Abstract
Wild-type herpes simplex virus type 1 (HSV-1) infection triggers apoptosis in human cells. The subsequent synthesis of infected cell proteins between 3 and 6 h postinfection (hpi) acts to block this process from killing the cells. The factors produced during this window also prevent cell death induced by environmental staurosporine or sorbitol (M. Aubert, J. O'Toole, and J. A. Blaho, J. Virol. 73:10359-10370, 1999). We now report that (i) during the prevention window, HSV-1(F) also inhibited apoptosis induced by tumor necrosis factor alpha (TNF-α) plus cycloheximide (CHX) treatment. While deciphering the mechanism of this inhibition, we observed that (ii) the transcription factor NF-κB translocated from the cytoplasm into the nuclei of infected cells, and (iii) this migration initiated at 3 hpi. (iv) The complete inhibition of protein synthesis at 3 hpi by the addition of CHX precluded NF-κB translocation, while CHX additions at 6 hpi or later did not elicit this effect. This result confirms that infected cell protein synthesis is required for the nuclear import of NF-κB. (v) The detection of NF-κB in nuclei correlated with the ability of HSV-1(F), HSV-1(KOS1.1), or HSV-1(R7032), a replication-competent recombinant virus containing a deletion in the gene encoding the gE glycoprotein, to prevent apoptosis. (vi) NF-κB did not bind its κB DNA recognition site and remained cytoplasmic in cells actively undergoing apoptosis following infection with HSV-1(vBSΔ27), a virus with the key regulatory protein ICP27 deleted. (vii) Prestimulation of NF-κB by the addition of a phorbol ester prevented HSV-1(vBSΔ27)-induced apoptosis. (viii) Retention of NF-κB in the cytoplasm by the addition of a pharmacological antagonist of its release from IκBα led to an increase in death factor processing during HSV-1(F) infection. (ix) A novel HEp-2 clonal cell line, termed IκBαDN, was generated which expresses a dominant-negative form of IκBα. Treatment of IκBαDN cells with TNF-α in the absence of CHX resulted in apoptotic death due to the inability of NF-κB to become activated in these cells. Finally, (x) infection of IκBαDN cells with HSV-1(F) or HSV-1(KOS1.1) resulted in apoptosis, demonstrating that (xi) the nuclear translocation of NF-κB between 3 and 6 hpi (the prevention window) is necessary to prevent apoptosis in wild-type HSV-1-infected human HEp-2 cells.
Herpes simplex virus type 1 (HSV-1) is a large, enveloped, double-stranded DNA virus with a genome of approximately 150 kbp that encodes over 80 proteins, many of whose functions are still unknown (reviewed in reference 48). Productive HSV-1 infection occurs in a sequentially ordered cascade in which α (immediate-early or IE) proteins precede the synthesis of β (early or E) and γ (late or L) proteins (22, 23). During the course of lytic infection, HSV-1 takes over the host cell, subjugating the cell's biomolecular synthesis machinery to serve its own reproduction. Cells react to viral infection by attempting to block viral replication, undergoing apoptosis, or by signaling neighboring cells to activate antiviral systems (reviewed in reference 31).
The interplay between HSV-1 and its host involves numerous factors, and the virus employs several mechanisms to combat the many antiviral responses enacted by an infected cell. One of the most dramatic cellular responses to viral infection is the induction of apoptosis or programmed cell death. Apoptosis is characterized by cell shrinkage, membrane blebbing, redistribution of phosphatidylserine to the outer leaflet of the plasma membrane, fragmentation of nuclei (karyorrhexis), chromosomal DNA (pyknosis), and oligosomal DNA laddering (29, 30, 58). These structural modifications are a consequence of the activation of effector (executioner) caspases (cysteine aspartases), one of which is caspase 3, and the cleavage of poly(ADP-ribose) polymerase (PARP) and structural proteins (29, 30, 39, 57, 58). Apoptosis functions to limit virus spread by preventing viral replication, saving other cells from infection (34). HSV-1 has developed a mechanism to counteract this antiviral cell death process (3).
The interaction of HSV-1 with its host cell results in the triggering of the apoptotic cell death program (2, 4, 33). During infection by wild-type HSV-1, caspase 3 is activated (4), the death factor DFF45 is completely cleaved (4), and phosphatidylserine is flipped from the inner to the outer membrane leaflet (25), indicating that replication-competent HSV-1 triggers the apoptosis death program and infected cells are actively in an apoptotic state. However, cells infected with wild-type HSV-1 do not show features of apoptosis, because infected cell proteins produced between 3 and 6 h postinfection (hpi), termed the apoptosis “prevention window” (4), prevent the process from killing the cells (4). Thus, human HEp-2 cells infected with either (i) viruses containing a deletion in the key viral regulators ICP4 or ICP27 or (ii) wild-type virus plus the addition of cycloheximide (CHX) at the time of infection, die by apoptosis (2, 33, 37). Although most recent efforts have focused on identifying viral gene products involved in apoptosis prevention during infection, such as ICP4, ICP27, and US3 (2, 4, 5, 24, 37, 38, 59), little is known of the role that cellular survival factors play in the process. While it had been shown by this lab and others that HSV-1 infection could block apoptosis induced by extracellular stimulants such as tumor necrosis factor alpha (TNF-α), anti-FAS antibody, CHX, staurosporine, ceramide, osmotic shock using sorbitol or ethanol, and hyperthermia (2, 4, 19, 25, 33, 35, 38), we demonstrated that infected cell proteins produced during the apoptosis prevention window inhibited cell death triggered by staurosporine and sorbitol (4).
It was our initial goal to determine whether the ability of wild-type HSV-1 to block the effects of TNF-α treatment was dependent on factors produced during the apoptosis prevention window. In response to TNF-α treatment, initiator caspases, such as caspase 8, activate the executioner caspases to effect apoptosis, unless this process is inhibited by the actions of the transcriptional regulatory factor NF-κB; IκB modulates the activity of NF-κB by retaining it in the cytoplasm (8, 9, 27, 39, 40, 54, 55, 57). During the course of our studies, we observed that NF-κB began to translocate to the nuclei of infected HEp-2 cells during the time period in which the apoptosis-preventing infected cell proteins were produced. While earlier reports (1, 45) have described the nuclear location of NF-κB during infection, the consequences of this activation for the infected cell remain unknown. Thus, our goal was to determine whether NF-κB is associated with apoptosis prevention by HSV-1. Based on our findings, it is our contention that the translocation of NF-κB to the nuclei of infected cells is a necessary component of the apoptosis prevention process during HSV-1 infection.
MATERIALS AND METHODS
Cell lines and viruses.
Vero and HEp-2 cells were obtained from the American Type Culture Collection (Rockville, Md.). All cells were maintained in Dulbecco's modified Eagle's medium containing 5% fetal bovine serum (5% FBS). The 2.2 cells are a derivative Vero cell line expressing the HSV-1 immediate-early protein ICP27 under its own promoter and were originally obtained from Roz Sandri-Goldin (University of California, Irvine) (51). The Vero 2.2 cells used in this study were provided by Saul Silverstein (Columbia University) and were maintained in 5% FBS containing 300 μg of G418/ml (2). The HSV-1(F) and HSV-1(R7032) viruses were obtained from Bernard Roizman (University of Chicago). HSV-1(F) is the wild-type strain used in the majority of these studies. HSV-1(R7032) is a recombinant virus derived from HSV-1(F) and contains a deletion in the gene encoding glycoprotein E (gE) (41). The wild-type HSV-1(KOS1.1) and recombinant mutant HSV-1(vBSΔ27) viruses were provided by Saul Silverstein. vBSΔ27 is an ICP27-null mutant derived from KOS1.1 that contains a replacement of the α27 gene with the Escherichia coli lacZ gene and must be propagated on an ICP27-complementing cell line such as Vero 2.2 (52). HEp-2 cell monolayers to be infected were maintained in Dulbecco's modified Eagle's medium containing 5% newborn calf serum (5% NBCS) for 24 h prior to infection. Cells were infected with multiplicities of infection (MOIs) of either 5 or 10 PFU/cell. Standard HSV-1 inoculations were carried out in 199 medium containing 2% FBS (199V) at 37°C for 1 h. In experiments with vBSΔ27, adsorptions were carried out for 2 h. Following adsorption, infected cells were maintained in 5% NBCS for the various times indicated in the text. All tissue culture reagents were purchased from Life Technologies.
Preparation of infected cell extracts.
Infected cells were harvested by scraping directly into the medium. After low-speed centrifugation at 800 × g, cell pellets were washed in phosphate-buffered saline (PBS) containing a 10 μM concentration each of the protease inhibitors N-tosyl-l-phenyl-alanine-chloromethylketone (TPCK), phenylmethylsulfonyl fluoride (PMSF), and tosyl-l-lysine-chloromethylketone (TLCK) (PBS*). Cells were resuspended in a lysis solution of 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA containing TPCK, TLCK, and PMSF (buffer A*). Infected cells were disrupted by sonication on ice three times for 10 s at an output level of 2 with a Branson sonifier. The protein concentration of each sample was determined by a modified Bradford protein assay as recommended by the manufacturer (Bio-Rad). All biochemical reagents were obtained from Sigma unless stated otherwise in the text.
Preparation of cytoplasmic and nuclear fractions.
The subfractionation of infected cells into nuclear and cytoplasmic extracts was performed as previously described (12, 47). All manipulations were performed on ice. Cytoplasmic and nuclear extracts were made at the times postinfection indicated in the text. Infected cells were scraped directly into the medium and pelleted by centrifugation at 800 × g for 3 min. Resuspended pellets were washed once with PBS*, transferred to a 1.5-ml tube, and pelleted again in a microcentrifuge at 1,000 × g for 3 min. Cells were lysed by gentle inversion after resuspending in 150 μl of PBS* containing 0.4% NP-40, followed by recentrifuging for 3 min. The supernatants (cytoplasmic extract) were removed and placed in fresh tubes. The nuclear pellets were washed once with 100 μl of PBS* containing 0.1% NP-40, pelleted at 1,000 × g for 3 min, and resuspended in 100 μl of PBS* containing 0.4% NP-40. Mixtures containing nuclei were frozen at −80°C for at least 30 min and rethawed on ice, as this increases the extraction of nuclear proteins from the insoluble material (M. L. Goodkin and J. A. Blaho, unpublished results). Finally, the nuclear samples were sonicated on ice three times for 5 s, and these constituted the nuclear fractions. In experiments where whole extracts and subcellular (cytoplasmic and nuclear) fractions were prepared from the same cells, infected cells were resuspended in 250 μl of PBS* and 50 and 200 μl were processed, respectively.
Denaturing gel electrophoresis and immunoblotting techniques.
Approximately 50 μg of infected cell proteins from whole extracts or subcellular fractions was electrophoretically separated in 15% sodium dodecyl sulfate-polyacrylamide gels cross-linked with N,N′-diallyltartardiamide (12), electrically transferred to nitrocellulose using a tank apparatus (Bio-Rad), and probed for a minimum of 2 h with the appropriate primary antibodies described below. Secondary goat anti-rabbit and anti-mouse antibodies conjugated to alkaline phosphatase were obtained from Southern Biotechnology. Anti-rabbit or anti-mouse antibodies conjugated to horseradish peroxidase were used for chemiluminescence detection as recommended by the vendor (Amersham). Specific proteins were detected following development with chemiluminescence reagents (Amersham) and autoradiography at 25°C using X-Omat film (Kodak, Rochester, N.Y.). Prestained molecular mass markers (GibcoBRL) were included in all acrylamide gels (lanes are not shown in most figures). Equal protein loadings were determined by Ponceau S staining prior to immunoblotting. All immunoblots and autoradiograms were digitized at 600 dots per inch using an AGFA Arcus II scanner linked to a Macintosh G3 PowerPC workstation. Raw digital images, saved as tagged image files using Adobe Photoshop, were organized into figures using Adobe Illustrator. Grayscale prints of figures were obtained using a Codonics dye-sublimation printer.
Immunological reagents.
The antibodies used to detect viral and cellular proteins were the following. RGST22 is a rabbit polyclonal antibody specific for ICP22 (14). RGST49 is a rabbit polyclonal antibody specific for VP22 (10). 1114 is a mouse monoclonal antibody specific for ICP4 (Goodwin Institute for Cancer Research). Mouse monoclonal antibodies specific for the p65 subunit of NF-κB, IκBα, caspase 3, and PARP were obtained from Santa Cruz Biotechnology, Imgenex, Transduction Laboratories, and Pharmingen, respectively. Full-length PARP (116 kDa) is cleaved during the execution phase of apoptosis, and our antibody reacts with both the 116-kDa form and its 85-kDa cleavage product (4, 28). PARP cleavage was quantitated (4, 28) using the public domain NIH Image program (developed at the National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). Background was subtracted using the 2D rolling ball and 2D streak removal filters. Densities of the 116- and 85-kDa bands were measured, and the percentage of PARP cleavage was calculated as follows: {(density of 85-kDa band)/[(density of 85-kDa band) + (density of 116-kDa band)]} × 100. During apoptosis, the inactive form of caspase 3 (procaspase 3) becomes cleaved. While our antibody recognizes both the uncleaved form (32 kDa) and one of the processed forms (20 kDa), under our gel electrophoresis conditions only the uncleaved (inactive) form is resolved. Therefore, loss of caspase 3 reactivity corresponds to apoptosis induction in our system (4). For indirect immunofluorescence, anti-NF-κB p65 subunit, ICP22, and VP22 antibodies were used at a dilution of 1:1,000 in PBS containing 1% bovine serum albumin (BSA). Fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (Boehringer Mannheim), diluted 1:1,000, and tetramethylrhodamine-isothiocyanate (Texas Red)-conjugated goat anti-rabbit immunoglobulin G (Vector Laboratories), diluted 1:150, were used as secondary antibodies.
Indirect immunofluorescence and microscopy.
