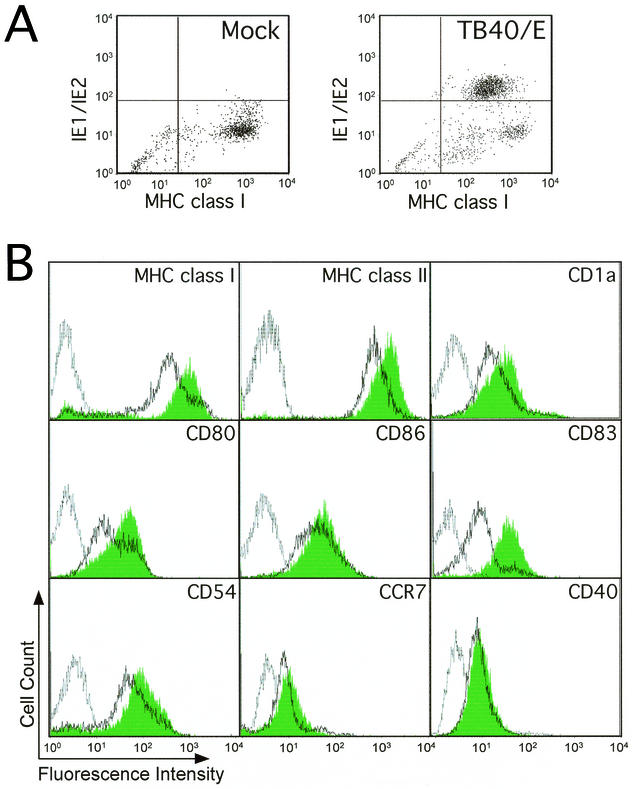
mDCs play roles in activation of naive and primed T lymphocytes during the induction of an antiviral immune response and are also likely to be targets of immune clearance. Both of these processes depend upon antigen presentation by MHC class I and class II proteins, as well as the additional costimulatory functions. Human CMV dramatically reduces the levels of key immune recognition proteins involved in the immune response (39). The impact of virus infection on the expression of specific mDC cell surface markers was evaluated by flow cytometry. Mock- and TB40/E-infected mDCs were collected at day 2 p.i., when infected cultures contained ca. 5% ± 2% more dead cells than mock-infected mDCs, as determined by PI staining. Dead cells were excluded from all analyses. Cells were stained for MHC class I, as well as for viral IE1/IE2 antigen. Approximately 60% of the cells expressed the IE1/IE2 antigen (Fig. 4A). The median MHC class I fluorescence value of 316 in the IE1/IE2-positive cells was much lower than the 644 value in mock-infected, viral antigen-negative mDCs. Similarly, IE1/IE2-positive cells expressed lower levels of CD83 (median fluorescence of 10 versus 34 in mock-infected cells) and MHC class II (median fluorescence of 661 versus 1,144 in mock-infected cells) (data not shown). The cell surface levels of MHC class I, MHC class II, CD1a, CD80, CD86, CD83, CD54 (ICAM-1), CCR7, and CD40 were evaluated on populations of mock- and virus-infected mDC (Fig. 4B). A majority of cells in all virus-infected cultures evaluated were IE1/IE2 positive. The cell surface levels of all cell markers except CD40 and CCR7 were downregulated by viral infection between 20 and 80% based on median fluorescence values. The fivefold downmodulation of CD83 was most dramatic. Consistent results were obtained for each of these in at least five independent experiments. To determine whether exposure to virus particles or expression of virus antigen contributed to the downmodulation, we compared the percentage of cells with downmodulated cell surface proteins after exposure to either AD169varATCC or TB40/E. These two strains differ markedly in the percentage of cells that express viral antigen when used at an equivalent MOI of 100. We found a correlation between the percentage of antigen-positive cells and the percentage of cells that exhibited downmodulation of MHC class I, MHC class II, or CD83 (data not shown). Uninfected cells incubated with virus-free supernatant from TB40/E-infected mDCs did not show any changes in these cell surface markers, suggesting little contribution of cytokines released during infection to this phenotype (data not shown). Our results clearly indicate that human CMV downmodulates a number of surface markers on infected LC-derived mDCs with a potential impact on functions such as antigen presentation, costimulation, and adhesion to T lymphocytes.
FIG. 4.
