Abstract
Measles virus, a paramyxovirus of the Morbillivirus genus, is responsible for an acute childhood illness that infects over 40 million people and leads to the deaths of more than 1 million people annually (C. J. Murray and A. D. Lopez, Lancet 349:1269-1276, 1997). Measles virus infection is characterized by virus-induced immune suppression that creates susceptibility to opportunistic infections. Here we demonstrate that measles virus can inhibit cytokine responses by direct interference with host STAT protein-dependent signaling systems. Expression of the measles V protein prevents alpha, beta, and gamma interferon-induced transcriptional responses. Furthermore, it can interfere with signaling by interleukin-6 and the non-receptor tyrosine kinase, v-Src. Affinity purification demonstrates that the measles V protein associates with cellular STAT1, STAT2, STAT3, and IRF9, as well as several unidentified partners. Mechanistic studies indicate that while the measles V protein does not interfere with STAT1 or STAT2 tyrosine phosphorylation, it causes a defect in IFN-induced STAT nuclear accumulation. The defective STAT nuclear redistribution is also observed in measles virus-infected cells, where some of the STAT protein is detected in cytoplasmic bodies that contain viral nucleocapsid protein and nucleic acids. Interference with STAT-inducible transcription may provide a novel intracellular mechanism for measles virus-induced cytokine inhibition that links innate immune evasion to adaptive immune suppression.
Cytokine signal transduction is essential for normal immune function and controls the quality of responses to a wide variety of microbial infections. Innate and adaptive host responses to virus infections are regulated by autocrine and paracrine cytokine signaling systems. For most cytokines, receptor binding triggers an intracellular signaling cascade involving one or more signal transducer and activator of transcription (STAT) proteins. Diverse cytokine and growth factor signaling pathways produce active STAT transcription factors that specify mRNA induction profiles (26). For example, the alpha and beta interferon (IFN-α/β) family is of primary importance for both innate and adaptive antiviral immunity (reviewed in references 1, 49, and 53). In the innate antiviral system, IFN-α/β initiates a receptor-mediated signaling system that produces an activated STAT1-STAT2-IRF9 heterotrimeric transcription complex known as ISGF3 (27). The ISGF3 complex translocates to the nucleus, where it can bind to target gene promoter elements and induce the transcription of host antiviral genes. Similarly, IFN-γ, a cytokine that mediates both innate and adaptive immune responses critical for defense against microbial infections and cancer (21), activates a homodimeric STAT1 transcription factor, GAF (9). Interleukin 6 (IL-6), a cytokine required for acute-phase responses, liver regeneration, B-cell maturation, and macrophage differentiation, activates STAT3 homodimers, a response in common with growth factor pathways (69) and oncogenic tyrosine kinases such as v-Src (68). The combined output of these and other cytokine signals determines the effectiveness of the immune response in engaging and neutralizing pathogens.
The success of many pathogens is linked to the evasion of host defense mechanisms. Many viruses encode gene products that are capable of inhibiting IFN systems (22). For negative-strand RNA viruses of the family Paramyxoviridae, host evasion activities have been ascribed to the viral V protein. Paramyxovirus V proteins have no cellular homologues but are readily identifiable by a highly conserved cysteine-rich zinc-binding domain at their C terminus (25, 41, 55). This C-terminal domain (CTD) is approximately 50% identical between all paramyxovirus V proteins. A diverse range of host evasion activities, including prevention of apoptosis (15, 60), cell cycle alterations (29), inhibition of double-stranded RNA signaling (15, 43), and prevention of IFN biosynthesis (15, 43, 60), have been ascribed to paramyxovirus V proteins. Moreover, V protein expression has also been demonstrated to inhibit the activities of IFN-responsive STAT proteins. Acute expression of V proteins from Rubulavirus species induces specific ubiquitylation and proteasome-dependent degradation of STATs (11, 24, 37, 56, 57, 64, 67). While simian virus 5 (SV5) V protein can target STAT1 for degradation, type II human parainfluenza virus V protein targets STAT2 and mumps virus V protein can target both STAT1 (35) and STAT3 (57). The Rubulavirus STAT degradation mechanism involves V protein-dependent degradation complexes (VDCs) that contain the V protein, STAT1 and STAT2 (and STAT3 in mumps virus), and a number of additional cellular proteins including the UV-damaged DNA-binding protein DDB1 (2, 29, 30, 56) and Cul4A, a member of the cullin family of ubiquitin ligase subunits (56). The mumps virus V protein can also interact with cellular RACK1, possibly influencing IFN receptor activity (23). Newcastle disease virus also encodes a V protein that has been demonstrated to antagonize the avian IFN system (40).