Indirect immunofluorescence experiments were performed as previously described (47). Infected cells grown on glass coverslips in 35-mm dishes were washed with PBS and fixed using 2.5% methanol-free formaldehyde (Polysciences) in PBS. After 20 min of incubation in formaldehyde, cells were washed twice with PBS, permeabilized with 100% acetone at −20°C for 4 min, and washed twice again with PBS. Because HSV-1-infected cells express the viral gE/gI Fc receptor complex (26), it was necessary to block any nonspecific antibody binding that the complex may cause by incubating in PBS containing 1% BSA plus 10 μg of pooled human immunoglobulin (Sigma)/ml for at least 1 h (32). Fixed, blocked cells were incubated with primary antibodies in 1% BSA for 1 h. Cells were washed twice with PBS and incubated in the dark for 1 h with secondary antibody. Finally, cells were washed twice with PBS and mounted onto glass microscope slides using a 0.1% Mowiol solution (Sigma) containing 2.5% DABCO as the antibleaching agent. The edges of the coverslips were sealed with clear nail polish to keep the slips in place, and the slides were stored at 4°C. Chromatin condensation was visualized using 0.05 μg of Hoechst 33258 (Sigma)/ml as previously described (2). Hoechst dye was added to cells 1 h prior to fluorescence microscopy. The number of apoptotic cells with condensed chromatin and fragmented nuclei, as well as the total number of cells in representative fields, were counted. Mean values were determined from two independent experiments in which a minimum of 300 and a maximum of 500 cells were counted for each condition. The percentage of apoptotic cells was calculated as follows: (number of apoptotic cells/total number of cells) × 100. Fluorescence was observed with an Olympus IX70/IX-FLA inverted fluorescence microscope, and images were acquired using a Sony DKC-5000 digital photo camera linked to a PowerMac G3 and processed through Adobe Photoshop.
Preparation of infected cell nuclear fractions for EMSA.
Infected cells were scraped directly into the medium and pelleted by centrifugation at 800 × g for 3 min. Pellets were washed once with cold PBS, transferred to a 1.5-ml tube, and spun down in a microcentrifuge at 1,000 × g for 30 s. Cells were resuspended in 400 μl of electrophoretic mobility shift assay (EMSA) buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA) containing 1 mM dithiothreitol (DTT) and 1 mM PMSF and incubated on ice for 15 min, after which 25 μl of 10% NP-40 was added. The mixture was vortexed vigorously for 10 s and centrifuged at 1,000 × g for 1 min. The supernatant was carefully removed and discarded. The nuclear pellet was washed once by layering 1 ml of cold EMSA buffer A over it and pelleting again for 1 min. The supernatant was again removed and discarded, while the pellet was centrifuged again to remove any remaining supernatant. Next, 50 μl of cold EMSA buffer C (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA) containing 1 mM DTT and 1 mM PMSF was added to the nuclear pellet. The nuclei were gently tapped until they were able to float and were rotated for 30 min at 4°C. Following this extraction step, the mixture was centrifuged at 1,000 × g for 1 min, the supernatant (nuclear extract) was transferred to a new 1.5-ml tube, and aliquots were stored at −70°C.
EMSA.
The DNA probe for EMSA studies was generated by annealing two complementary oligonucleotides (forward, 5′-CTA GTG GGG ACT TTC CAC CTG GGG ACT TTC CAC CT-3′; reverse, 5′-CTA GAG GTG GAA AGT CCC CAG GTG GAA AGT CCC CA-3′) and 5′-end labeling with [γ-32P]ATP (6,000 Ci/mmol; Amersham) and T4 polynucleotide kinase (New England Biolabs) to an average activity of approximately 200,000 cpm/ng of oligonucleotide fragment. Aliquots of nuclear extract protein (approximately 3 μg) were added to a mixture of EMSA binding buffer (5% glycerol, 1 mM EDTA, 50 mM NaCl, 10 mM Tris-HCl [pH 7.5]) containing 10 mM DTT and 4 μg of poly(dI-dC) (Sigma) and allowed to incubate at 25°C for 10 min. Approximately 0.5 ng of radiolabeled DNA probe was added, and the samples were allowed to incubate for an additional 30 min at the same temperature. For “super-shifting” experiments, 0.4 μg of anti-NF-κB p65 antibody was added to the reaction mixture and allowed to incubate at 25°C for 10 min prior to the addition of radiolabeled DNA. Samples were electrophoretically separated in a 5% nondenaturing polyacrylamide gel and dried under vacuum at 80°C for 75 min, and DNA-protein complexes were visualized by autoradiography. Unlabeled wild-type and mutant probes were used to competitively inhibit oligonucleotide binding. The sequences of the mutant oligonucleotides were as follows (mutated residues are indicated with bold type): forward, 5′-CTA GTG GCG ACT TTC CAC CTG GCG ACT TTC CAC CT-3′; reverse, 5′-CTA GAG GTG GAA AGT CGC CAG GTG GAA AGT CGC CA-3′.
Plasmids used for transfections and the generation of stable cell lines.
A dominant-negative IκBα mutant containing serine-alanine substitutions at positions 32 and 36 (16, 53) was subcloned into the Peak8 vector, which contains the gene for puromycin resistance (Edge Biosystems, Gaithersburg, Md.), and expressed under the control of the Ef1α promoter to generate plasmid pPEAK8-IκBαDN. Plasmids generated using the same vector but containing green fluorescent protein (GFP) or CD14 in the polylinker site were used as controls (pPEAK8-GFP and pPEAK8-CD14, respectively). Plasmid DNA (10 μg/10-cm dish) was introduced into HEp-2 cells by using the calcium phosphate transfection method (46), and the cells were maintained in 5% FBS. After 3 days, the medium was changed to 5% FBS containing 1 μg of puromycin/ml to select for transfected cells and maintained in this medium for 5 days. At the same time, a titration of HEp-2 cell susceptibility to puromycin indicated that a concentration of 2 μg/ml was sufficient to kill nontransfected cells within 3 to 4 days. The concentration of puromycin used in the medium for the transfected cells was then increased to 2 μg/ml at 3 days posttransfection, and the cells were maintained at this level for 2 weeks, changing the medium every 4 to 5 days. The observation of green fluorescence in the pPeak8-GFP-transfected cells indicated the presence of stably transfected cultures.
Biochemical and pharmacological (i) inhibition of infected cell protein synthesis, (ii) induction of apoptosis, (iii) inhibition of NF-κB translocation, and (iv) activation of NF-κB in HEp-2 cells.
CHX addition at the time of HSV-1 infection inhibits infected cell protein synthesis, ultimately leading to apoptosis of human HEp-2 cells (2, 33). The time course of the CHX addition experiment that was used to define the HSV-1 apoptosis prevention window was described in detail previously (4). The basis of this technique is the following. Wild-type HSV-1 triggers apoptosis, but infected cell proteins produced after 3 hpi prevent the process from killing the cell. Thus, CHX addition to an HSV-1 infection at 3 hpi or before leads to apoptosis, while CHX addition at 6 hpi or later does not. At the times indicated below in Results, CHX (10 μg/ml) in 5% NBCS was added to infected cells and maintained to 18 hpi. As a positive control to induce apoptosis, TNF-α (10 ng/ml) and CHX were added to mock-infected HEp-2 cells. Lyophilized TNF-α (Sigma) was dissolved in sterile PBS containing 1% BSA. Aliquots of TNF-α stocks at a concentration of 10 μg/ml were stored at −80°C and thawed once prior to using. The antioxidant and metal chelator pyrrolidine dithiocarbamate (PDTC) is a potent NF-κB inhibitor (15, 36, 50). PDTC (Sigma) was dissolved directly in 5% NBCS medium at a final concentration of 400 μM. This concentration was empirically determined to be the optimal amount necessary to prevent NF-κB nuclear import induced by TNF-α treatment in HEp-2 cells (M. L. Goodkin and J. A. Blaho, unpublished results). Cells were pretreated in 5% NBCS medium containing PDTC for 2 h at 37°C prior to infection. Following viral adsorption in PDTC medium at 37°C for 1 h, cells were maintained in the presence of the drug to 6 hpi. At this time, cells were washed twice with sterile PBS, the medium was replaced with 5% NBCS devoid of PDTC, and the infections proceeded to 18 hpi. Thus, the cells were maintained in the presence of PDTC for the first 6 h of infection. The rationale for this was to inhibit NF-κB during the apoptosis prevention window. The phorbol ester phorbol-12-myristate-13-acetate (PMA; Sigma) was resuspended in 100% ethanol at a concentration of 100 μg/ml. The PMA was diluted to final concentrations of 1, 5, and 10 ng/ml in 5% NBCS or 199V medium. Cells were pretreated for 1 h in 5% NBCS medium containing PMA prior to HSV-1(vBSΔ27) infection. Mock-treated control cells (minus PMA) were incubated with ethanol only. Following viral adsorption in 199V medium containing PMA for 2 h at 37°C, cells were maintained in 5% NBCS in the presence of PMA until the end of the experiment at 18 h.
RESULTS
HSV-1(F)-infected cell proteins synthesized between 3 and 6 hpi block apoptosis triggered by TNF-α plus CHX treatment.
We previously reported that infected cell proteins produced between 3 and 6 hpi by HSV-1 strain KOS1.1 had the ability to prevent infection-, staurosporine-, and sorbitol-induced apoptosis in human HEp-2 cells (4). TNF-α is a potent inducer of the caspase 8-dependent cell death pathway (8, 40). When TNF-α induction occurs in the presence of the protein synthesis inhibitor CHX, cultured human cells rapidly die by apoptosis (55). A previous report indicated that HSV-1 strain F could block apoptosis induced by TNF-α (19). Following the lead of these researchers, we set out to determine whether this blocking of TNF-α-triggered apoptosis occurred during the apoptosis prevention window that our laboratory had described previously (4).
Human HEp-2 cells were mock- or HSV-1(F)-infected, and TNF-α plus CHX was added at either 3 or 6 hpi and maintained until the end of the experiment. At 17 hpi, infected cells were stained with Hoechst DNA dye to detect infected cell nuclei and subsequently visualized by phase-contrast microscopy and fluorescence microscopy at 18 hpi, as described in Materials and Methods. Controls included mock-infected and HSV-1(F)-infected cells that were left untreated. The results of this experiment (Fig. 1A) were as follows.
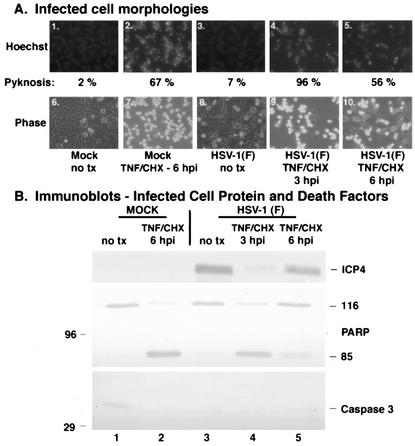
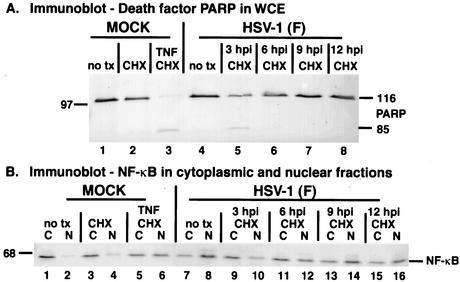
FIG. 1.
Infected cell proteins produced by wild-type HSV-1(F) infection by 6 hpi block TNF-α-induced apoptosis. Fluorescence and phase-contrast images of infected cells (A) and immune reactivities of infected cell proteins and cellular death factors (B) were obtained as described in Materials and Methods. HEp-2 cells were infected with HSV-1(F) (MOI = 5) or mock infected, TNF-α plus CHX was added at 3 or 6 hpi, Hoechst dye was added 60 min prior to harvesting, and the number of cells (percent) with pyknotic nuclei (Pyknosis) was determined from two independent experiments. Infected whole-cell extracts were prepared at 18 hpi (TNF-α-plus-CHX treatments) or 24 hpi (without treatment), separated in a denaturing gel, and transferred to nitrocellulose, and immunoblotting was performed with anti-ICP4 and anti-PARP antibodies. 116 and 85 refer to full-length and processed PARP, respectively. “no tx” refers to no TNF-α-plus-CHX treatment. Magnification, ×40.
The morphologies of cells infected with the wild-type HSV-1(F) virus appeared flat and elongated, similar to mock-infected cells (Fig. 1A, compare panel 6 with 8). In addition, the nuclei of these cells were large and rounded (panels 1 and 3). Only 2 and 7% of the mock-infected and HSV-1(F)-infected cells had condensed chromatin, respectively. In contrast, mock-infected cells that received TNF-α plus CHX treatment at 6 hpi and HSV-1(F)-infected cells treated at 3 hpi exhibited condensed chromatin and nuclear fragmentation (panels 2 and 4) as well as cell shrinkage and membrane blebbing (panels 7 and 9). The number of apoptotic cells detected appears low due to a consequence of the death process; cells lifted off the plate and floated out of the plane of focus and, thus, were not visible in the photographs (2). Infected cells that were treated with TNF-α plus CHX at 6 hpi had features of both nonapoptotic and apoptotic cells (panels 5 and 10). The number of apoptotic cells (56%) was smaller than that of the 3-hpi samples (96%) (compare panels 5 and 10 with 4 and 9) and the mock-infected cells treated at 6 hpi (67%). These results suggest that delaying the addition of TNF-α plus CHX until 6 hpi leads to a reduction in apoptosis.
To further characterize the extent of apoptosis, the experiment above was repeated and a biochemical analysis was performed specifically looking at the cleavage of PARP, a hallmark of apoptosis (39). Whole extracts of infected cells were prepared at 18 hpi, separated in a denaturing gel, transferred to nitrocellulose, and probed with an antibody to PARP. Immunoblotting for the IE viral ICP4 protein was used as an infection control. As expected, the uninfected cells did not show cleavage of PARP (Fig. 1B, lane 1). Similarly, HSV-1(F)-infected cells did not show the 85-kDa cleavage product of PARP (lane 4). When the HSV-1(F)-infected cells were treated with TNF-α plus CHX at 3 hpi, cleavage of PARP was observed (lane 5). The extent of this death factor processing was comparable to that of the positive control sample in which TNF-α plus CHX was added to mock-infected cells at 6 hpi (compare lanes 5 and 3). Significantly less PARP cleavage was observed in the HSV-1(F)-infected cells treated with TNF-α plus CHX at 6 hpi than in the mock infected control (compare lane 6 with 3). Most importantly, less death factor processing was observed with the 6-hpi additions than with the 3-hpi additions (compare lanes 6 and 5). These findings suggest that infected cell proteins made at 6 but not 3 hpi are capable of blocking apoptosis triggered by the addition of TNF-α plus CHX. The fact that productive infection occurred in these cells was confirmed by probing the membrane for the IE viral protein ICP4. Little to no ICP4 was observed when the cells were treated with CHX at 3 hpi (lane 5). When treated at 6 hpi, ICP4 production progressed to a detectable level (lane 6). In untreated infected cells, there was an abundance of this viral protein at 18 hpi (lane 4).