Human CMV infection alters the cell surface expression of key molecules involved in mDC function. (A) Dot plot showing expression of MHC class I molecules on the surface of mock infected (left) or CMV strain TB40/E-infected LC-derived mDCs (right) at day 2 p.i. Cells were stained with a PE-conjugated anti-MHC class I monoclonal antibody (x axis), as well as with an FITC-conjugated anti-IE1/IE2 monoclonal antibody (y axis). (B) Flow cytometry analysis of expression levels of MHC class I, MHC class II, CD1a, CD80, CD86, CD83, CD54, CCR7, and CD40 on the surface of mock-infected and CMV strain TB40/E-infected LC-derived mDCs at day 2 p.i. Filled histograms, mock-infected cells; open histograms, TB40/E-infected cells; gray line, isotype controls.
Attenuation of the alloreactive T-cell response.
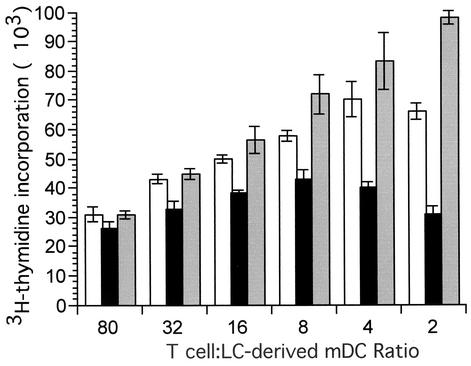
To examine the functional consequences of human CMV infection on the ability of LC-derived mDCs to stimulate T-cell proliferation, we performed an allostimulation assay (Fig. 5). Graded numbers of mock- and TB40/E-infected mDCs (from 103 to 4 × 104) collected at day 2 p.i. were irradiated and cocultivated with 8 × 104 allogeneic T cells for 3 days. As a positive control, mock-infected mDCs were treated with 10 μg of LPS/ml for 2 days prior to coculture with T cells. T-cell proliferation was assessed by the incorporation of [3H]thymidine. The capacity of infected mDCs to stimulate T-cell proliferation was reduced by approximately twofold compared to controls. As expected, T-cell proliferation observed with LPS-treated mDCs was greater than mock- or virus-infected mDCs. Results similar to these were obtained when T-cell proliferation was measured 1 day earlier (day 2), as well as 1 day later (day 4) (data not shown). Three independent experiments performed with mDCs derived from three different donors gave similar results. These data indicate that productively infected cultures of LC-derived mDCs have a decreased capacity to stimulate T cells. This CMV-dependent attenuation of the ability of these mDCs to stimulate a T-cell response may correspond to CMV-induced immune modulation during natural infection.
FIG. 5.
Human CMV infection of mDCs impairs their ability to stimulate T-cell proliferation. A representative experiment showing T-cell proliferation after coculture of 8 × 104 allogeneic T cells with various numbers of stimulatory mock-infected- or CMV strain TB40/E-infected LC-derived mDCs at day 2 p.i. Reduced T-cell proliferation was observed at the higher numbers of stimulator cells, suggesting that a subpopulation within the infected DC cultures may have been responsible for this activity. T-cell proliferation was measured as counts per minute of [3H]thymidine incorporated into nucleic acids. As a positive control, LC-derived mDCs were treated with 10 μg of LPS/ml for 2 days. White bars, mock-infected cells; black bars, TB40/E-infected cells; gray bars, LPS-treated cells. Means and standard deviations of [3H]thymidine incorporation from triplicate wells are shown.
DISCUSSION
Tissue-resident DCs, in particular LCs in the mucosal epithelia, are among the first cells to encounter CMV during natural infection. LCs are professional phagocytes and would be expected to carry virus and infected cell debris from the periphery to lymph nodes, where they mature and prime the T-cell immune response. The data we have presented suggests that the initial interaction with these immature DCs is nonproductive, but that susceptibility to virus infection is acquired as cells mature. LCs exposed to virus in the periphery may therefore either become permissive as they migrate to lymphoid tissues or act as vehicles bringing virus to susceptible LC-derived mDCs located in these tissues. The susceptibility of mDCs to infection by CMV and the consequent modulation of mDC function suggests that this interaction may promote dissemination, as well as delay the antiviral T-cell response, a combination that would facilitate the prolonged persistent replication that has been observed in infected individuals (54, 80).