Nipah virus, a species of the recently emerged and deadly Henipavirus genus, encodes a V protein that is not conserved outside of the CTD. Nipah virus V protein can nonetheless antagonize IFN responses in both human and avian systems (40, 48). Rather than degrading STATs, Nipah virus V protein sequesters both STAT1 and STAT2 in high-molecular-weight cytoplasmic complexes, thereby preventing IFN-dependent and basal STAT protein nuclear accumulation, an efficient means of stopping host antiviral responses (48).
The discovery of functional diversity within the context of STAT inhibition suggested the possibility that other paramyxoviruses might encode V proteins that possess distinct STAT-directed interference properties. Measles virus, a prototype of the Morbillivirus genus, encodes a V protein distinct from those of the Rubulavirus and Henipavirus genera. The measles virus V protein has 37% overall amino acid sequence identity to the SV5 V protein (7% outside the CTD) but is homologous to the Nipah virus V protein only within the CTD.
The virulence and pathogenicity of measles virus have been well correlated with cellular IFN responses. It has been reported that infection with some strains of measles virus can induce IFN biosynthesis (34) and that measles infection (18) or expression of the measles virus N protein (54) can activate NF-κB and IRF3 transcription factors that are required for IFN-β gene induction. In addition, infection with measles virus activates the IFN-induced STAT pathways to yield activated ISGF3 and GAF signaling complexes (18). Both wild-type measles virus and vaccine strains induce immediate-early genes and genes linked to antiviral responses (5, 17). Recombinant measles viruses with defective V proteins can grow in cell lines that do not produce IFN (51), but in vivo studies demonstrate an important role of the V proteins as virulence factors (42), and analysis of thymic xenografts revealed that V-deficient virus replication was delayed and prolonged compared to that of wild-type or V-overproducing viruses (58). These phenotypes are consistent with a role for V protein in measles pathogenesis and might be explained by defective host evasion capabilities in the V-deficient virus.
To test the hypothesis that measles virus might also have evolved to use V-dependent mechanisms to inhibit STAT signaling, assays for V-dependent IFN signaling inhibition were performed. The results demonstrate that measles virus V protein is an efficient inhibitor of IFN signal transduction but acts via a mechanism distinct from the previously characterized activities of both Rubulavirus and Henipavirus V proteins. Measles virus V protein prevents both IFN-α/β- and IFN-γ-induced transcriptional responses and inhibits STAT3-dependent signaling via IL-6 or v-Src. Measles virus V protein copurifies STAT1, STAT2, STAT3, and IRF9 but does not copurify other known cellular components of Rubulavirus VDC ubiquitin ligases. It does not eliminate IFN-induced STAT activation by tyrosine phosphorylation but effectively prevents IFN-induced STAT nuclear translocation. In addition to these effects revealed by ectopic expression of measles virus V protein, a dramatic redistribution of cellular STAT proteins is observed in measles virus-infected cells, suggesting that additional virus-encoded factors may also participate in immune evasion.
MATERIALS AND METHODS
Cells and viruses.
Human 2fTGH, 293T, and 293 Tet-On (Clontech) cell lines and murine NIH 3T3 cells were maintained in Dulbecco's modified Eagle's medium (Gibco-BRL) supplemented with 10% Cosmic calf serum (HyClone) and 1% penicillin-streptomycin (Gibco-BRL) as described previously (38). Measles virus (Edmonston strain; ATCC VR-24) was cultivated and subjected to titer determination in Vero cells.