Taking these results together, we conclude the following. (i) Human HEp-2 cells are susceptible to the induction of apoptosis by the addition of TNF-α plus CHX to the cell culture medium. The apoptotic features we observed include cell shrinkage, membrane blebbing, condensed chromatin (pyknosis), nuclear fragmentation (karyorrhexis), and death factor (PARP) processing. (ii) Infected cell proteins present at 6 hpi are able to reduce the amount of apoptosis triggered by TNF-α plus CHX. This finding corroborates our earlier findings showing that the period between 3 and 6 hpi during wild-type HSV-1 infection constitutes a prevention window in which apoptosis triggered by various external stimuli is inhibited (4). (iii) The combination of HSV-1 infection at 3 hpi with the addition of TNF-α plus CHX leads to a greater amount of apoptosis than when TNF-α plus CHX is added during infection at 6 hpi. This conclusion is based on the finding that, under these conditions, the number of apoptotic cells (Fig. 1A) was greater with the HSV-1(F) infection treated at 3 hpi (96% pyknosis) than with the treated mock-infected control (67% pyknosis). A possible explanation for this is that viral infection may provide an initial trigger of apoptosis which is then enhanced by the TNF-α plus CHX treatment, since infected cell prevention factors are not present at that time (3 hpi).
NF-κB partitions in the nuclear fraction of HSV-1(F)-infected human epithelial cells.
The translocation of cellular NF-κB to the nuclei of HSV-1-infected cells has previously been reported (1, 45). However, the role that NF-κB plays during infection remains unknown. In apoptosis-competent human cells, HSV-1 triggers apoptosis upon infection (2, 33), but infected cell proteins produced later in infection prevent the process from killing the cells (4). While recent efforts have focused on identifying potential viral antiapoptotic gene products (2, 4, 5, 24, 37, 38, 59), little is known about the role of cellular survival factors in this prevention process. As NF-κB has been shown to have antiapoptotic functions in other systems (7, 8, 44, 54, 56), we wished to determine whether NF-κB may play a similar role during HSV-1 infection.
First, we set out to determine whether the NF-κB nuclear translocation phenomenon was observable in HSV-1-infected human epithelial HEp-2 cells, our prototype apoptosis-competent cell system (2). HEp-2 cells were mock or HSV-1(F) infected, and subcellular cytoplasmic and nuclear fractions were prepared at 3, 6, and 9 hpi. The time period between 3 and 6 hpi corresponds approximately to the transition from the immediate-early to early phase of HSV-1 replication (22, 23) and was specifically chosen to capture the boundaries of the viral cell death prevention window (3, 4). Infected cell polypeptides were separated in a denaturing gel, electrically transferred to nitrocellulose, and probed with anti-ICP4, anti-ICP22, and anti-NF-κB antibodies as described in Materials and Methods. ICP4 and ICP22 are representative IE proteins that accumulate in the nuclei of infected cells at late times postinfection (11, 13). The results (Fig. 2) were as follows.
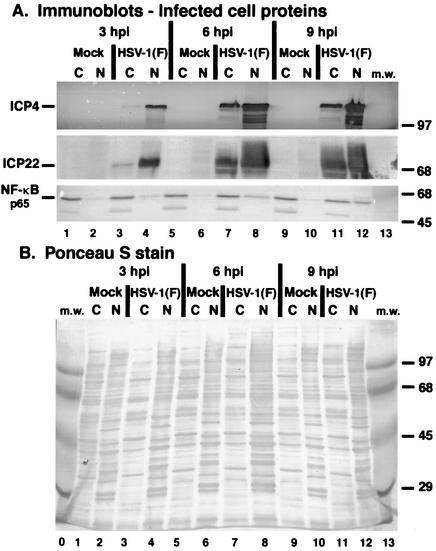
FIG. 2.
Immune reactivities (A) and Ponceau S staining (B) of infected cell extracts indicate that NF-κB partitions in the nuclear fraction of HSV-1(F)-infected cells. HEp-2 cells were mock infected or infected with HSV-1(F) (MOI = 5), cytoplasmic (C) and nuclear (N) extracts were prepared at 3, 6, and 9 hpi, and infected cell proteins were separated in a denaturing gel and transferred to nitrocellulose as described in Materials and Methods. Immune reactivities with anti-ICP4 and anti-NF-κB antibodies were determined using alkaline phosphatase detection methods, while reactivities with anti-ICP22 antibody were determined by using a chemiluminescence technique. The anti-NF-κB recognizes the p65 subunit of the protein. Ponceau S staining prior to immunoblotting was used to demonstrate equal protein loadings and to show that efficient separation of nuclear and cytoplasmic fractions had occurred. The sizes of molecular mass (m.w.) markers (in kilodaltons) are shown in the righthand margins.
At 3 hpi, both ICP4 and ICP22 predominated in the nuclear fractions (lane 4), as expected (47). These two viral proteins then accumulated to essentially the same levels at 6 and 9 hpi (lanes 8 and 12), indicating that our infections were successful and proceeded as expected. While these representative viral IE proteins accumulated to high levels in the nuclear fractions, significant amounts of the proteins were also observed in the cytoplasmic fractions. The cytoplasmic forms of these proteins likely represent newly synthesized protein. The Ponceau S stain of the membrane (Fig. 2B) demonstrated that no contamination between the fractions occurred.
Little to none of the p65 subunit of NF-κB was observed in the nuclear fractions of mock-infected cells at all time points (Fig. 2A, lanes 2, 6, and 10). This finding was expected (9, 27) since, in the absence of stimulation, NF-κB is mainly cytoplasmic (lanes 1, 5, and 9). During HSV-1(F) infection, a low amount of NF-κB was detected in the nuclear fraction at 3 hpi (lane 4), and it increased at 6 and 9 hpi (lanes 8 and 12). Based on these observations, we conclude that NF-κB partitions in the nuclei during HSV-1(F) infection of human HEp-2 cells. Since the levels of ICP4 and ICP22 were high at 3 hpi but the amount of nuclear NF-κB remained low compared to that at the later time points, these results suggest that the translocation of NF-κB may follow de novo viral immediate-early protein synthesis.
NF-κB nuclear translocation follows the immediate-early phase and precedes the late phase of HSV-1 replication.
To define more precisely the kinetics of NF-κB translocation from the cytoplasm to the nucleus during HSV-1 infection in HEp-2 cells, we used indirect immunofluorescence to compare this process with the intracellular transport of specific viral proteins. ICP22 and VP22 were our determinants of the early and late stages of infection, respectively (47). Mock- or HSV-1(F)-infected HEp-2 cells were fixed at 3, 6, 9, and 12 hpi, followed by staining with antibodies specific for NF-κB, the IE protein ICP22, or the L (γ1) viral protein VP22, as described in Materials and Methods.
In Fig. 3A, NF-κB localized exclusively to the cytoplasm of mock-infected cells at 9 hpi. Essentially all NF-κB in the infected cells at 3 hpi was in the cytoplasm, and its localization was similar to mock infection. ICP22 was observed in the infected cells at 3 hpi. As the duration of infection increased, ICP22 accumulated in the cells such that by 9 hpi, dark nuclear staining was readily observed. There were some cells that were clearly infected (6 hpi) but did not have maximum levels of nuclear NF-κB. This finding indicates that the nuclear translocation of NF-κB was independent of ICP22 synthesis and ICP22 nuclear transport. By 9 hpi, infected cells with large amounts of ICP22 in their nuclei showed NF-κB nuclear staining. These observations are consistent with our biochemical fractionation results (Fig. 2).
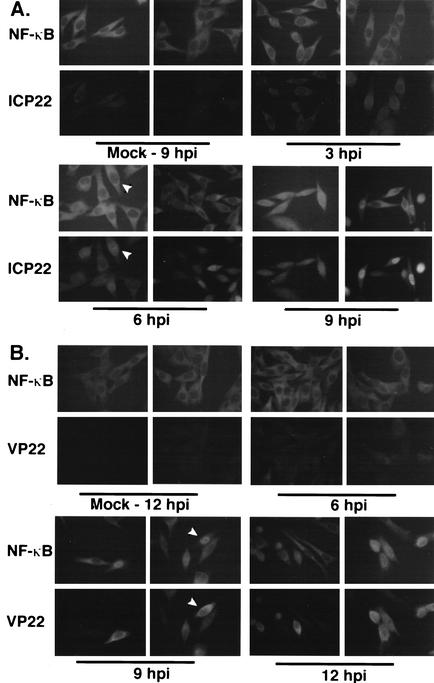
FIG. 3.
NF-κB translocation to HSV-1(F)-infected cell nuclei follows the immediate-early phase and is complete by the L phase of viral replication. HEp-2 cells were mock infected or infected with HSV-1(F) (MOI = 5), and at 3, 6, 9, and 12 hpi the infected cells were prepared for indirect immunofluorescence and double stained with antibodies specific for NF-κB (A and B) plus either IE ICP22 (A) or L VP22 (B) as described in Materials and Methods. Arrowheads in panel A mark a single cell with nuclear ICP22 and cytoplasmic NF-κB; in panel B, the arrowheads mark a cell with nuclear NF-κB and cytoplasmic VP22. Two representative microscopic fields are shown for each condition. Magnification, ×110.
The L viral VP22 protein accumulates in the cytoplasm at early times during HSV-1 infection, and its translocation to the nucleus at late times requires microtubule rearrangement (12, 47). In Fig. 3B, mock- and HSV-1(F)-infected HEp-2 cells were prepared at 6, 9, and 12 hpi for indirect immunofluorescence staining with antibodies specific for NF-κB and VP22. Minimal amounts of VP22 could be detected in the cytoplasm of infected cells at 6 hpi, while cytoplasmic VP22 was most abundant at 9 hpi. As expected, the majority of infected cells at 9 hpi had nuclear NF-κB. Thus, NF-κB nuclear translocation precedes the nuclear transport of VP22. At 12 hpi, most VP22 was nuclear and essentially all infected cells exhibited nuclear NF-κB staining. These results indicate that NF-κB translocation to the nuclei of infected cells initiates following the immediate-early phase and is completed by the L phase of HSV-1 replication. Thus, it is unlikely that the early interactions of the virus with the cell, for example, receptor binding and capsid entry, trigger a rapid activation and translocation of NF-κB in HEp-2 cells. If such early events stimulate NF-κB, another infected cell activity must then function to delay its translocation. In addition, our results suggest that NF-κB nuclear translocation occurs independently of the regulated nuclear import of VP22 (32). Taken together, these data confirm earlier findings (1, 45) and extend to human epithelial HEp-2 cells the observation that HSV-1 infection induces NF-κB nuclear translocation.
NF-κB nuclear translocation correlates with apoptosis prevention during HSV-1 infection.
The time postinfection at which NF-κB nuclear translocation occurred (3 to 6 hpi) corresponds exactly to the period during which infected cell proteins are produced that block apoptosis triggered by either the virus itself or environmental agents (Fig. 1) (4). Thus, the goal of this experiment was to determine whether the presence of NF-κB in nuclei is associated with apoptosis prevention during infection. Two series of experiments were performed. In the first set, HEp-2 cells were mock infected or infected with HSV-1(F) and, at 3 and 6 hpi, the cells were treated with the protein synthesis inhibitor CHX. At 18 hpi, whole-cell extracts or subcellular fractions were prepared, and infected cell proteins were separated in a denaturing gel, transferred to nitrocellulose, and probed with anti-ICP4, anti-VP22, anti-PARP, anti-caspase 3, and anti-NF-κB antibodies as described in Materials and Methods. In our system, the activation of caspase 3 is evident by the disappearance of pro-caspase 3. Controls included mock- and HSV-1(F)-infected extracts or fractions that did not receive CHX treatment and mock-infected cells treated with CHX for 18 h. The control for apoptosis induction was mock-infected cells treated with TNF-α plus CHX for 18 h. The results from this portion of the study (Fig. 4) were as follows.
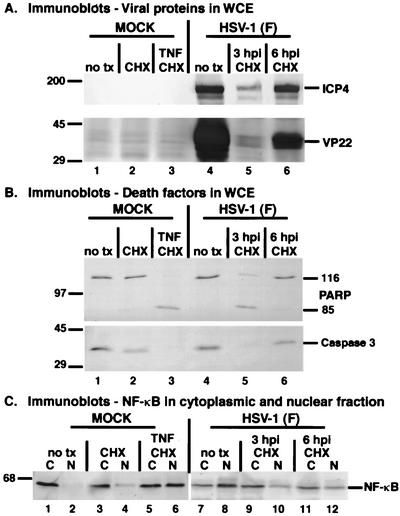
FIG. 4.
Immune reactivities of viral proteins (A), cellular death factors (B), and NF-κB (C) indicate that NF-κB nuclear translocation correlates with apoptosis prevention during HSV-1(F) infection. HEp-2 cells were mock infected or infected with HSV-1(F) (MOI = 10), and at 3 and 6 hpi the medium of the HSV-1(F)-infected cells was replaced with medium containing CHX (10 μg/ml). At 18 hpi, whole-cell extracts (panel A) or cytoplasmic (C) and nuclear (N) extracts (panels B and C) were prepared, separated in denaturing gels, transferred to nitrocellulose membranes, and probed with specific antibodies as described in Materials and Methods. Membranes containing infected cell extracts were reacted with anti-PARP, anti-caspase 3, anti-ICP4, and anti-VP22 antibodies, while membranes containing cytoplasmic and nuclear fractions were probed with anti-NF-κB antibody. Mock-infected cells were treated with either CHX alone or with CHX plus TNF-α (10 ng/ml) at the time of infection and maintained for 18 h. 116 and 85 refer to full-length and processed PARP, respectively. “no tx” refers to cells that did not receive CHX or CHX-plus-TNF-α treatments. Locations of molecular mass markers (in kilodaltons) are indicated in the left margins.