Interestingly, our observations are in contrast to studies on PB monocyte-derived interstitial DCs, where immature cells have been shown to be permissive (25, 56) and maturation either decreases (43) or maintains (51) susceptibility to virus infection. Interstitial and LC-type DCs have been proposed to represent two distinct lineages (4) from which a number of mature DC types may arise depending on the signals (20, 34). These DC subsets differ in cell surface marker profiles and function (34). For example, PB monocyte-derived, interstitial DCs, produce IL-10, activate naive B cells and play a major role in humoral immune responses, whereas LCs produce less interleukin-10 (IL-10) and play major roles in priming CD8+-T-cell responses (9, 10, 34, 42). Source and maturation stimuli may influence the susceptibility of different DC lineages to CMV infection, a finding consistent with phenotypic variation observed when LCs are driven to mature with different inducers, TNF-α, LPS, or CD40L (20). These populations of mature DCs differ in their patterns of T-cell stimulation, IL-12 production, and intracellular localization of MHC proteins. Such a complexity in immature and mature DC types predicts that DCs may impact a variety of different stages of viral infection and latency, such as tissue sites of virus replication or persistence, dissemination via lymph and/or blood, as well as viral antigen presentation and clearance.
Here, we have studied an in vitro system. Evidence that DCs play important roles during natural infection has been suggested by studies evaluating murine CMV infection of mice. First, a DC-like mononuclear leukocyte is responsible for the dissemination of murine CMV (68). Second, interstitial DCs found in spleen are direct targets of infection (1). Similar to the behavior of human interstitial DCs (43), immature DCs appear susceptible to infection and maturation does not increase susceptibility. Third, after inoculation in footpads, virus-infected cells appear in the popliteal lymph node and reach maximal levels within a day (57), which suggests that a tissue-resident cell type carries infectious virus from the periphery into this organ. Fourth, DCs are the cell type most likely to be responsible for carrying virus from a site of inoculation to draining lymph nodes. CD40 deficient mice exhibit dramatically reduced levels of virus in popliteal lymph nodes compared to controls (S. A. Aguirre, J. Huang, and E. S. Mocarski, unpublished observations). Although LC-type DCs have not been studied directly in mice, roles for different DC populations in the initial stages of virus infection and dissemination are strongly supported by studies in this animal model.
Our results are consistent with models of DC function that have described functional differences of DCs derived from different tissue origins (34). Whereas immature PB monocyte-derived interstitial DCs support the replication of endotheliotropic strains of human CMV (25, 56), we found that fewer than 5% of immature CD34+ progenitor-derived LC-type DCs are susceptible to productive infection by these strains. Our finding that permissiveness develops during LC maturation into APC is reminiscent of observations on PB monocyte-derived macrophages (24, 31), where a role for cellular differentiation in susceptibility to CMV has been clearly established. Activated macrophages, but not unstimulated monocytes, are permissive hosts for CMV (24, 31, 66). Monocytes or myeloid progenitors in which the viral genome resides during lifelong latency gain susceptibility to virus replication when maturation is driven by proinflammatory cytokines (23, 24, 31, 38, 66), and these cytokines are a component of conditions that reactivate latent virus (65). In our experiments on LC-derived mDCs, maturation with the noninflammatory cytokine GM-CSF and the costimulatory inducer CD40L models a process that naturally occurs when LCs encounter virus in peripheral tissues and migrate to draining lymph nodes. Given the changes in susceptibility, LC-type DCs appear to be programmed to mature into virus-susceptible mDCs independent of proinflammatory cytokines. This dependence on maturation suggests that LC-type DCs may be an important reservoir of viral latency, where maturation may serve as a reactivation signal. Whether a common set of cellular factors contribute to susceptibility of both macrophages and mDCs remains to be determined. Ultimately, an understanding of the cellular factors dictating the behavior and the susceptibility of these cells will come from a better elucidation of the block to viral replication in immature cells that is relieved by maturation.
Our findings also suggest that the ability of different virus strains to infect and replicate in DCs depends on the complement of genes encoded by these strains. Endotheliotropic strains of this virus, typified by TB40/E and VHL/E, have an extended host cell range in cell culture compared to viruses propagated exclusively on HFs. Initially characterized by their ability to enter and replicate in endothelial cells (60) (59), these types of strains also retain maximal tropism for monocytes/macrophages (25), immature interstitial DCs (56) and, as shown here, mature LC-type DCs. Based on genome sequence analysis carried out on strains AD169, Towne, and Toledo (14, 15, 50), CMV strains propagated in HFs develop deletions and rearrangements within their genomes. This has the effect of removing or altering the normal complement of viral genes. It is highly likely that the differences observed here will map to specific genes encoded by endotheliotropic virus strains. Previous differences in the susceptibility of epithelial cells (7) shown for strain Toledo, compared to laboratory-adapted strains AD169varATCC or TownevarRIT3, appear to be unrelated to the ability to replicate in mDCs.