For the tetracycline-inducible measles virus V cell line, 293 Tet-On cells (Clontech) were stably transfected with a pBI-FLAG-measles virus V plasmid and a puromycin-resistant plasmid (pBABE-puro [32]). Individual clones were selected and then probed by FLAG immunoblotting for inducible V expression regulated by 1 μg of doxycycline per ml. Positive clones were further screened for doxycycline-inducible suppression of IFN-α-induced ISRE-Luciferase reporter gene activity (data not shown). The Tet-On measles virus V cell line was maintained in Dulbecco's modified Eagle's medium supplemented with 10% Cosmic calf serum, 1% penicillin-streptomycin, 2 μg of puromycin per ml, and 500 μg of G418 per ml.
Plasmids, transfection, and reporter gene assays.
A cDNA copy of the measles virus V mRNA was amplified by PCR from reverse-transcribed polyadenylate RNA isolated from infected Vero cells by oligo(dT) chromatography. Primers were used to add restriction endonuclease recognition sequences for direct cloning of the PCR products into a mammalian expression plasmid downstream of in-frame N-terminal FLAG or HA epitope tags. Several independently transcribed and amplified cDNAs were sequenced, and V cDNAs were recognized by the presence of non-templated nucleotides at the “editing site.” All full-length V cDNAs were found to differ from the GenBank database sequence entry at two positions (C at nucleotide 423; G at nucleotide 825) without influencing the amino acid sequence.
Luciferase assays in 293T and 2fTGH cells were carried out exactly as described previously (57). In all cases, data represent the average values for triplicate samples normalized to cotransfected β-galactosidase activity, expressed as a percentage of the value for the control stimulated sample. In the experiments in some figures, P values were calculated by the t test using SigmaPlot software.
Indirect immunofluorescence.
For immunofluorescence, cells were grown on Permanox chamber slides (Nalgene Nunc) and transfected and stained exactly as described previously (48). Images were obtained using a Leica TCSSP confocal microscope, and representative fields illustrating transfected and untransfected cells in the same field are illustrated whenever possible. For infections, 2fTGH cells were infected on chamber slides with 2 or 10 PFU of measles virus per cell and stained with measles virus N antibody (10 μg/ml; Chemicon International). Nucleic acids were visualized by staining with 48 nM TOTO3 (Molecular Probes). Representative fields containing both transfected and untransfected cells are illustrated, but effects of measles virus V protein on STATs were observed with 100% penetrance in several independent experiments.
Cell extracts, immunoblotting, and immunoprecipitation.
For preparation of cell extracts, samples were washed once with ice-cold phosphate-buffered saline and subsequently lysed with whole-cell extract buffer (WCEB) as described previously (38, 56). For immunoblotting, proteins were separated, transferred to nitrocellulose, probed with antibodies, and visualized by chemiluminescence (NEN Life Sciences). For immunoprecipitation, lysates were prepared in WCEB and precleared with protein A-agarose. Antibody-protein complexes were purified with protein A beads and washed with WCEB. After elution with sodium dodecyl sulfate (SDS), proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and processed for immunoblotting. The antibodies used were STAT1 (Santa Cruz C20), STAT2 (Santa Cruz, C24), STAT3 (Santa Cruz C20), IRF9 (Santa Cruz 496), FLAG (Sigma), DDB1 (Abcam 9194), Cullin 4A (Santa Cruz 8557), STAT1 phosphotyrosine 701 (Cell Signaling Technologies 9171), and STAT2 phosphotyrosine 690 (Upstate Biotechnology 07-224).
Affinity purification.
Affinity purification was carried out exactly as described previously (56) using FLAG affinity gel (Sigma). After binding, the beads were washed with WCEB and protein complexes were eluted with 100 μg of FLAG peptide per ml. Eluates were denatured in SDS loading buffer, separated by SDS-PAGE, and processed for either silver staining (Bio-Rad Silver Stain Plus) or immunoblotting.
RESULTS
Measles virus V protein inhibits IFN-α and IFN-γ transcriptional responses.