High levels of ICP4 and VP22 were observed in untreated, infected cells at 18 hpi (Fig. 4A, lane 4), as expected. When CHX was added at 3 hpi, levels of these proteins were dramatically reduced (lane 5). This low level of ICP4 is consistent with the results in Fig. 1 and 2. In the 6-hpi CHX addition sample, the ICP4 level was almost as high as that of the untreated control. We have previously detected very low levels of VP22 at early infection times (47). While the VP22 level was greater at 6 hpi than that at 3 hpi in the treated samples, it was still significantly lower than its amount in the untreated sample (compare lane 6 with 4). Thus, CHX addition worked as expected: the representative IE ICP4 protein was produced at optimal levels under the 6-hpi treatment condition while the L VP22 protein was significantly reduced.
Treatment of the mock-infected cells with TNF-α plus CHX for 18 h resulted in complete cleavage of PARP and caspase 3 activation (Fig. 4B, lane 3). While CHX addition to mock-infected cells in the absence of TNF-α induced a small amount of caspase 3 activation (compare lane 2 with 1), no PARP processing was observed, as expected (4). This minimal background level of processing is similar to that observed by Galvan and Roizman, who reported that CHX treatment alone has a slight but reproducible effect of accelerating the degradation of DNA in SK-N-SH cells (19). Additionally, no PARP processing was observed with HSV-1(F)-infected cells that were either untreated (lane 4) or treated with CHX at 6 hpi (lane 6). However, HSV-1(F) infection of these cells did induce caspase 3 cleavage (compare lanes 6 and 4 with 1) in a manner similar to that previously observed with HSV-1(KOS1.1) (4). Our earlier studies showed that infection of HEp-2 cells by wild-type HSV-1 activates caspase 3 but does not result in apoptosis (2, 4). When CHX was added at 3 hpi, complete PARP cleavage and caspase 3 activation occurred (lane 5), since infected cell proteins preventing apoptosis were not produced (4). These results confirm our previous findings defining the apoptosis prevention window as being between 3 and 6 hpi during wild-type HSV-1 infection (4).
Treatment of mock-infected cells with TNF-α plus CHX induced the nuclear translocation of NF-κB, as expected (9), and an approximately equal if not greater amount of NF-κB was detected in the nuclear fraction compared to the cytoplasmic fraction (Fig. 4C, compare lanes 6 and 5). A small amount of nuclear NF-κB was detected in mock-infected cells treated with CHX alone (compare lanes 4 and 2). This level of nuclear NF-κB is comparable in magnitude to the amount of caspase 3 processing observed in these cells (Fig. 4B, compare lanes 2 and 1).
Consistent with the results in Fig. 2, HSV-1(F) infection in the absence of any treatment resulted in the partitioning of NF-κB in the nuclear fraction at 18 hpi (Fig. 4C, compare lanes 8 and 7). The amount of nuclear NF-κB in these infected cells was greater than that in the cytoplasm. While TNF-α-plus-CHX-treated mock-infected cells showed approximately equal levels of nuclear and cytoplasmic NF-κB at 18 h, HSV-1(F) infection yielded more nuclear than cytoplasmic NF-κB (compare lanes 7 and 8 with 5 and 6). This finding suggests that infection may be a more potent stimulator of NF-κB translocation than TNF-α treatment in these cells. Combining these results with those of Fig. 2 suggests that NF-κB continues to accumulate in nuclei as infection proceeds in HEp-2 cells.
When CHX was added to the infected cells at 3 hpi, significantly less NF-κB was in the nuclear fraction (Fig. 4C, compare lanes 10 and 8) than in the cytoplasm (compare lanes 9 and 7) compared to the untreated, infected cells. This result correlates with activation of caspase 3 and PARP cleavage (Fig. 4B, lane 5). While there appeared to be more NF-κB in the cytoplasm than in the nuclei of the infected cells treated at 6 hpi, the amount of nuclear NF-κB was much greater than that of mock-infected cells receiving CHX alone (Fig. 4C, compare lanes 12 and 4). The 6-hpi treated, infected cells also had less caspase 3 activation than the treated, mock-infected cells, and both conditions retained unprocessed PARP (Fig. 4B, compare lanes 6 and 2). This observation might suggest that the extent of NF-κB nuclear translocation correlates with the extent of caspase 3 activation under certain experimental conditions.
In the second set of experiments, we focused on determining whether NF-κB remained in the infected cell nuclei during the later phases of infection. Our rationale for considering this possibility was based on two findings. First, the results in Fig. 2 and 4 suggested that the amount of nuclear NF-κB increased as HSV-1 infection progressed. Second, more nuclear NF-κB was detected in infected cells at 18 hpi than in mock-infected cells treated with TNF-α plus CHX for the same duration (Fig. 4C, compare lanes 6 and 8). HEp-2 cells were infected with HSV-1(F) as above, and at 3, 6, 9, and 12 hpi were treated with CHX. Cells were harvested at 18 hpi, and infected cell proteins from cytoplasmic and nuclear fractions were analyzed by immunoblotting using anti-PARP and anti-NF-κB antibodies. The results (Fig. 5) were as follows.
FIG. 5.
NF-κB accumulation in HSV-1(F)-infected cell nuclei up to 12 hpi directly coincides with apoptosis prevention. HEp-2 cells were mock infected or infected with HSV-1(F) (MOI = 10), and CHX was added at 3, 6, 9, and 12 hpi. Mock-infected cells were treated with either CHX or CHX plus TNF-α at the time of infection. At 18 hpi, whole-cell extract (panel A) or cytoplasmic (C) and nuclear (N) extracts (panel B) were prepared, separated in denaturing gels, transferred to nitrocellulose membranes, and probed with anti-PARP (A) and anti-NF-κB (B) antibodies as described in Materials and Methods. 116 and 85 refer to full-length and processed PARP, respectively. “no tx” refers to cells that did not receive CHX or CHX-plus-TNF-α treatments. Locations of molecular mass markers (in kilodaltons) are indicated in the left margins.
As expected (4), apoptosis leading to PARP cleavage occurred in HSV-1(F)-infected cells in which CHX was added at 3 hpi but not at 6, 9, or 12 hpi (Fig. 5A, lanes 5 to 8). These results indicate that infected cell proteins produced between 3 and 6 hpi block the apoptotic process. At 3 hpi, there was significantly more cytoplasmic than nuclear NF-κB (Fig. 5B, compare lanes 10 and 9), consistent with the results in Fig. 4. Additionally, the amount of nuclear NF-κB increased at 6 hpi (compare lanes 12 and 10). The amount of NF-κB in the nucleus reached its maximum at 9 hpi, and this level was maintained at 12 and 18 hpi (lanes 14, 16, and 8, respectively). Thus, the nuclear translocation of NF-κB increases during the period in which HSV-1(F)-infected cell proteins are produced that block the cell death process. These findings indicate that there is a direct correlation of nuclear NF-κB translocation with apoptosis prevention in HSV-1-infected cells. Blocking total protein synthesis at 3 hpi precludes this nuclear import. This result indicates that de novo synthesis of infected cell proteins is required for the translocation to occur, thus confirming an earlier indication reported by Patel et al. (45).
NF-κB nuclear translocation correlates with apoptosis prevention during infection with a recombinant HSV-1 strain with gE deleted.
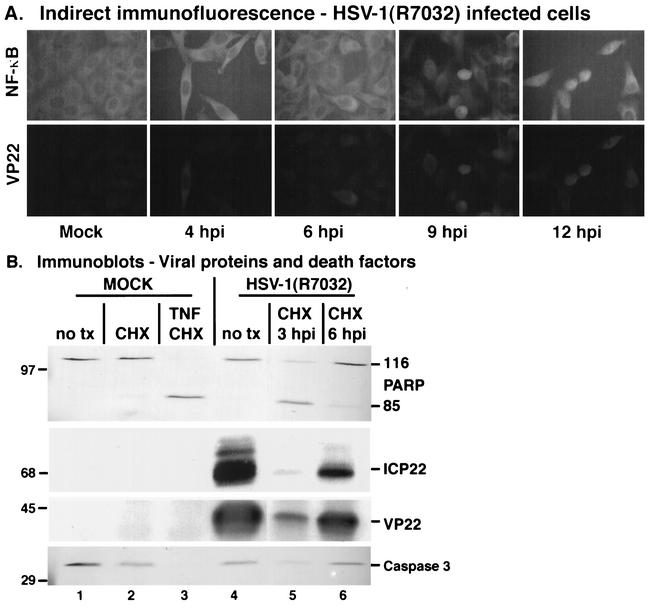
One potential hazard inherent in the use of polyclonal antibodies to study HSV-1 infection is that of the gE/gI glycoprotein complex. This complex acts as a receptor, binding human and rabbit immunoglobulin molecules (26). The potential exists for it to bind our anti-ICP22 or anti-VP22 polyclonal antibodies during indirect immunofluorescence analyses of wild-type HSV-1-infected cells. To eliminate this phenomenon as a potential confounder, we chose to confirm our findings by using the related HSV-1(R7032) virus which contains a deletion of the gene encoding gE (41). HEp-2 cells were infected with HSV-1(R7032) or mock infected and fixed for indirect immunofluorescence at 4, 6, 9, and 12 hpi using anti-NF-κB and anti-VP22 antibodies as described in Materials and Methods. The results (Fig. 6A) indicate that NF-κB was cytoplasmic at 4 and 6 hpi during HSV-1(R7032) infection, and these cells had a staining pattern similar to mock-infected cells at 18 h. Consistent with our earlier findings (Fig. 3), nuclear NF-κB was detected at 9 and 12 hpi. Nuclear NF-κB was observed at the same time postinfection that we detected VP22, thus confirming our findings (Fig. 3) on the timing of NF-κB translocation. The detection of VP22 in these HSV-1(R7032)-infected cells also indicates that our VP22 immunostaining is not related to nonspecific gE receptor binding activity. Inspection of the data in Fig. 6A and comparison with those in Fig. 3B indicate that more nuclear VP22 was observed at 9 hpi with HSV-1(R7032) than with HSV-1(F). This finding is consistent with our laboratory's recent observations (32) suggesting a role for gE in VP22 nuclear import.
FIG. 6.
(A) Indirect immunofluorescence and immune reactivities indicate that HSV-1(R7032), which contains a deletion in the viral gE, induces and then blocks apoptosis and stimulates NF-κB nuclear translocation during infection. HEp-2 cells were mock infected or infected with HSV-1(R7032) (MOI = 10), prepared for indirect immunofluorescence at 4, 6, 9, and 12 hpi, and double stained with anti-NF-κB or anti-VP22 antibodies as described in Materials and Methods. Magnification, ×85. (B) In a separate analysis, CHX was added to the infected cells at 3 and 6 hpi, while CHX or TNF-α plus CHX was added to mock-infected cells at the time of infection. Infected cell extracts were prepared for immunoblotting of viral proteins and death factors at 18 hpi, and immune reactivities were measured using anti-PARP, anti-ICP22, anti-VP22, and anti-caspase 3 antibodies. Viral and cellular proteins were detected using chemiluminescence and alkaline phosphatase techniques, respectively. 116 and 85 refer to full-length and processed PARP, respectively. “no tx” refers to cells that did not receive CHX or CHX-plus-TNF-α treatments. Locations of molecular mass markers (in kilodaltons) are indicated in the left margins. For clarity, a blank lane was removed between lanes 4 and 5.
Next, HEp-2 cells were mock infected or infected with HSV-1(R7032), and at 3 and 6 hpi the cells were treated with CHX. At 18 hpi, whole-cell extracts were prepared, and immunoblotting analyses were performed with anti-ICP22, anti-VP22, anti-PARP, and anti-caspase 3 antibodies as described in Materials and Methods. The results (Fig. 6B) showed that HSV-1(R7032) infection without CHX (lane 4) or with CHX at 6 hpi (lane 6) did not elicit a high level of PARP cleavage, while significant cleavage occurred during infection with CHX added at 3 hpi (lane 5). A slight activation of caspase 3 upon infection was observed in the untreated and 6-hpi-treated cells, while a high level of activation was seen with the 3-hpi treatment sample (lane 5). Immunostaining for viral ICP22 and VP22 proteins indicated that infection occurred in these cells. These results indicate that the HSV-1(R7032) virus triggers apoptosis, but infected cell proteins produced during infection prevent the process from killing the cells. This finding represents the first demonstration of a virus containing a deletion in the viral gE glycoprotein preventing apoptosis during infection.
These data suggest that, along with wild-type HSV-1, any recombinant replication-competent strain of HSV-1 capable of blocking apoptosis facilitates the induction of NF-κB nuclear translocation. That a virus with a deletion in a viral glycoprotein (gE) still blocks cell death is significant, as other viral glycoproteins have been implicated in the prevention process (24, 59).
NF-κB does not translocate to the nuclei of apoptotic HSV-1(vBSΔ27)-infected cells.
All of the previous results (Fig. 1 to 6) were obtained using replication-competent viruses that first trigger, then prevent apoptosis during productive infection. It was our desire to confirm our findings using an apoptotic replication-defective virus. We previously demonstrated that infection by viruses possessing mutations in the essential regulatory ICP27 protein induce apoptosis and are unable to synthesize infected cell proteins that prevent the process from killing the infected cells (2, 3). HEp-2 cells were mock infected or infected with HSV-1(F), HSV-1(KOS1.1), or HSV-1(vBSΔ27), and nuclear and cytoplasmic extracts were prepared at 12 hpi. HSV-1(vBSΔ27) is a mutant derived from HSV-1(KOS1.1) and contains a deletion in the essential IE gene α27 that encodes the ICP27 protein (52). Two separate sets of infections using different preparations of this virus were performed to insure reproducibility. Infected cell proteins were separated in a denaturing gel, transferred to nitrocellulose, and reacted with anti-ICP4, anti-NF-κB, and anti-caspase 3 antibodies as described in Materials and Methods. Ponceau S staining of the membrane prior to immunoblotting indicated that identical amounts of infected cell proteins were loaded in each lane (data not shown). The results were as follows (Fig. 7A).