In all APC cell types examined to date, some of the cell surface proteins involved in antigen presentation have been shown to be modulated by productive viral infection. MHC class I levels have been consistently observed to be downmodulated in many cell types and are altered more than MHC class II levels in the LC-derived mDCs we have used, as well as in interstitial DCs studied by others (43, 51). Downmodulation of antigen presentation function would likely slow the priming of the CD8+-T-cell-mediated antiviral immune response and reduce the ability of T cells to recognize and clear virus-infected cells. MHC class I levels are controlled by the CMV US2, US3, US6, and US11 gene products via several distinct mechanisms (21, 39). These functions have been studied most extensively in cell types other than DCs, and only a subset of these may impact MHC class I levels in DCs (53).
CMV infection clearly downmodulates MHC class II levels on mDCs, although less dramatically than MHC class I levels, perhaps due to the longer half-life of MHC class II on the cell surface (13). CMV was shown to inhibit gamma interferon-induced MHC class II levels on endothelial cells and HFs (58) due to disruption of MHC class II transactivator (CIITA) expression (37). The ability to modulate constitutive levels of MHC class II has been observed more recently in infected macrophages (45) and can be modeled in U373 MG astrocytoma cells transduced with CIITA (12, 75). Evidence from these systems suggests that CMV may interfere with transcription of MHC class II genes, as well as trafficking of gene products. In contrast to implications of studies on host and viral IL-10 (52, 67), we do not find that reduced surface levels of either MHC class II or class I results from the production of a soluble factor by mDCs. Similar changes are not caused by exposure to supernatants from infected cultures or by treatment with recombinant human IL-10 (data not shown). Thus, it appears that a viral gene product directly modulates MHC class II in the mDCs we have studied here.
Viral downmodulation of MHC levels on infected mDCs would be predicted to hinder the priming of virus-specific CD8+ and CD4+ T cells and reduce the intensity of the effector cell response. Our finding that infection of mature LC-type DCs attenuates the alloreactive T-cell response indicates that CMV disrupts the productive interaction of DCs with T cells. Multiple mechanisms may underlie this effect (51). Consistent with the ability of the virus to disable critical APC functions of mDCs, there is growing evidence that the primary response to CMV is slow and that primary infection is prolonged in a manner distinct from other virus infections (54, 80). Nevertheless, large numbers of both effector and memory CD8+ and CD4+ T cells ultimately develop in healthy CMV-seropositive individuals and persist for life (3, 27, 72, 78). Cross-presentation (4) may be one host countermeasure (2, 73) balancing the direct modulation of mDCs by virus infection.
In addition to modulation of antigen-presenting proteins themselves, we observed changes in other mDC surface markers with immune function. Similar to findings in immature interstitial DCs (43), we observed a reduction in costimulatory molecules, with CD80 affected more than CD86, in CMV-infected cells. Most striking was the downmodulation of CD83, a result that differs from unchanged levels on infected mature interstitial DCs (51). CD83, a protein highly restricted to mature DCs whose cognate binding partner(s) are unknown, has been strongly implicated in DC-mediated T-cell activation (32), as well as in the maturation of CD4+ thymocytes (19). Infection of mature monocyte-derived DC with herpes simplex virus type 1 reduces the expression of CD83 by degradation (30). Exposure of immature interstitial DCs to supernatant from CMV-infected HFs has been reported to induce CD83 expression (2). It will be of interest to elucidate the mechanism(s) underlying the dramatic alteration of CD83 in mDCs and to determine whether this has functional consequences.
Acknowledgments
L.H. and V.G.L. contributed equally to this work.
We thank Stanley Plotkin, Christian Sinzger, and W. James Waldman for providing CMV strains Toledo, TB40/E, and VHL/E, respectively; David C. Johnson for kindly providing the US2N and US3N rabbit antisera; and Immunex Corporation for providing recombinant CD40L.
This work was supported by Public Health Service grants AI33852, CA49605 (E.S.M.), and AI48212 (E.D.M.) and by The Leukemia and Lymphoma Society (V.G.L.).