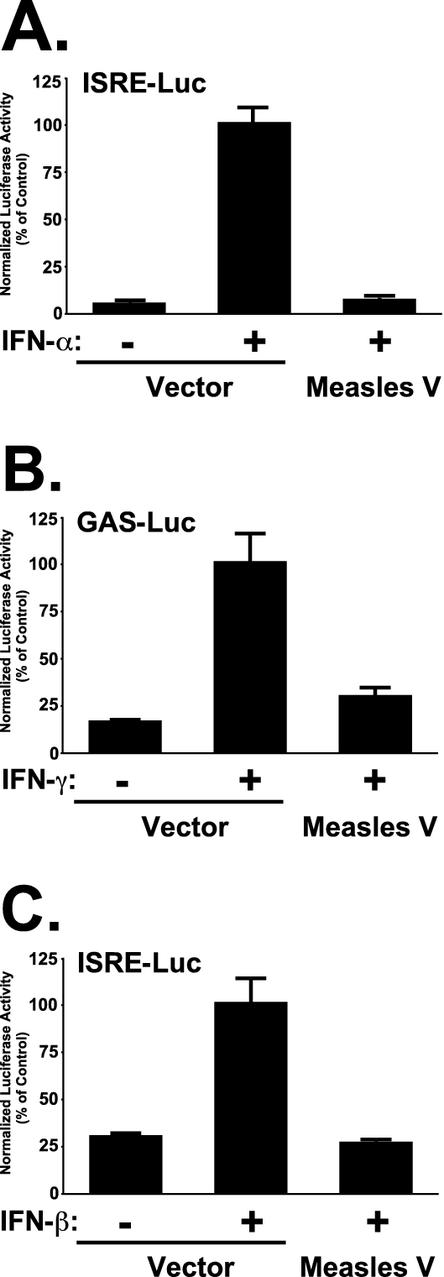
A cDNA copy of the measles virus V mRNA from the Edmonston strain was isolated and tested in assays for IFN signaling inhibition (Fig. 1). Stimulation of cells with IFN-α potently activated the transcription of an ISGF3-responsive ISRE-luciferase reporter gene. In contrast, expression of measles virus V protein interfered with the ability of IFN-α to induce a transcriptional response (Fig. 1A). Similarly, the response to IFN-γ was tested using a STAT1-dependent γ-IFN activation sequence (GAS)-luciferase reporter gene. Expression of measles virus V protein also inhibited the transcriptional response to IFN-γ (Fig. 1B). These results indicate that isolated expression of the measles virus V protein is sufficient to suppress STAT-dependent signaling by IFNs. For SV5, differences between human and murine STAT2 orthologues provide a barrier to SV5-induced STAT1 degradation in the mouse system (39). To test the measles virus V protein for species-restricted IFN signaling inhibition, mouse NIH 3T3 fibroblast cells were used for the ISGF3 transcription assay. While IFN stimulation induced the ISRE-luciferase reporter gene, expression of the measles virus V protein efficiently antagonized IFN-β signaling in the murine system (Fig. 1C). Together, these results indicate that measles virus V protein is an effective inhibitor of IFN-α/β and IFN-γ signaling and functions in both human and murine systems.
FIG. 1.
Measles virus V protein blocks IFN signal transduction. (A) 293T cells were transfected with an ISRE-luciferase reporter gene and either empty vector or measles virus V protein expression vector as indicated. Cells were treated with 1,000 U of IFN-α per ml for 14 h prior to lysis and luciferase assays (+) or left untreated (−). (B) The same experiment was carried out using a GAS-luciferase reporter gene and treatment with 5 ng of IFN-γ per ml. (C) Murine NIH3T3 fibroblasts were subject to ISRE-luciferase assays as above, but using 1,000 U of murine IFN-β per ml.
Measles virus V protein forms complexes with STAT1, STAT2, STAT3, and IRF9.