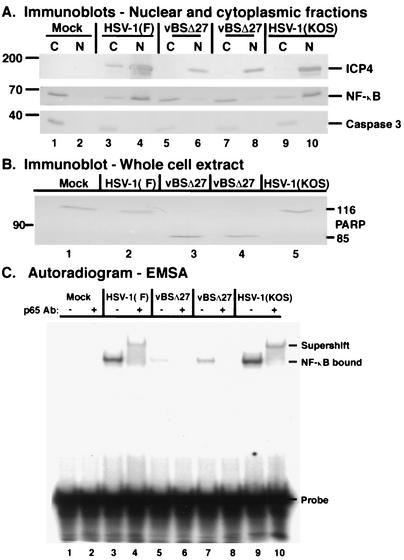
FIG. 7.
NF-κB does not translocate to the nuclei of apoptotic HSV-1(vBSΔ27)-infected cells and does not bind DNA. Immune reactivities of nuclear and cytoplasmic fractions (A) and whole-cell extract (B) of infected cell proteins and autoradiographic images (C) of radiolabeled DNA-protein complexes are shown. HEp-2 cells were mock infected or infected with HSV-1(F), HSV-1(vBSΔ27), or HSV-1(KOS1.1) (MOI = 10) and at 12 hpi, cytoplasmic (C) and nuclear (N) extracts were prepared, separated in denaturing gels, transferred to nitrocellulose, and probed with anti-ICP4, anti-caspase 3, and anti-NF-κB antibodies as described in Materials and Methods. To ensure consistency in this study, two separate infections were performed with two independent stocks of HSV-1(vBSΔ27) (lane 5 to 8). All immune reactivities were detected using an alkaline phosphatase technique. The locations of molecular mass markers (in kilodaltons) are shown in the left margin. Duplicate sets of HEp-2 cells were mock infected or infected with HSV-1(F), HSV-1(KOS), and HSV-1(vBSΔ27) (MOI = 10), and at 12 hpi, nuclear or whole-cell extracts were prepared. Whole-cell extracts were used for immunoblotting and probed with an anti-PARP antibody. Approximately 3 μg of nuclear extract was reacted with a 32P-labeled NF-κB site probe and electrophoresed in a nondenaturing polyacrylamide gel, and the gel was dried and exposed to autoradiographic film as described in Materials and Methods. A control anti-NF-κB p65 antibody was added (+) prior to addition of the labeled DNA probe. NF-κB that bound to the DNA probe and that supershifted by the anti-p65 antibody is indicated.
As expected, mock-infected cells showed little to no detectable NF-κB in the nuclear extract (Fig. 7A, lane 2) and no degradation of cytoplasmic caspase 3 (lane 1). Both wild-type HSV-1(F) (lanes 3 and 4) and HSV-1(KOS1.1) (lanes 9 and 10) viruses had significant amounts of nuclear NF-κB at 12 hpi compared to that in the mock-infected control (compare lanes 4 and 10 with 2). Both viruses also produced high levels of ICP4. Consistent with previous findings (Fig. 1, 4, and 6) (4), there were slight reductions in the amounts of caspase 3 observed in these infected samples relative to that in mock-infected cells (compare lanes 3 and 9 with 1). These reductions were expected and indicate that these two wild-type viruses first trigger and then prevent the apoptotic program. These findings confirm our results in Fig. 4 and 5 and demonstrate that the phenomenon of NF-κB nuclear translocation also occurs in HSV-1(KOS1.1)-infected HEp-2 cells. Cells infected with the HSV-1(vBSΔ27) mutant virus showed reductions in the amounts of ICP4 and had the greatest amount of caspase 3 activation (Fig. 7A, compare lanes 5 and 7 with 1, 3, and 9), as expected (2, 4). The vast majority of NF-κB in the HSV-1(vBSΔ27)-infected cells was in the cytoplasmic extracts (lanes 5 and 7). The slight amounts of nuclear NF-κB detected in the HSV-1(vBSΔ27)-infected cells likely reflect the fact that these cells are quite apoptotic and the nuclear membranes may no longer be completely intact. Detailed control experiments indicated that while this background “contamination” of apoptotic nuclei with NF-κB increased with longer times post-HSV-1(vBSΔ27) infection, these background amounts never approached the levels of nuclear NF-κB observed during wild-type HSV-1 infections (M. L. Goodkin and J. A. Blaho, unpublished results).
From these findings, we conclude that (i) all replication-competent HSV-1 strains induce NF-κB nuclear translocation, inasmuch as our results using HSV-1(KOS1.1) were identical to those obtained with HSV-1(F) and HSV-1(R7032). (ii) Replication-defective HSV-1(vBSΔ27) is unable to induce NF-κB nuclear translocation in HEp-2 cells. HSV-1(vBSΔ27) does not produce E and L viral proteins in these cells (2). Thus, it is likely that infected cell factors required for NF-κB import are not produced during HSV-1(vBSΔ27) infection. (iii) This lack of nuclear localization of NF-κB correlates with the observation (references 2 and 4 and this study) of apoptosis in HSV-1(vBSΔ27)-infected HEp-2 cells. These results further support our hypothesis that NF-κB participates in the prevention of apoptosis in HSV-1-infected cells.
Absence of NF-κB DNA binding activity in the nuclei of apoptotic HSV-1(vBSΔ27)-infected human cells.
Our results (Fig. 7A) indicate that little to no NF-κB translocated to the nuclei of infected human cells during infection with the apoptotic HSV-1(vBSΔ27) virus. The purpose of this experiment was to determine the ability of nuclear NF-κB to bind DNA during viral infection. Duplicate sets of HEp-2 cells were either mock infected or infected with HSV-1(F), HSV-1(KOS1.1), or HSV-1(vBSΔ27). At 12 hpi, one set of samples were prepared for EMSA analysis, while the other was used for the preparation of whole-cell extracts for immunoblotting using an anti-PARP antibody, as described in Materials and Methods. The results (Fig. 7B and C) show the following.
We observed complete PARP processing during both HSV-1(vBSΔ27) infections (Fig. 7B, lanes 3 and 4). This was in contrast to the wild-type HSV-1(F)- and HSV-1(KOS1.1)-infected cells (lanes 2 and 5), where no PARP processing was detected. In the EMSA analysis, no NF-κB binding to DNA occurred in mock-infected cells (Fig. 7C, lanes 1 and 2). Both wild-type HSV-1(F)- (lanes 3 and 4) and HSV-1(KOS1.1)- (lanes 9 and 10) infected cells had high levels of DNA binding activity that were shifted with an antibody specific for the p65 subunit of NF-κB, thus confirming the specificity of the bound protein. Our findings extend the recently reported observations that HSV-1 infection of primary human fibroblasts increases nuclear NF-κB levels and DNA binding (18). Substantially less NF-κB binding to the DNA was observed in both of the HSV-1(vBSΔ27) infections (lanes 5 to 8). From these findings, we conclude that the lack of nuclear localization observed in Fig. 7A and the lack of DNA binding activity of NF-κB correlates with the observation (references 2 and 4 and this study) of apoptosis in HSV-1(vBSΔ27)-infected HEp-2 cells.
Activation of NF-κB prior to HSV-1(vBSΔ27) infection decreases death factor processing.
The results above illustrated that NF-κB did not localize to or bind DNA in the nuclei of HEp-2 cells infected with the replication-defective HSV-1(vBSΔ27) virus. This apparent lack of NF-κB activation correlated with cellular death substrate processing. To test further our hypothesis that NF-κB activation during HSV-1 infection suppresses apoptosis, we examined whether the preactivation of NF-κB would have an effect on apoptosis induced by the HSV-1(vBSΔ27) virus. Experimentally, NF-κB can be activated by treating cells with the phorbol ester PMA, which activates IκB kinase (IKK) via a protein kinase C-dependent mechanism (17, 44). Activated IKK subsequently phosphorylates IκBα, which results in the degradation of IκBα, thus allowing NF-κB subunits to translocate to the nucleus (9, 20, 27). HEp-2 cells were pretreated for 1 h with increasing amounts of PMA in 5% NBCS prior to infection with HSV-1(vBSΔ27), and the PMA was maintained throughout the course of infection. At 18 hpi, whole-cell and subcellular extracts were prepared for use in immunoblotting analyses using anti-ICP4, anti-PARP, anti-caspase 3, and anti-NF-κB. Percentages of PARP cleavage and relative amounts of ICP4 were determined as described in Materials and Methods. The results (Fig. 8) were as follows.
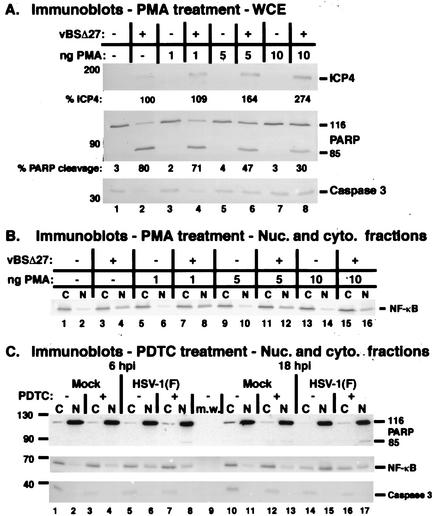
FIG. 8.
Pharmacological modulation of NF-κB. Immune reactivities following PMA (A and B) or PDTC (C) treatments of infected cells. HSV-1(vBSΔ27)-infected (MOI = 10) HEp-2 cells were treated with increasing concentrations of PMA for 18 h, and whole-cell extracts (panel A) or cytoplasmic (C) and nuclear (N) extracts (panels B and C) were prepared, separated in denaturing gels, transferred to nitrocellulose, and probed with anti-NF-κB, anti-ICP4, anti-PARP, and anti-caspase 3 antibodies. Amounts of ICP4 and PARP cleavage were determined using NIH Image software as described in Materials and Methods. HEp-2 cells treated with (+) or without (−) PDTC were mock infected or infected with HSV-1(F) (MOI = 10), and at 6 and 18 hpi cytoplasmic (C) and nuclear (N) extracts were prepared for immunoblotting and probed with anti-PARP, anti-caspase 3, and anti-NF-κB antibodies. Immune reactivities with anti-NF-κB, anti-ICP4, anti-PARP (A), and anti-caspase 3 antibodies were performed using an alkaline phosphatase detection method, while reactivities with the anti-PARP antibody (C) utilized a chemiluminescence technique.
Essentially no PARP (<4%) or caspase 3 cleavage was observed in mock-infected cells or cells treated with PMA in the absence of viral infection (Fig. 8A, lanes 1, 3, 5, and 7). As expected, HSV-1(vBSΔ27)-infected HEp-2 cells in the absence of PMA displayed PARP and caspase 3 cleavage (lane 2). However, in PMA-treated, HSV-1(vBSΔ27)-infected cells, there was a striking dose-dependent decrease in PARP and caspase 3 cleavage (compare lanes 4, 6, and 8 with 2). Treatment with 1 ng of PMA/ml had little effect on death substrate processing, since PARP cleavage (71%) was similar to that of untreated, infected cells (80%) (compare lanes 4 and 2). An increase to 5 ng of PMA/ml reduced the apoptotic processing by almost half (to 47%) (lane 6) and at 10 ng/ml yielded a further reduction in PARP cleavage (to 30%) (lane 8). Concurrent with this decrease in PARP cleavage, a decrease in caspase 3 cleavage was observed in these cells compared to HSV-1(vBSΔ27)-infected cells not treated with PMA. Finally, PMA treatment during HSV-1(vBSΔ27) infection led to an increase in the accumulation ICP4 (compare lanes 4, 6, and 8 with 2). Previously, we reported that ICP4 is produced in very low amounts in the HSV-1(vBSΔ27)-infected cells (2). Here, we observed an approximately two- to threefold increase in the amount of ICP4 in the PMA-treated, HSV-1(vBSΔ27)-infected cells compared to the untreated, infected control.
Subcellular fractionation of the cells examined in Fig. 8A showed the following. There were minimal background amounts of nuclear NF-κB that did not change with time in mock-infected (Fig. 8B, lane 2) or PMA-treated cells (lanes 6, 10, and 14) at the time of harvest, indicating that the NF-κB stimulated by PMA treatment had translocated back to the cytoplasm some time during the 18-h treatment. Control experiments indicated that under these conditions, NF-κB in the mock-infected cells remains nuclear at least until 6 h posttreatment, at which time it appears to begin migrating back into the cytoplasm (data not shown). A small amount of nuclear NF-κB was evident in HSV-1(vBSΔ27)-infected cells at 18 hpi (lane 4). As mentioned above, this finding is consistent with the results of previous time course of infection experiments (data not shown), in which NF-κB began to appear in the nuclear fraction of HSV-1(vBSΔ27)-infected cells at approximately 15 hpi but never reached the levels observed with wild-type HSV-1.
HSV-1(vBSΔ27)-infected HEp-2 cells treated with PMA also showed nuclear translocation of NF-κB. However, the amount of nuclear localization appeared to decrease slightly with an increase in PMA concentration. This seemingly paradoxical result is likely due to the fact that by 18 hpi, HSV-1(vBSΔ27)-infected cells are fully apoptotic and nuclear membrane integrity has been lost. The amount of nuclear NF-κB in infected cells treated with 1 ng of PMA/ml appeared similar to that of HSV-1(vBSΔ27)-infected cells (Fig. 8B, compare lanes 3 and 4 with 7 and 8) and correlates with the apoptotic processing seen in Fig. 8A. An increase to 5 ng/ml led to a decrease in apoptotic processing (Fig. 8A, lane 6), and less nuclear NF-κB was observed. A concentration of 10 ng/ml dramatically decreased the appearance of apoptosis in infected cells and was associated with the least nuclear NF-κB. The most likely explanation for these observations is that as apoptosis is prevented during HSV-1(vBSΔ27) infection by the addition of PMA, the cells survive, organelle integrity is maintained, and there is less mixing of cellular contents. From these experiments, we conclude that activation of NF-κB by PMA treatment during infection with HSV-1(vBSΔ27) decreases apoptosis in the infected cells.
Inhibition of NF-κB translocation in HSV-1(F)-infected cells increases death factor processing.