REFERENCES
- 1.Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077-1084. [DOI] [PubMed] [Google Scholar]
- 2.Arrode, G., C. Boccaccio, J. P. Abastado, and C. Davrinche. 2002. Cross-presentation of human cytomegalovirus pp65 (UL83) to CD8+ T cells is regulated by virus-induced, soluble-mediator-dependent maturation of dendritic cells. J. Virol. 76:142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asanuma, H., M. Sharp, H. T. Maecker, V. C. Maino, and A. M. Arvin. 2000. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J. Infect. Dis. 181:859-866. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 6.Bolovan-Fritts, C. A., E. S. Mocarski, and J. A. Wiedeman. 1999. Peripheral blood CD14+ cells from healthy subjects carry a circular conformation of latent cytomegalovirus genome. Blood 93:394-398. [PubMed] [Google Scholar]
- 7.Brown, J. M., H. Kaneshima, and E. S. Mocarski. 1995. Dramatic interstrain differences in the replication of human cytomegalovirus in SCID-hu mice. J. Infect. Dis. 171:1599-1603. [DOI] [PubMed] [Google Scholar]
- 8.Caux, C., C. Massacrier, B. Dubois, J. Valladeau, C. Dezutter-Dambuyant, I. Durand, D. Schmitt, and S. Saeland. 1999. Respective involvement of TGF-β and IL-4 in the development of Langerhans cells and non-Langerhans dendritic cells from CD34+ progenitors. J. Leukoc. Biol. 66:781-791. [DOI] [PubMed] [Google Scholar]
- 9.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, B. de Saint-Vis, C. Dezutter-Dambuyant, C. Jacquet, D. Schmitt, and J. Banchereau. 1997. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. Adv. Exp. Med. Biol. 417:21-25. [DOI] [PubMed] [Google Scholar]
- 10.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, I. Durand, M. Cella, A. Lanzavecchia, and J. Banchereau. 1997. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha. II. Functional analysis. Blood 90:1458-1470. [PubMed] [Google Scholar]
- 11.Caux, C., B. Vanbervliet, C. Massacrier, C. Dezutter-Dambuyant, B. de Saint-Vis, C. Jacquet, K. Yoneda, S. Imamura, D. Schmitt, and J. Banchereau. 1996. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J. Exp. Med. 184:695-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cebulla, C. M., D. M. Miller, Y. Zhang, B. M. Rahill, P. Zimmerman, J. M. Robinson, and D. D. Sedmak. 2002. Human cytomegalovirus disrupts constitutive MHC class II expression J. Immunol. 169:167-176. [DOI] [PubMed] [Google Scholar]
- 13.Cella, M., A. Engering, V. Pinet, J. Pieters, and A. Lanzavecchia. 1997. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 388:782-787. [DOI] [PubMed] [Google Scholar]
- 14.Cha, T. A., E. Tom, G. W. Kemble, G. M. Duke, E. S. Mocarski, and R. R. Spaete. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. I. Hutchison, T. Kouzarides, J. A. Martignetti, E. Preddie, S. C. Satchwell, P. Tomlinson, K. M. Weston, and B. G. Barrell. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-170. [DOI] [PubMed] [Google Scholar]
- 16.Chougnet, C., G. M. Shearer, and A. L. Landay. 2002. The role of antigen-presenting cells in HIV pathogenesis. Curr. Infect. Dis. Rep. 4:266-271. [DOI] [PubMed] [Google Scholar]
- 17.Courcelle, C. T., J. Courcelle, M. N. Prichard, and E. S. Mocarski. 2001. Requirement for uracil-DNA glycosylase during the transition to late-phase cytomegalovirus DNA replication. J. Virol. 75:7592-7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flores-Romo, L. 2001. In vivo maturation and migration of dendritic cells. Immunology 102:255-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimoto, Y., L. Tu, A. S. Miller, C. Bock, M. Fujimoto, C. Doyle, D. A. Steeber, and T. F. Tedder. 2002. CD83 expression influences CD4+ T-cell development in the thymus. Cell 108:755-767. [DOI] [PubMed] [Google Scholar]
- 20.Gatti, E., M. A. Velleca, B. C. Biedermann, W. Ma, J. Unternaehrer, M. W. Ebersold, R. Medzhitov, J. S. Pober, and I. Mellman. 2000. Large-scale culture and selective maturation of human Langerhans cells from granulocyte colony-stimulating factor-mobilized CD34+ progenitors. J. Immunol. 164:3600-3607. [DOI] [PubMed] [Google Scholar]
- 21.Gewurz, B. E., R. Gaudet, D. Tortorella, E. W. Wang, and H. L. Ploegh. 2001. Virus subversion of immunity: a structural perspective. Curr. Opin. Immunol. 13:442-450. [DOI] [PubMed] [Google Scholar]