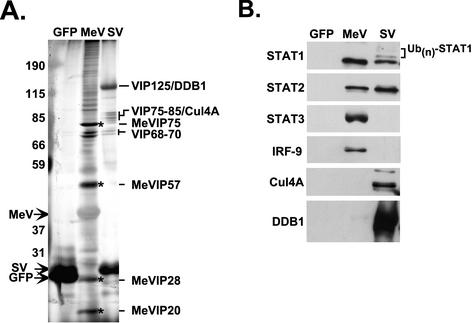
STAT protein targeting by SV5, HPIV2, and mumps viruses requires a multisubunit VDC Ub ligase complex that is composed of cellular proteins including STATs, DDB1, and Cul4A (56, 57), and Nipah virus V protein assembles high-molecular-weight STAT-containing complexes (48). To isolate a measles virus V protein STAT-targeting complex, FLAG-tagged measles virus V was subjected to affinity chromatography along with FLAG-tagged SV5 V and green fluorescent protein (GFP) controls. Analysis of the affinity-purified material by SDS-PAGE and silver staining revealed the expected pattern of polypeptide species for SV5 V that ranged in apparent molecular mass between 40 and 300 kDa, in agreement with previously described SV5 V-interacting protein (VIP) complexes (56, 57). The measles virus V affinity preparation had a distinct composition, with only a few bands in common with SV5 V, notably in the range of VIP68 to VIP70. In addition, the eluted material contained several unique measles virus V-interacting protein (MeVIP) species with the most abundant MeVIP bands migrating in the range of ∼20, ∼28, ∼57, and ∼75 kDa (Fig. 2A). Interestingly, a more heterogenous ladder of higher-molecular-mass proteins that migrate between ∼90 and >200 kDa were found in the measles virus V affinity preparation. Overall, the patterns of cellular proteins associated with the two viral proteins are strikingly distinct, consistent with a hypothesis that these complexes function differently in STAT signaling inhibition.
FIG. 2.
Affinity purification of a measles virus V protein-dependent complex. (A) Silver stain of the eluate from FLAG affinity purification. Migration positions of prestained molecular mass markers and FLAG-GFP, FLAG-SV5 V (SV), or FLAG-measles virus V (MeV) are indicated on the left in kilodaltons. SV5 VIP subunits are indicated on the right, as are prominent MeVIPs (indicated by asterisks). (B) Identification of MeVIP polypeptides. Parallel samples from the experiment in panel A were processed for immunoblotting with antisera for STAT1, STAT2, STAT3, IRF9, DDB1, or Cul4A.
Immunoblotting with specific antisera (Fig. 2B) was used to probe the composition of the measles virus and SV5 V protein affinity preparations. The STAT1α immunoblot revealed a single band for measles virus V, in contrast to the STAT1 laddering pattern observed for SV5 V protein, consistent with the reported SV5 V-mediated STAT1 polyubiquitylation (56, 57). STAT2 was also present in both V protein affinity preparations. In contrast, antiserum specific for IRF9 reacted with the affinity preparation from measles virus V protein but not with that from the SV5 V protein. Similarly, immunoblotting with antiserum specific for STAT3 revealed that measles virus V formed associations with STAT3. Analysis for the VDC ubiquitin ligase subunits DDB1 and Cul4A revealed that these proteins were not significant components of the measles virus V affinity preparation, although a trace amount of DDB1 could be detected. Importantly, none of these partner proteins were detected in the FLAG-GFP control. These results support a model wherein the measles virus V protein induces the formation of STAT1-, STAT2-, STAT3-, and IRF9-containing complex(es) distinct in composition from the interactions nucleated by the SV5 V protein.
Measles virus V protein interferes with STAT3-dependent IL-6 and v-Src signaling.