All of our evidence up to this point argues that the translocation of NF-κB to the nuclei of infected cells is associated with apoptosis prevention. The goal of this study was to confirm this hypothesis by performing infections under conditions in which NF-κB nuclear import is inhibited, and the NF-κB antagonist PDTC was utilized for this purpose. PDTC is a pyrrolidine derivative of dithiocarbamate that functions as both a metal chelator and an antioxidant and is readily taken up by cultured mammalian cells (15). As PDTC scavenges reactive oxygen species within cells, it antagonizes the release of NF-κB from IκB by inhibiting the degradation of IκB (36, 50). Consequently, NF-κB is not free to translocate to the nucleus in response to inducing stimuli. As the effects of this compound on human HEp-2 cells were unknown, it was imperative for us to perform control experiments to ensure that this drug was efficacious in our cells. We empirically determined the PDTC treatment conditions that inhibit TNF-α-induced NF-κB translocation using HEp-2 cells (data not shown). Titration experiments indicated that a 400 μM concentration of the compound was the optimal effective concentration. During this analysis, we discovered that PDTC treatment leads to a low (background) level of NF-κB nuclear translocation that is independent of dosage. These results indicate that the drug functions in HEp-2 cells as has been reported for other cell lines, including the presence of its biphasic effect (50).
Next, we used PDTC to determine the effect of inhibiting NF-κB nuclear translocation on apoptosis prevention during HSV-1 infection. Duplicate sets of HEp-2 cells were pretreated with 400 μM PDTC in 5% NBCS for 2 h prior to infection. Pairs of mock- and HSV-1(F)-infected cells were maintained in PDTC medium until 6 hpi, at which time the medium of one set was changed to 5% NBCS devoid of PDTC, and these cells were incubated to 18 hpi. Subcellular extracts were prepared from the other set at 6 hpi. This treatment procedure was chosen to determine the effects of the compound during the HSV-1 prevention window and represents the standard protocol for the use of this inhibitor (50). Cytoplasmic and nuclear extracts were prepared at either 6 or 18 hpi, and polypeptides were separated in a denaturing gel, transferred to a membrane, and probed with anti-PARP, anti-NF-κB, and anti-caspase 3 antibodies as described in Materials and Methods.
The results (Fig. 8C) showed that at 6 hpi, PDTC treatment led to an increase in caspase 3 cleavage in mock-infected cells along with the localization of a small (background) amount of NF-κB in the nuclear extract (compare lanes 3 and 4 with 1 and 2). However, no PARP processing was detected in treated mock-infected cells. In contrast, a very slight amount of PARP processing was seen in the infected nuclear extract in the presence of PDTC (lane 8). Although the PARP cleavage signal was low, this result is significant, since it represents the first time that we have ever observed PARP processing at 6 hpi during a wild-type HSV-1 infection. At 18 hpi, the majority of NF-κB was located in the nuclear extract of HSV-1(F)-infected cells (lanes 14 and 15), as expected. Cells infected in the presence of PDTC had significantly less nuclear NF-κB than their untreated infected counterparts (compare lane 17 with 15), confirming the efficacy of the treatment. The PDTC-treated infected cells had more caspase 3 cleavage than the control samples at 18 hpi (compare lane 16 with 12 and 14). Finally, detectable PARP processing was observed only in the infected, PDTC-treated cells but not in any of the other cells at 18 hpi (compare lane 17 with 11, 13, and 15). Equal amounts of the viral ICP4 protein were observed in the nuclear extracts by immunoblotting (data not shown), indicating that PDTC treatment of the cells did not prohibit HSV-1 infectivity.
Based on these results, we conclude that (i) PDTC blocks the nuclear translocation of NF-κB in both mock-infected and HSV-1(F)-infected HEp-2 cells. (ii) The prevention of NF-κB nuclear import in HSV-1(F)-infected cells leads to an increase in the processing of the apoptotic death factors caspase 3 and PARP. The results of this analysis support our contention that NF-κB participates in the apoptosis prevention process during HSV-1 infection.
Generation and characterization of an IκBα dominant-negative HEp-2 cell line.
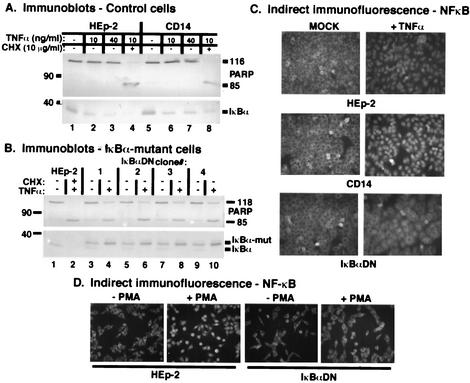
To confirm the necessity of NF-κB in apoptosis prevention during HSV-1 infection, we set out to develop a molecular genetic system in which the activity of NF-κB was prohibited inside of cells. We made several attempts to transiently transfect a nonphosphorylatable IκBα into HEp-2 cells (M. L. Goodkin and J. A. Blaho, unpublished results). These attempts were unsuccessful due to (i) low transfection efficiencies, (ii) toxicities associated with the plasmid constructs to the human HEp-2 cells, and (iii) sensitivity of the HEp-2 cells to transfection in itself, leading to a high background of NF-κB activation in these cells as determined by immunoblotting of subcellular fractions. Our lack of success with transient-transfection experiments led us to generate stably transfected HEp-2 cell lines by using a plasmid expressing a dominant-negative IκBα possessing a stabilizing serine-to-alanine mutation at the two key serines of the protein (serines 32 and 36). This construct contains the mutant IκBα under the control of the human Ef1α promoter. It also contains a puromycin resistance gene for the purpose of selection. The mutated IκBα contains a FLAG tag which increases the size of the protein, allowing differentiation between the wild-type and mutant IκBα forms when probed by immunoblotting with an anti-IκBα antibody. Similar plasmids containing GFP (Peak8-GFP, as a transfection control) and CD14 (Peak8-CD14, as a vector control) were also generated. The specifics of the selection and isolation of these clonal cell lines is described in detail in Materials and Methods. Several of the resulting clonal cell lines were tested for their ability to inhibit NF-κB activation (Fig. 9).
FIG. 9.
Characterization of IκBαDN and control CD14 HEp-2 cell lines based on immune reactivities (A [control cells] and B [IκBα mutant cells]) and indirect immunofluorescence (C and D). Clonal CD14-transfected cells and nontransfected HEp-2 cells were left untreated or treated with either TNF-α (10 or 40 ng/ml) or TNF-α (10 ng/ml) plus CHX (10 mg/ml). Clonal IκBαDN cells were treated with TNF-α while control, nontransfected HEp-2 cells were treated with TNF-α (10 ng/ml) plus CHX (10 μg/ml). At 18 h posttreatment, whole-cell extracts were prepared, separated in denaturing gels, transferred to nitrocellulose, and probed with anti-PARP and anti-IκBα antibodies as described in Materials and Methods. Immune reactivities were detected using alkaline phosphatase methods, and IκBα-mut marks the location of the slower-migrating, FLAG-tagged mutant form of IκBα. IκBαDN cells and control cells were also plated onto glass coverslips and either treated with TNF-α for 1 h or treated with PMA for 45 min, and the cells were fixed and permeabilized for immunofluorescence using anti-NF-κB p65 antibody as described in Materials and Methods. Magnification, ×40.
If the dominant-negative IκBα behaved as expected, treatment with TNF-α in the absence of CHX should lead to apoptosis (36, 50). As initial controls for this study, parental HEp-2 and vector control cells expressing CD14 were treated with 10 or 40 ng of TNF-α/ml in the absence of CHX to test whether these cells would undergo TNF-α-induced apoptosis. At 18 h posttreatment, whole-cell extracts were analyzed by immunoblotting with anti-PARP and anti-IκBα antibodies. As expected, neither the parental line nor the control lines showed apoptotic processing in response to TNF-α treatment (Fig. 9A, lanes 2, 3, 6, and 7). However, when these cells were treated with TNF-α in the presence of CHX, both the parental HEp-2 cells and the CD14 control exhibited apoptotic processing (Fig. 9A, lanes 4 and 8). Thus, the control CD14 clone behaved similarly to the parental cell line.
Next, we treated a series of four independently selected dominant-negative IκBα clonal cell lines with 10 ng of TNF-α/ml in the absence of CHX, comparing them to HEp-2 cells treated with TNF-α plus 10 μg of CHX/ml, and immunoblotting with anti-PARP and anti-IκBα antibodies was performed (Fig. 9B). As expected, TNF-α-plus-CHX treatment of HEp-2 cells led to the disappearance of IκBα and the cleavage of PARP (lane 2), while untreated HEp-2 cells showed no PARP cleavage (lane 1). All of the clonal lines showed the same pattern when compared to the HEp-2 cell controls. First, both the wild-type and mutant forms of IκBα were observed in each of the clonal lines (lanes 3, 5, and 7). The slower-migrating, mutant forms also reacted with specific anti-FLAG antibodies (data not shown). Second, TNF-α treatment of the clonal lines showed a dramatic increase in PARP cleavage compared to untreated clones and to the HEp-2 cells (lanes 4, 6, and 8). Finally, comparison of the TNF-α-treated and untreated clonal lines demonstrated that only the wild-type IκBα band disappeared from the treated cells (compare lane 3 with 4, 5 with 6, and 7 with 8). These results indicate that the TNF-α signaling cascade which leads to the degradation of IκBα was intact in these cells. Interestingly, an increase in the amount of the mutant IκBα in the treated clonal lines was observed (lanes 4, 6, and 8). The basis for this is currently unknown. Clonal cell line number 3 was chosen for further characterization and renamed IκBαDN.
Initially, IκBαDN′s response to TNF-α treatment was carried out. HEp-2, CD14, and IκBαDN cells were treated for 1 h with 10 ng of TNF-α/ml in 5% FBS and then fixed for indirect immunofluorescence using an anti-NF-κB p65 antibody as described in Materials and Methods. Microscopic analyses (Fig. 9C) indicated that no untreated cells possessed nuclear NF-κB. TNF-α treatment led to the detection of NF-κB in the nuclei of all HEp-2 and CD14 (vector control) cells. In contrast, the IκBαDN cells maintained NF-κB in their cytoplasm following TNF-α treatment. Based on these results, we conclude that the mutant IκBα form expressed in IκBαDN cells is capable of inhibiting NF-κB translocation to nuclei in response to TNF-α treatment as an inducing stimulus.
Next, these cells were tested for their ability to prevent NF-κB nuclear localization in response to treatment with PMA. PMA was used previously in our pharmacological assays (Fig. 8) to stimulate NF-κB nuclear localization during infection. HEp-2 and IκBαDN cells were treated with 10 ng of PMA/ml in 5% FBS or left for 45 min, and the cells were again analyzed by indirect immunofluorescence using the anti-NF-κB p65 antibody (Fig. 9D). Both mock-treated HEp-2 and IκBαDN cells showed no nuclear localization of NF-κB. IκBαDN cells not treated with PMA shared this phenotype. PMA-treated HEp-2 cells clearly showed nuclear localization of NF-κB, while the treated IκBαDN cells did not. This last study served a dual purpose. First, it indicated that the IκBαDN cells had a defect in NF-κB nuclear localization in response to the PMA treatment stimulus. Second, it reiterated that HEp-2 cells respond to PMA by inducing the nuclear translocation of NF-κB, thus confirming our pharmacological studies discussed above.
Based on our characterization of the IκBαDN cells, we concluded that these cells are capable of inhibiting NF-κB nuclear localization in response to environmental stimulation by two well-characterized activators of NF-κB. As a consequence of the inhibition of NF-κB's translocation to the nuclei by the dominant-negative form of IκBα, the IκBαDN cells are sensitive to TNF-α-induced apoptosis in the absence of the protein synthesis inhibitor CHX. This finding strongly suggests that under these conditions, there is an absence of NF-κB-stimulated gene transcription in the IκBαDN cells.
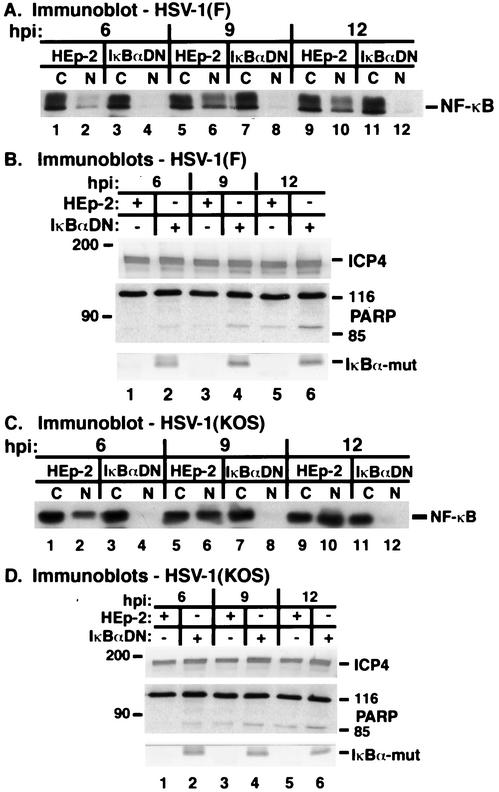
HSV-1 infection of an IκBα dominant-negative HEp-2 cell line.
The goal of this study was to access the impact of the dominant-negative IκBα on apoptosis during HSV-1 infection. HEp-2 and IκBαDN cells were infected with HSV-1(F) or HSV-1(KOS1.1), and at 6, 9, and 12 hpi whole-cell or subcellular extracts were prepared, separated in a denaturing polyacrylamide gel, transferred to nitrocellulose, and probed with anti-ICP4, anti-PARP, anti-IκBα, and anti-NF-κB antibodies as described in Materials and Methods. The results obtained using these wild-type HSV-1(F) (Fig. 10A and B) and HSV-1(KOS1.1) (Fig. 10C and D) viruses were as follows.
FIG. 10.