- 22.Girolomoni, G., C. Caux, S. Lebecque, C. Dezutter-Dambuyant, and P. Ricciardi-Castagnoli. 2002. Langerhans cells: still a fundamental paradigm for studying the immunobiology of dendritic cells. Trends Immunol. 23:6-8. [DOI] [PubMed] [Google Scholar]
- 23.Hahn, G., R. Jores, and E. S. Mocarski. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 95:3937-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibanez, C. E., R. Schrier, P. Ghazal, C. Wiley, and J. A. Nelson. 1991. Human cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 65:6581-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jahn, G., S. Stenglein, S. Riegler, H. Einsele, and C. Sinzger. 1999. Human cytomegalovirus infection of immature dendritic cells and macrophages. Intervirology 42:365-372. [DOI] [PubMed] [Google Scholar]
- 26.Jones, T. R., and L. Sun. 1997. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J. Virol. 71:2970-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kern, F., E. Khatamzas, I. Surel, C. Fr#mmel, P. Reinke, S. L. Waldrop, L. J. Picker, and H. D. Volk. 1999. Distribution of human CMV-specific memory T cells among the CD8(+) subsets defined by CD57, CD27, and CD45 isoforms. Eur. J. Immunol. 29:2908-2915. [DOI] [PubMed] [Google Scholar]
- 28.Kondo, K., H. Kaneshima, and E. S. Mocarski. 1994. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. USA 91:11879-11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo, K., J. Xu, and E. S. Mocarski. 1996. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc. Natl. Acad. Sci. USA 93:11137-11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruse, M., O. Rosorius, F. Kratzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber, and A. Steinkasserer. 2000. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J. Virol. 74:7127-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lathey, J. L., and S. A. Spector. 1991. Unrestricted replication of human cytomegalovirus in hydrocortisone-treated macrophages. J. Virol. 65:6371-6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lechmann, M., D. J. Krooshoop, D. Dudziak, E. Kremmer, C. Kuhnt, C. G. Figdor, G. Schuler, and A. Steinkasserer. 2001. The extracellular domain of CD83 inhibits dendritic cell-mediated T-cell stimulation and binds to a ligand on dendritic cells. J. Exp. Med. 194:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin, M. J., C. R. Rinaldo, Jr., P. L. Leary, J. A. Zaia, and M. S. Hirsch. 1979. Immune response to herpesvirus antigens in adults with acute cytomegaloviral mononucleosis. J. Infect. Dis. 140:851-857. [DOI] [PubMed] [Google Scholar]
- 34.Liu, Y. J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106:259-262. [DOI] [PubMed] [Google Scholar]
- 35.Marie, J. C., J. Kehren, M. C. Trescol-Biemont, A. Evlashev, H. Valentin, T. Walzer, R. Tedone, B. Loveland, J. F. Nicolas, C. Rabourdin-Combe, and B. Horvat. 2001. Mechanism of measles virus-induced suppression of inflammatory immune responses. Immunity 14:69-79. [DOI] [PubMed] [Google Scholar]
- 36.Mendelson, M., S. Monard, P. Sissons, and J. Sinclair. 1996. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J. Gen. Virol. 77:3099-3102. [DOI] [PubMed] [Google Scholar]
- 37.Miller, D. M., B. M. Rahill, J. M. Boss, M. D. Lairmore, J. E. Durbin, J. W. Waldman, and D. D. Sedmak. 1998. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J. Exp. Med. 187:675-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minton, E. J., C. Tysoe, J. H. Sinclair, and J. G. Sissons. 1994. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J. Virol. 68:4017-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mocarski, E. S. 2002. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 10:332-339. [DOI] [PubMed] [Google Scholar]
- 40.Mocarski, E. S., Jr., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication., p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 41.Mocarski, E. S., L. Pereira, and N. Michael. 1985. Precise localization of genes on large animal virus genomes: use of lambda gt11 and monoclonal antibodies to map the gene for a cytomegalovirus protein family. Proc. Natl. Acad. Sci. USA 82:1266-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mortarini, R., A. Anichini, M. Di Nicola, S. Siena, M. Bregni, F. Belli, A. Molla, A. M. Gianni, and G. Parmiani. 1997. Autologous dendritic cells derived from CD34+ progenitors and from monocytes are not functionally equivalent antigen-presenting cells in the induction of melan-A/Mart-1(27-35)-specific CTLs from peripheral blood lymphocytes of melanoma patients with low frequency of CTL precursors. Cancer Res. 57:5534-5541. [PubMed] [Google Scholar]