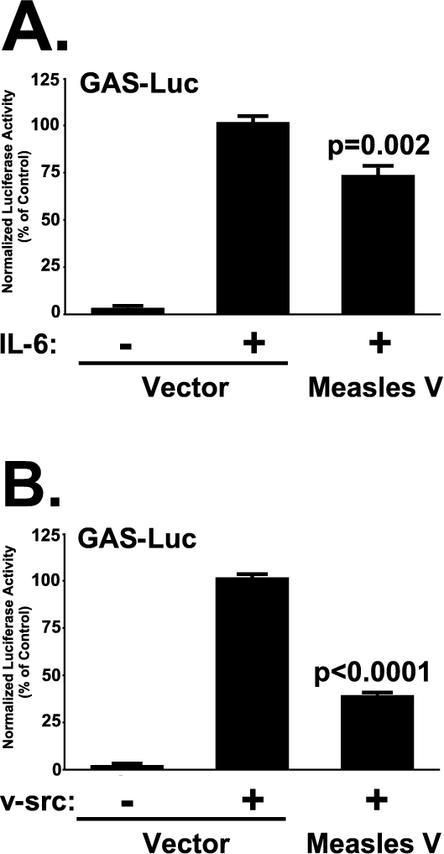
The discovery of STAT3 as a MeVIP prompted us to investigate the impact of V protein expression on STAT3-dependent transcription. STAT3 activation and transcription factor activity has been well studied for cytokine signaling systems similar to IL-6 (1, 16), a cytokine induced by measles virus infections (3, 18). Treatment of 2fTGH cells with IL-6 potently induced reporter gene expression from a STAT3-responsive GAS-luciferase construct, but expression of measles virus V protein reduced IL-6-induced reporter gene activity to 72% of control values (P = 0.002 [Fig. 3A ]). STAT3 is also an important effector for intracellular tyrosine kinase signaling (6, 7). To determine the ability of measles virus V protein to block STAT3 signaling induced by an intracellular stimulus, the v-Src oncogenic tyrosine kinase was used to activate STAT3. Expression of v-Src potently induced reporter gene activity, but this activity was reduced to 38% of control values by expression of measles virus V protein (P < 0.0001 [Fig. 3B]). Together, these results demonstrate that the measles virus V protein is capable of inhibiting both extracellular and intracellular STAT3-dependent signaling.
FIG. 3.
Measles virus V protein STAT3 interference inhibits IL-6 and v-Src transcriptional responses. (A) Measles virus V inhibits IL-6 signaling. GAS-luciferase reporter gene assays were carried out with 2fTGH cells as in Fig. 1, but the cells were transfected with empty vector or measles virus V expression vector as indicated and then treated with IL-6 (400 ng/ml) plus soluble IL-6 receptor (500 ng/ml) or left untreated, as described previously (14, 57). (B) Measles virus V inhibits v-Src signaling. The GAS-luciferase assay was carried out as in panel A, but with cotransfected v-Src expression vector as the STAT3 activator.
Effect of measles virus V protein on STAT activation and dimerization.
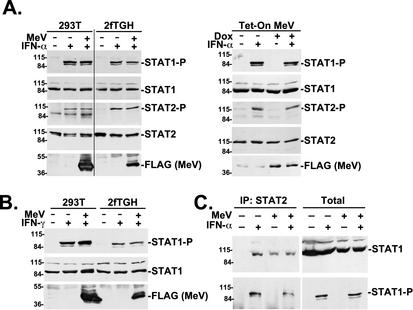
It has been reported that ISGF3 activation is induced by measles virus infection (18). However, since multiple STAT proteins were found to copurify as MeVIPs and V-dependent IFN signaling inhibition was observed, we more closely examined the early steps of IFN signaling: STAT protein activating tyrosine phosphorylation and STAT1-STAT2 heterodimerization. Stimulation of cells with IFN induces phosphorylation of STAT2 on tyrosine 690 and of STAT1 on tyrosine 701. Immunoblotting with STAT phosphotyrosine-specific antisera revealed that the expression of measles virus V protein did not detectably reduce IFN-induced tyrosine phosphorylation of either STAT1 or STAT2 in transfected 2fTGH cells or 293T cells or in a stable cell line harboring a tetracycline-inducible measles virus V protein (Fig. 4A). Similarly, no inhibition of IFN-γ-induced STAT1 tyrosine 701 phosphorylation was observed (Fig. 4B). These data indicate that IFN-dependent STAT protein activating tyrosine phosphorylation is not targeted by measles virus V protein.
FIG. 4.