Apoptosis in IκBαDN cells following HSV-1(F) (A and B) and HSV-1(KOS1.1) (C and D) infection. Duplicate sets of HEp-2 or IκBαDN cells were infected with HSV-1(F) or HSV-1(KOS1.1) (MOI = 10) and at 6, 9, and 12 hpi, cytoplasmic (C) and nuclear (N) and whole-cell extracts were prepared, separated in denaturing gels, transferred to nitrocellulose, and probed with anti-NF-κB, anti-ICP4, anti-PARP, and anti-IκBα antibodies as described in Materials and Methods. IκBα-mut denotes the mutant protein, as the native form gets degraded and is not visible on the immunoblot. Anti-ICP4 and anti-IκBα immune reactivities were detected using alkaline phosphatase methods, while anti-PARP and anti-NF-κB reactivities were detected using a chemiluminescence technique.
While we detected an increase over time in the amount of NF-κB localized to the nuclei of both HSV-1(F)- and HSV-1(KOS1.1)-infected HEp-2 cells (Fig. 10A and C, lanes 2, 6, and 10), little to no translocation of NF-κB was observed in the infected IκBαDN cells (lanes 4, 8, and 12). The results indicate that the dominant-negative IκBα in our IκBαDN cells was active during the HSV-1(F) and HSV-1(KOS1.1) infections. There was also a loss of wild-type IκBα in the infected HEp-2 and IκBαDN cells (Fig. 10B and D, compare lanes 1, 3, and 5 with 2, 4, and 6). However, the mutant IκBα remained intact and was not degraded during infection of IκBαDN cells. Immune reactivities against the IE ICP4 protein were essentially the same for both viruses in infected IκBαDN and HEp-2 cells. Together, these findings confirm and extend those of Patel et al., who also reported that there was no decrease in the production of IE proteins in HSV-1(KOS) infection of an IκBα dominant-negative C33 cell line (45). In addition, our findings confirm the prediction that HSV-1 infection-induced NF-κB activation proceeds via the degradation of IκBα (45).
Finally, we observed an increase in the cleavage of the apoptotic death substrate PARP in the infected IκBαDN cells compared to that in the HEp-2 cells (Fig. 10B and D, compare lanes 1, 3, and 5 with 2, 4, and 6). The extent of PARP cleavage in the IκBαDN cells increased as the infection duration increased. This increase in the amount of PARP cleavage in infected IκBαDN cells coincided with the lack of IκBα degradation and the absence of nuclear NF-κB. In additional control studies (data not shown), we observed the same results using the IκBα dominant-negative clone number 4 (Fig. 9). These results indicate that there is an enhanced level of apoptosis in IκBαDN cells infected with wild-type HSV-1. The difference in PARP cleavage between the infected IκBαDN and HEp-2 cells is especially evident at early times (6 and 9 hpi), thus corroborating our data above that showed the importance of the kinetics of NF-κB activation during HSV-1 infection.
Based on these investigations, we conclude that (i) the viral induction of NF-κB nuclear localization proceeds via an IκBα degradation-dependent mechanism in infected HEp-2 cells. (ii) In IκBαDN cells, the inhibition of IκBα degradation and the subsequent inhibition of NF-κB activation leads to the processing of apoptotic substrates in infected cells. Thus, our results indicate that (iii) NF-κB activation and translocation to the nucleus is necessary for optimal prevention of apoptosis during HSV-1 infection.
DISCUSSION
Wild-type HSV-1 induces and then prevents apoptosis in human cells. This prevention requires infected cell proteins produced between 3 and 6 hpi. Interestingly, wild-type HSV-1 is capable of blocking apoptosis stimulated by staurosporine or sorbitol treatments, and these antiapoptotic functions also require infected cell protein produced during this cell death prevention window. The initial goal of our study was to determine whether putative infected cell antiapoptotic factors produced within this time frame during HSV-1 replication also block cell death induced by the addition of TNF-α plus CHX. Our investigation into the molecular basis of this phenomenon led us to focus on the behavior of the cellular transcription factor NF-κB during HSV-1 infection. The significant findings of our study may be summarized as follows.
(i) Proteins synthesized between 3 and 6 h post-HSV-1(F) infection block apoptosis induced by TNF-α plus CHX.
This conclusion is based on the observation that HEp-2 cells infected with this wild-type virus and treated with TNF-α-plus-CHX at 3 hpi exhibited apoptotic properties which resembled positive control, treated, uninfected cells. In contrast, infected cell proteins present at 6 hpi precluded apoptosis resulting from the addition of the proapoptotic agents. This result is comparable to our earlier findings showing that HSV-1(KOS1.1) blocked apoptosis induced by staurosporine or sorbitol during the identical prevention window (4). Thus, it appears that wild-type HSV-1 infection activates potent, broad-based antiapoptotic effectors that are produced between 3 and 6 hpi, and these activities appear to be independent of the virus strain. TNF-α initiates activation of the proapoptotic caspase cascade within cells, leading to cleavage of death substrates (40). This pathway is balanced by the antiapoptotic action of NF-κB, and protein synthesis is required for its protective effects (8, 54).
(ii) NF-κB translocates to the nuclei of HSV-1(F)-, HSV-1(R7032)-, or HSV-1(KOS1.1)-infected HEp-2 cells.
Our results are derived from a series of indirect immunofluorescence and subcellular fractionation experiments using these viruses. This general phenomenon has been reported by two other groups (1, 45). It is of interest that, in our hands, HSV-1(F) infection yielded more nuclear than cytoplasmic NF-κB at late infection times, while TNF-α-plus-CHX treatment of mock-infected cells resulted in approximately equal levels of nuclear and cytoplasmic NF-κB at the same time points. This finding was unexpected and suggests that infection may be a more potent stimulator of NF-κB translocation than TNF-α plus CHX treatment in these cells. An alternative, and perhaps more likely, explanation for this is that NF-κB may be free to shuttle in the treated, uninfected cells. Virus infection presumably short circuits this cycling, resulting in a persistent (45) activation of NF-κB. The possibility also exists that it is not a viral protein that directly stimulates NF-κB. The interaction of viral proteins with cellular protein(s) may lead to the translocation of this cell survival factor.
NF-κB is a transcription factor that functions in immune and inflammatory responses, apoptotic pathways, and the interferon induction pathway (7). In response to stimuli such as the TNF-α and interleukin-1 cytokines (9), bacterial lipopolysaccharide, or viral double-stranded RNA (20), a phosphorylation cascade is set in motion, leading ultimately to the activation of NF-κB. Inactive NF-κB is complexed with IκB in the cytoplasm. IκB degradation is induced following phosphorylation by IKK, and NF-κB is then released (17). This release unmasks the nuclear localization signal on NF-κB, allowing it to translocate to the nucleus, where it binds κB sites in promoters of target genes. As a result, NF-κB is a potent transcriptional activator of many factors, including antiapoptotic proteins, cytokines, cell adhesion molecules, growth factors, immune modulators, and assorted enzymes (44). It is currently not known whether nuclear NF-κB participates in regulating the expression of HSV-1 genes during infection. It is important to note that the genome of HSV-1 contains multiple NF-κB binding sites (49). It will be important to determine the significance of these NF-κB loci in the viral apoptosis prevention process.
(iii) Infected cell proteins produced after 3 hpi are required for the translocation of NF-κB.
NF-κB nuclear translocation followed the synthesis of the viral IE ICP22 protein inasmuch as nuclear ICP22 existed in cells where NF-κB was cytoplasmic. This finding extends earlier studies (45) using replication-defective ICP4-minus and ICP27-minus viruses that failed to show nuclear NF-κB activity during infection. Additionally, we observed that NF-κB is not detected in infected cell nuclei when CHX is added at 3 hpi and maintained to 18 hpi. This finding verifies the necessity of de novo-infected cell protein synthesis for the induction of NF-κB nuclear translocation. That NF-κB translocation appears to be delayed during HSV-1 replication seems counterintuitive, since the initiation of many cell responses to viral invasion are very early events (reviewed in reference 31). For example, it was recently discovered that HSV-1 entry into cells induces a cellular antiviral response that is blocked by IE proteins (42, 43). While we do not know the mechanism by which NF-κB nuclear translocation is stimulated, it is conceivable that viral rather than cellular factors play the dominant roles in regulating this phenomenon. It stands to reason that if the nuclear import of NF-κB is related to the early antiviral response, there must be some sort of a block preventing the translocation until after 3 hpi. It is conceivable that a preexisting protein, such as a component of the incoming virion, might act to “stall” NF-κB, and then a factor produced at later times functions to remove this impediment. Additional experiments are currently under way to address these and other questions regarding the activation of NF-κB during HSV-1 infection.
The initiation of NF-κB nuclear import precedes the late stage of viral infection, but the protein continues to accumulate in the nucleus up to 9 hpi and is maintained at this level to 18 hpi. The late phase of HSV-1 infection corresponds to the period in which a dramatic reorganization of cytoplasmic microtubules occurs (6, 32). We recently discovered that this rearrangement facilitates the translocation of the major virion tegument protein VP22 into the nucleus (32). It seemed conceivable that the import of NF-κB might be regulated in a similar manner. However, several pieces of data argue against this appealing hypothesis. First, there is no difference in the timing of the translocation of NF-κB between the HSV-1(F) and HSV-1(R7032) strains. In contrast, the kinetics of VP22 nuclear import differs between the two viruses, which led us to propose previously that glycoprotein gE plays a role in the virus-dependent microtubule reorganization process (32). Second, microtubules reassemble at late infection times (6, 32), but NF-κB is predominantly nuclear at 18 and 24 hpi. Finally, NF-κB was detected in the nuclei of cells in which VP22 was still localized in the cytoplasm.
(iv) Infected cells which possess nuclear NF-κB do not undergo apoptosis.
NF-κB nuclear translocation in productively infected HEp-2 cells is a gradual, increasing process. The prevention of apoptosis is also a time-dependent process (4) in these infected cells. As these two events occur within the same window of time, there is a correlation of the translocation of NF-κB to the nucleus with the prevention of apoptosis. Three out of the four viruses we analyzed in this study stimulated NF-κB nuclear import during infection. HSV-1(F), HSV-1(KOS1.1), and HSV-1(R7032) are replication-competent viruses that trigger apoptosis but then prevent the process from killing the infected cells. Initially, these correlations suggested that NF-κB participates in apoptosis prevention during productive HSV-1 infection. To test this hypothesis, we utilized the replication-defective HSV-1(vBSΔ27) virus. From previous studies (2), it was known that HSV-1(vBSΔ27) was an apoptotic virus (3). During HSV-1(vBSΔ27) infection, NF-κB remained cytoplasmic and the cells underwent apoptosis. The observation in these experiments that HSV-1(vBSΔ27)-infected HEp-2 cells produce reduced amounts of viral ICP4 supports the model that infected cell proteins are required for NF-κB nuclear import.
The nuclear translocation of NF-κB also appears to coincide with the activation of caspase 3 under certain conditions. Our findings indicate that this correlation holds in both infected and uninfected cells. This theory is based on two findings. First, a small amount of nuclear NF-κB was detected in mock-infected cells that were treated with CHX alone, and this level was comparable in magnitude to the amount of caspase 3 processing observed in these cells. Caspase 3 is not activated in mock, untreated cells, and no nuclear NF-κB is detected in them. Second, infections of both untreated cells and cells treated with CHX at 6 hpi led to caspase 3 activation, and NF-κB was detected in the nuclei of these cells. Interestingly, minimal nuclear NF-κB is detected when caspase 3 is completely degraded, and cells die by apoptosis (e.g., CHX addition at 3 hpi during infection). All HSV-1 strains which express IE genes that have been tested to date activate caspase 3 during infection (3). Since productive HSV-1 infection also leads to the nuclear translocation of NF-κB, it seems likely that the import of NF-κB may be a direct consequence of the activation of caspase 3. Studies designed to block the activation of caspase 3 during infection should confirm this model.
(v) The viral glycoprotein gE is not required for the prevention of apoptosis during HSV-1 infection.
Nuclear localization of NF-κB occurs during infection of HEp-2 cells with a virus containing a deletion in the gene encoding gE, and the kinetics of the NF-κB nuclear import are identical to those of HSV-1(F). Similarly, cells infected with the gE-minus virus were not killed by apoptosis. Thus, gE is not essential for the prevention of apoptosis. At least three other viral proteins implicated in preventing apoptosis in HSV-1-infected cells are also not absolutely required for viral replication (3, 4, 24, 38). ICP22, US3, and US5 are, therefore, accessory factors (3), while ICP27, ICP4, and gD are required for apoptosis prevention during infection (2, 37, 59). Studies are currently under way to determine the effect of the IE ICP27 and ICP4 proteins on the activation of NF-κB.
(vi) NF-κB does not bind DNA in the nuclei of apoptotic HSV-1(vBSΔ27)-infected human cells.
There is little to no DNA binding activity of NF-κB in HSV-1(vBSΔ27)-infected cells, while its DNA binding activity in wild-type HSV-1(F)- or HSV-1(KOS1.1)-infected cells is substantial compared to that of mock infection. In the HSV-1(vBSΔ27)-infected cells, where NF-κB did not bind DNA, apoptotic death factor processing was detected. The implication of these data is that NF-κB activation seems necessary for the inhibition of apoptosis. Thus, an HSV-1 virus that is unable to activate NF-κB may not prevent the apoptosis that its early viral processes induce. Infected cell factors necessary for the activation of NF-κB are either not synthesized or not active during HSV-1(vBSΔ27) infection. It is remains unclear whether it is the ICP27 protein itself or the proteins whose synthesis it regulates that actually modulate NF-κB.
(vii) Activation of NF-κB prior to infection with apoptotic HSV-1(vBSΔ27) rescues the infected cell from apoptosis.
Interestingly, the decrease in apoptotic processing observed during PMA treatment of HSV-1(vBSΔ27)-infected cells was accompanied by an increase in the accumulation of viral proteins. As mentioned above, potential NF-κB binding sites exist in some viral promoters. It is possible that PMA treatment enabled NF-κB to promote viral replication, thus overcoming the defects caused by deletion of ICP27 from the mutant virus. However, it is more likely that the activated NF-κB participated in the synthesis of antiapoptotic mediators (7, 8, 54, 56), giving HSV-1(vBSΔ27) the opportunity to replicate more efficiently. Supporting this idea is our finding that prevention of apoptosis by the addition of caspase inhibitors leads to the increased accumulation of viral proteins (4). An appealing theory is that NF-κB may simply function to delay virus-triggered apoptosis until the point in which viral antiapoptotic factors have been produced and have accumulated to adequate levels to block infected cell death.