- 43.Moutaftsi, M., A. M. Mehl, L. K. Borysiewicz, and Z. Tabi. 2002. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99:2913-2921. [DOI] [PubMed] [Google Scholar]
- 44.Naniche, D., and M. B. Oldstone. 2000. Generalized immunosuppression: how viruses undermine the immune response. Cell Mol. Life Sci. 57:1399-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Odeberg, J., and C. Soderberg-Naucler. 2001. Reduced expression of HLA class II molecules and interleukin-10- and transforming growth factor β1-independent suppression of T-cell proliferation in human cytomegalovirus-infected macrophage cultures. J. Virol. 75:5174-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palucka, K., and J. Banchereau. 2002. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 14:420-431. [DOI] [PubMed] [Google Scholar]
- 47.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In D. Knipe and P. Howley (ed.), Fields virology. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 48.Plachter, B., W. Britt, R. Vornhagen, T. Stamminger, and G. Jahn. 1993. Analysis of proteins encoded by IE regions 1 and 2 of human cytomegalovirus using monoclonal antibodies generated against recombinant antigens. Virology 193:642-652. [DOI] [PubMed] [Google Scholar]
- 49.Plotkin, S. A., S. E. Starr, H. M. Friedman, E. Gonczol, and R. E. Weibel. 1989. Protective effects of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. J. Infect. Dis. 159:860-865. [DOI] [PubMed] [Google Scholar]
- 50.Prichard, M. N., M. E. Penfold, G. M. Duke, R. R. Spaete, and G. W. Kemble. 2001. A review of genetic differences between limited and extensively passaged human cytomegalovirus strains. Rev. Med. Virol. 11:191-200. [DOI] [PubMed] [Google Scholar]
- 51.Raftery, M. J., M. Schwab, S. M. Eibert, Y. Samstag, H. Walczak, and G. Schonrich. 2001. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15:997-1009. [DOI] [PubMed] [Google Scholar]
- 52.Redpath, S., A. Angulo, N. R. Gascoigne, and P. Ghazal. 1999. Murine cytomegalovirus infection downregulates MHC class II expression on macrophages by induction of IL-10. J. Immunol. 162:6701-6707. [PubMed] [Google Scholar]
- 53.Rehm, A., A. Engelsberg, D. Tortorella, I. J. Korner, I. Lehmann, H. L. Ploegh, and U. E. Hopken. 2002. Human cytomegalovirus gene products US2 and US11 differ in their ability to attack major histocompatibility class I heavy chains in dendritic cells. J. Virol. 76:5043-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Revello, M. G., M. Zavattoni, A. Sarasini, E. Percivalle, L. Simoncini, and G. Gerna. 1998. Human cytomegalovirus in the blood of immunocompetent persons during primary infection: prognostic implications for pregnancy. J. Infect. Dis. 177:1170-1175. [DOI] [PubMed] [Google Scholar]
- 55.Riddell, S. R., and P. D. Greenberg. 1995. Principles for adoptive T-cell therapy of human viral diseases. Annu. Rev. Immunol. 13:545-586. [DOI] [PubMed] [Google Scholar]
- 56.Riegler, S., H. Hebart, H. Einsele, P. Brossart, G. Jahn, and C. Sinzger. 2000. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Gen. Virol. 81:393-399. [DOI] [PubMed] [Google Scholar]
- 57.Saederup, N., S. A. Aguirre, T. E. Sparer, D. M. Bouley, and E. S. Mocarski. 2001. Murine cytomegalovirus CC chemokine homolog MCK-2 (m131-129) is a determinant of dissemination that increases inflammation at initial sites of infection. J. Virol. 75:9966-9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sedmak, D. D., A. M. Guglielmo, D. A. Knight, D. J. Birmingham, E. H. Huang, and W. J. Waldman. 1994. Cytomegalovirus inhibits major histocompatibility class II expression on infected endothelial cells. Am. J. Pathol. 144:683-692. [PMC free article] [PubMed] [Google Scholar]
- 59.Sinzger, C., and G. Jahn. 1996. Human cytomegalovirus cell tropism and pathogenesis. Intervirology 39:302-319. [DOI] [PubMed] [Google Scholar]
- 60.Sinzger, C., M. Kahl, K. Laib, K. Klingel, P. Rieger, B. Plachter, and G. Jahn. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 81:3021-3035. [DOI] [PubMed] [Google Scholar]