(viii) Prevention of NF-κB nuclear import leads to an increase in apoptosis during HSV-1(F) infection.
We utilized two methods to inhibit NF-κB function. The pharmacological inhibitor PDTC antagonizes the release of cytoplasmic NF-κB from IκBα. Our dominant-negative cell lines contain a mutant form of IκBα that sequesters NF-κB in the cytoplasm. In both situations, reducing the amount of NF-κB nuclear import led to an increase in death factor processing during infection. The conclusion drawn from these data is that in the absence of NF-κB translocation and activation, wild-type HSV-1 is unable to optimally prevent infection-induced apoptosis. These findings strongly implicate a role for NF-κB in apoptosis prevention during HSV-1 infection. Our results are exciting because we have now developed a molecular genetic system in which wild-type HSV-1-infected cells exhibit phenotypes that are usually only observed in HSV-1(vBSΔ27)-infected cells.
Several human viruses have evolved ways to appropriate aspects of the NF-κB pathway (21, 31), and NF-κB is widely known to be involved in antiapoptotic actions. It is our hypothesis that this transcription factor is necessary, at least in part, for the prevention of apoptosis and promotion of survival in HSV-1-infected cells. It is not known whether viral or cellular proteins play dominant roles in regulating NF-κB nuclear import during infection. Patel et al. concluded that NF-κB localization to the nucleus is necessary for efficient viral replication (45) but did not address the mechanism underlying their observations. Our investigations with the IκBαDN cell line have established that the requirement for NF-κB activation during HSV-1 infection is based, at least in part, on its antiapoptotic activities. These results corroborate these earlier authors' findings, since conditions that result in the retention of NF-κB in the cytoplasm lead to apoptotic death of the infected cells. Thus, our data suggest that the reason that NF-κB is needed for optimal virus replication is because it acts to prevent apoptosis triggered by the virus.
In response to an inducing stimulus, IκBα is degraded to allow NF-κB to enter the nucleus, and this degradation is signaled by phosphorylation of IκBα by IKK (9, 17, 27). Investigations by Patel et al. and Amici et al. indicated that IκBα played a role in NF-κB activation during HSV-1 infection (1, 45). HSV-1 infection induces translocation of NF-κB to the nuclei of infected C33 cells (45). These authors reported that IκB was degraded and was not resynthesized during infection. Additionally, NF-κB translocation was reduced in cells in which IκB degradation was inhibited either by using a dominant-negative IκB (45) or by inhibiting IKK activity (1). Amici et al. also proposed that phosphorylation of IκBα by IKK was responsible for NFκB release (1). In their studies of a potential antiviral therapy, prostaglandin A1 was used to inhibit IKK kinase activity, and they observed that this compound hindered the ability of the virus to replicate, leading to a decrease in viral titer. We observed that HSV-1 has a reduced ability to replicate in our IκBαDN cells compared to HEp-2 cells. Thus, it is likely that the mechanism by which cytoplasmic NF-κB becomes activated during HSV-1 infection of HEp-2 cells involves the release from IκB.
The mechanism through which the persistent degradation of IκBα might occur during HSV-1 infection remains unknown. The maintenance of nuclear NF-κB which we observe at late infection times is likely a consequence of this perturbed regulation. We now propose that the function of this activated, nuclear NF-κB is to participate in apoptosis prevention during HSV-1 infection. Our development of these biochemical and molecular genetic experiment systems designed to modulate NF-κB activity during infection should continue to provide important new information on the role of NF-κB during HSV-1 infection.
Acknowledgments
We thank Elise Morton (Mount Sinai) for critically reading the manuscript; Saul Silverstein (Columbia University) for providing HSV-1(vBSΔ27), which was generated by Bob Soliman in his laboratory, and HSV-1(KOS1.1) viruses; and Bernard Roizman (University of Chicago) for providing low-passage isolates of HSV-1(R7032), which was originally generated by Richard Longnecker, Penelope Mavromara-Nazos, and Amy Sears in his laboratory, and its parental HSV-1(F).
These studies were supported in part by grants from the U.S. Public Health Service (AI38873 and AI48582 to J.A.B. and AI52417 to A.T.T.) and the American Cancer Society (JFRA 634 to J.A.B.). J.A.B. thanks the Lucille P. Markey Charitable Trust and the National Foundation for Infectious Diseases for their support. M.L.G. was a predoctoral trainee and was supported in part by a U.S. Public Health Service Institutional Research Training Award (GM 007280).
REFERENCES
- 1.Amici, C., G. Belardo, A. Rossi, and M. G. Santoro. 2001. Activation of I kappa b kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276:28759-28766. [DOI] [PubMed] [Google Scholar]
- 2.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert, M., and J. A. Blaho. 2001. Modulation of apoptosis during herpes simplex virus infection in human cells. Microbes Infect. 10:859-866. [DOI] [PubMed] [Google Scholar]
- 4.Aubert, M., J. O'Toole, and J. A. Blaho. 1999. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J. Virol. 73:10359-10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert, M., S. A. Rice, and J. A. Blaho. 2001. Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells. J. Virol. 75:1013-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avitabile, E., S. Di Gaeta, M. R. Torrisi, P. L. Ward, B. Roizman, and G. Campadelli-Fiume. 1995. Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J. Virol. 69:7472-7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkett, M., and T. D. Gilmore. 1999. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 18:6910-6924. [DOI] [PubMed] [Google Scholar]
- 8.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-alpha-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 9.Beg, A. A., T. S. Finco, P. V. Nantermet, and A. S. Baldwin, Jr. 1993. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IκBα: a mechanism for NF-κB activation. Mol. Cell. Biol. 13:3301-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaho, J. A., C. Mitchell, and B. Roizman. 1994. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J. Biol. Chem. 269:17401-17410. [PubMed] [Google Scholar]
- 11.Blaho, J. A., C. Mitchell, and B. Roizman. 1993. Guanylylation and adenylylation of the alpha regulatory proteins of herpes simplex virus require a viral beta or gamma function. J. Virol. 67:3891-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaho, J. A., and B. Roizman. 1998. Analyses of HSV proteins for posttranslational modifications and enzyme functions, p. 237-256. In S. M. Brown and A. R. Maclean (ed.), Methods in molecular medicine: herpes simplex virus protocols, vol. 10. Human Press Inc., Totowa, N.J. [DOI] [PubMed]
- 13.Blaho, J. A., and B. Roizman. 1991. ICP4, the major regulatory protein of herpes simplex virus, shares features common to GTP-binding proteins and is adenylated and guanylated. J. Virol. 65:3759-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaho, J. A., C. S. Zong, and K. A. Mortimer. 1997. Tyrosine phosphorylation of the herpes simplex virus type 1 regulatory protein ICP22 and a cellular protein which shares antigenic determinants with ICP22. J. Virol. 71:9828-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowie, A., and L. A. J. O'Neill. 2000. Oxidative stress and nuclear factor-κB activation. Biochem. Pharm. 59:13-23. [DOI] [PubMed] [Google Scholar]
- 16.Brockman, J. A., D. C. Scherer, T. A. McKinsey, S. M. Hall, X. Qi, W. Y. Lee, and D. W. Ballard. 1995. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol. Cell. Biol. 15:2809-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiDonato, J. A., F. Mercurio, and M. Karin. 1995. Phosphorylation of IκBα precedes but is not sufficient for its dissociation from NF-κB. Mol. Cell. Biol. 15:1302-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erlandsson, A. C., L. G. Bladh, P. Stierna, T. Yucel-Lindbeg, O. Hammarsten, T. Modeer, J. Harmenberg, and A. C. Wikstrom. 2002. Herpes simplex virus 1 infection and glucocorticoid treatment regulate viral yield, glucocorticoid receptor, and NF-κB levels. J. Endocrinol. 175:165-176. [DOI] [PubMed] [Google Scholar]
- 19.Galvan, V., and B. Roizman. 1998. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 95:3931-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilmore, T. D. 1999. The Rel/NF-κB signal transduction pathway: introduction. Oncogene 18:6842-6844. [DOI] [PubMed] [Google Scholar]
- 21.Hiscott, J., H. Kwon, and P. Genin. 2001. Hostile takeovers: viral appropriation of the NF-κB pathway. J. Clin. Investig. 107:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honess, R. W., and B. Roizman. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc. Natl. Acad. Sci. USA 72:1276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, H. Lee, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, US5 and US3. J. Virol. 73:8950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jerome, K. R., J. F. Tait, D. M. Koelle, and L. Corey. 1998. Herpes simplex virus type 1 renders infected cells resistant to cytotoxic T-lymphocyte-induced apoptosis. J. Virol. 72:436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, D. C., M. C. Frame, M. W. Ligas, A. M. Cross, and N. D. Stow. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 62:1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karin, M. 1999. How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene 18:6867-6874. [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann, S. H., S. Desnoyers, Y. Ottaviano, N. E. Davidson, and G. G. Poirier. 1993. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 53:3976-3985. [PubMed] [Google Scholar]
- 29.Kerr, F. R., and B. V. Harmon. 1991. Definition and incidence of apoptosis: an historical perspective, p. 5-29. In L. D. Tomei and F. O. Cope (ed.), Apoptosis: the molecular basis of cell death. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Kerr, J. F., A. H. Wyllie, and A. R. Currie. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26:239-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knipe, D. M., C. E. Samuel, and P. Palese. 2001. Virus-host interactions, p. 130-170. In D. M. Knipe and P. M. Howley (ed.), Virology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 32.Kotsakis, A., L. E. Pomeranz, A. Blouin, and J. A. Blaho. 2001. Microtubule reorganization during herpes simplex virus type 1 infection facilitates the nuclear localization of VP22, a major virion tegument protein. J. Virol. 75:8697-8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyama, A. H., and A. Adachi. 1997. Induction of apoptosis by herpes simplex virus type 1. J. Gen. Virol. 78:2909-2912. [DOI] [PubMed] [Google Scholar]
- 34.Koyama, A. H., T. Fukumori, M. Fujita, H. Irie, and A. Adachi. 2000. Physiological significance of apoptosis in animal virus infection. Microbes Infect. 2:1111-1117. [DOI] [PubMed] [Google Scholar]
- 35.Koyama, A. H., and Y. Miwa. 1997. Suppression of apoptotic DNA fragmentation in herpes simplex virus type 1-infected cells. J. Virol. 71:2567-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, R., P. Beaupariant, H. Elford, P. Ponka, and J. Hiscott. 1997. Selective inhibition of IκBα phosphorylation and HIV LTR-directed gene expression by novel antioxidant compounds. Virology 234:277-290. [DOI] [PubMed] [Google Scholar]
- 37.Leopardi, R., and B. Roizman. 1996. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc. Natl. Acad. Sci. USA 93:9583-9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 94:7891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, H., and J. Yuan. 1999. Deciphering the pathways of life and death. Curr. Opin. Cell Biol. 11:261-266. [DOI] [PubMed] [Google Scholar]
- 40.Liu, Z. G., H. Hsu, D. V. Goeddel, and M. Karin. 1996. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87:565-576. [DOI] [PubMed] [Google Scholar]
- 41.Meignier, B., R. Longnecker, P. Mavromara-Nazos, A. E. Sears, and B. Roizman. 1988. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162:251-254. [DOI] [PubMed] [Google Scholar]
- 42.Mossman, K. L., P. F. Macgregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholl, M. J., L. H. Robinson, and C. M. Preston. 2000. Activation of cellular interferon-responsive genes after infection of human cells with herpes simplex virus type 1. J. Gen. Virol. 81:2215-2218. [DOI] [PubMed] [Google Scholar]
- 44.Pahl, H. L. 1999. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 18:6853-6866. [DOI] [PubMed] [Google Scholar]
- 45.Patel, A., J. Hanson, T. I. McLean, J. Olgiate, M. Hilton, W. E. Miller, and S. L. Bachenheimer. 1998. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology 247:212-222. [DOI] [PubMed] [Google Scholar]
- 46.Pomeranz, L. E., and J. A. Blaho. 2000. Assembly of infectious herpes simplex virus type 1 virions in the absence of full-length VP22. J. Virol. 74:10041-10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pomeranz, L. E., and J. A. Blaho. 1999. Modified VP22 localizes to the cell nucleus during synchronized herpes simplex virus type 1 infection. J. Virol. 73:6769-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Virology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 49.Rong, B. L., T. A. Libermann, K. Kogawa, S. Ghosh, L. X. Cao, D. Pavan-Langston, and E. C. Dunkel. 1992. HSV-1-inducible proteins bind to NF-kappa B-like sites in the HSV-1 genome. Virology 189:750-756. [DOI] [PubMed] [Google Scholar]
- 50.Schreck, R., B. Meier, D. N. Mannel, W. Droge, and P. Baeuerle. 1992. Dithiocarbamates as potent inhibitors of nuclear factor κB activation in intact cells. J. Exp. Med. 176:1181-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sekulovich, R. E., K. Leary, and R. M. Sandri-Goldin. 1988. The herpes simplex virus type 1 alpha protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J. Virol. 62:4510-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ting, A. T., F. X. Pimentel-Muinos, and B. Seed. 1996. RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but not Fas/APO-1-initiated apoptosis. EMBO J. 15:6189-6196. [PMC free article] [PubMed] [Google Scholar]
- 54.Van Antwerp, D. J., S. J. Martin, T. Kafri, D. R. Green, and I. M. Verma. 1996. Suppression of TNF-alpha-induced apoptosis by NF-κB. Science 274:787-789. [DOI] [PubMed] [Google Scholar]
- 55.Wallach, D., E. E. Varfolomeev, N. L. Malinin, Y. V. Goltsev, A. V. Kovalenko, and M. P. Boldin. 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17:331-367. [DOI] [PubMed] [Google Scholar]
- 56.Wang, C. Y., M. W. Mayo, and A. S. Baldwin, Jr. 1996. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 274:784-787. [DOI] [PubMed] [Google Scholar]
- 57.Wolf, B. B., and D. R. Green. 1999. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J. Biol. Chem. 274:20049-20052. [DOI] [PubMed] [Google Scholar]
- 58.Wyllie, A. H., J. F. Kerr, and A. R. Currie. 1980. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68:251-306. [DOI] [PubMed] [Google Scholar]
- 59.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]