- 61.Sinzger, C., B. Plachter, A. Grefte, T. H. The, and G. Jahn. 1996. Tissue macrophages are infected by human cytomegalovirus in vivo. J. Infect. Dis. 173:240-245. [DOI] [PubMed] [Google Scholar]
- 62.Skaletskaya, A., L. M. Bartle, T. Chittenden, A. L. McCormick, E. S. Mocarski, and V. S. Goldmacher. 2001. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA 98:7829-7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slobedman, B., and E. S. Mocarski. 1999. Quantitative analysis of latent human cytomegalovirus. J. Virol. 73:4806-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1998. Growth of human cytomegalovirus in primary macrophages. Methods 16:126-138. [DOI] [PubMed] [Google Scholar]
- 65.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Reactivation of latent cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 66.Soderberg-Naucler, C., D. N. Streblow, K. N. Fish, J. Allan-Yorke, P. P. Smith, and J. A. Nelson. 2001. Reactivation of latent human cytomegalovirus in CD14+ monocytes is differentiation dependent. J. Virol. 75:7543-7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spencer, J. V., K. M. Lockridge, P. A. Barry, G. Lin, M. Tsang, M. E. Penfold, and T. J. Schall. 2002. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 76:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stoddart, C. A., R. D. Cardin, J. M. Boname, W. C. Manning, G. B. Abenes, and E. S. Mocarski. 1994. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J. Virol. 68:6243-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strobl, H., C. Bello-Fernandez, E. Riedl, W. F. Pickl, O. Majdic, S. D. Lyman, and W. Knapp. 1997. flt3 ligand in cooperation with transforming growth factor-β1 potentiates in vitro development of Langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood 90:1425-1434. [PubMed] [Google Scholar]
- 70.Strobl, H., E. Riedl, C. Bello-Fernandez, and W. Knapp. 1998. Epidermal Langerhans cell development and differentiation. Immunobiology 198:588-605. [DOI] [PubMed] [Google Scholar]
- 71.Strobl, H., E. Riedl, C. Scheinecker, C. Bello-Fernandez, W. F. Pickl, K. Rappersberger, O. Majdic, and W. Knapp. 1996. TGF-β1 promotes in vitro development of dendritic cells from CD34+ hemopoietic progenitors J. Immunol. 157:1499-1507. [PubMed] [Google Scholar]
- 72.Suni, M. A., L. J. Picker, and V. C. Maino. 1998. Detection of antigen-specific T-cell cytokine expression in whole blood by flow cytometry J. Immunol. Methods 212:89-98. [DOI] [PubMed] [Google Scholar]
- 73.Tabi, Z., M. Moutaftsi, and L. K. Borysiewicz. 2001. Human cytomegalovirus pp65- and immediate-early 1 antigen-specific HLA class I-restricted cytotoxic T-cell responses induced by cross-presentation of viral antigens. J. Immunol. 166:5695-5703. [DOI] [PubMed] [Google Scholar]
- 74.Tenney, D. J., and A. M. Colberg-Poley. 1991. Human cytomegalovirus UL36-38 and US3 immediate-early genes: temporally regulated expression of nuclear, cytoplasmic, and polysome-associated transcripts during infection J. Virol. 65:6724-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomazin, R., J. Boname, N. R. Hegde, D. M. Lewinsohn, Y. Altschuler, T. R. Jones, P. Cresswell, J. A. Nelson, S. R. Riddell, and D. C. Johnson. 1999. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 5:1039-1043. [DOI] [PubMed] [Google Scholar]
- 76.Viola, A., G. Iezzi, and A. Lanzavecchia. 1999. The role of dendritic cells in T-cell priming: the importance of being professional, p. 251-255. In M. T. Lotze and A. W. Thomson (ed.), In Dendritic cells: biology and clinical applications. Academic Press, Inc., New York, N.Y.
- 77.Waldman, W. J., W. H. Roberts, D. H. Davis, M. V. Williams, D. D. Sedmak, and R. E. Stephens. 1991. Preservation of natural endothelial cytopathogenicity of cytomegalovirus by propagation in endothelial cells. Arch. Virol. 117:143-164. [DOI] [PubMed] [Google Scholar]
- 78.Waldrop, S. L., K. A. Davis, V. C. Maino, and L. J. Picker. 1998. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J. Immunol. 161:5284-5295. [PubMed] [Google Scholar]
- 79.Weiland, K. L., N. L. Oien, F. Homa, and M. W. Wathen. 1994. Functional analysis of human cytomegalovirus polymerase accessory protein. Virus Res. 34:191-206. [DOI] [PubMed] [Google Scholar]
- 80.Zanghellini, F., S. B. Boppana, V. C. Emery, P. D. Griffiths, and R. F. Pass. 1999. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J. Infect. Dis. 180:702-707. [DOI] [PubMed] [Google Scholar]