Effect of measles virus V protein on STAT phosphorylation and dimerization. (A) IFN-α-induced STAT1 and STAT2 activating tyrosine phosphorylation was tested by immunoblotting with STAT phosphopeptide-specific antisera in 293T and 2fTGH cell lines transfected with empty vector (−) or measles virus V cDNA (MeV). Total STAT1 and STAT2 levels and V protein expression were analyzed in parallel. The results are shown in the left panels. A similar analysis was performed with a stable 293 Tet-On cell line harboring a doxycycline (Dox)-inducible measles virus V cDNA. The results are shown in the right panels. (B) IFN-γ-induced STAT1 tyrosine phosphorylation was tested as in panel A by immunoblotting with STAT1 phosphopeptide-specific antisera. (C) Measles virus V induces constitutive STAT association. Cells transfected with empty vector (−) or measles virus V cDNA (MeV) were treated with 1,000 U of IFN-α per ml for 30 min (+) or left untreated and whole-cell lysates were immunoprecipitated (IP) with STAT2-specific antiserum (left) or directly applied to the gel (right) and then processed for immunoblotting with STAT1 antiserum or STAT1 phosphopeptide-specific antiserum.
Following activation, STAT proteins dimerize by reciprocal SH2 domain-phosphotyrosine interactions (52). A coimmunoprecipitation assay was used to test STAT1-STAT2 heterodimerization. Cell lysates were immunoprecipitated with STAT2-specific antiserum, and immune complexes were processed for STAT1 immunoblotting. In control cells, STAT1 is detected in the STAT2 immune complex only following IFN stimulation (Fig. 4C). In contrast, in cells expressing the measles virus V protein, STAT1 was found in STAT2 immune complexes regardless of IFN stimulation (Fig. 4C), consistent with V-dependent complex formation. Immunoblotting with the STAT1 phosphotyrosine-specific antiserum indicates that activated STAT1 is not excluded from the coimmunoprecipitated material. One interpretation of these results is that the measles virus V-STAT complex(es) is capable of being presented to the IFN receptor-kinase complex but is deficient in a subsequent step of STAT signaling.
Measles virus V protein prevents IFN-induced STAT nuclear accumulation.
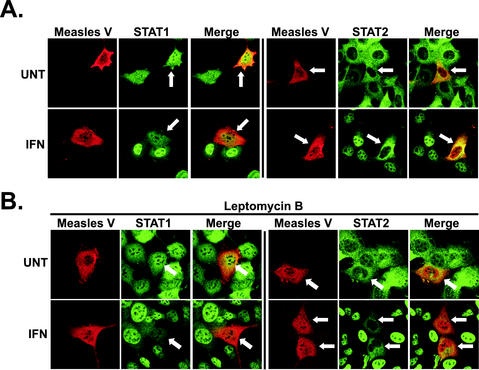
IFN signaling inhibition by the Nipah virus V protein relies on alteration of the subcellular distribution of STAT1 and STAT2 (48). To determine the effects of measles virus V protein on STAT protein distribution, indirect immunofluorescence was used to visualize the subcellular localization of measles virus V protein, STAT1, and STAT2. Epitope-tagged V protein was detected with tag-specific antibodies, and STAT1 and STAT2 were detected in the same cells by double staining. The measles virus V protein was found in both the nucleus and cytoplasm, and the distribution was not altered by IFN stimulation (Fig. 5A).
FIG. 5.
Measles virus V protein prevents IFN-induced STAT nuclear accumulation. (A) Human 2fTGH cells were transfected with HA-tagged measles virus V cDNA and were untreated (UNT) or treated with IFN for 30 min 36 h later. The cells were stimulated with IFN-α (1,000 U/ml) to visualize STAT2 nuclear redistribution and IFN-γ (5 ng/ml) to visualize STAT1 nuclear redistribution. They were fixed, permeabilized, and stained with antisera to HA tag (red), and STAT1 or STAT2 (green). Merged images illustrate overlap in yellow; arrows point to V-expressing cells for clarity. (B) Same as in panel A, except that the cells were also treated with LMB (10 ng/ml) for 5 h.
STAT1 is found in both the nucleus and cytoplasm in unstimulated cells, a result of balanced signal-independent basal nuclear shuttling (31) (Fig. 5). STAT2 also dynamically shuttles between the nucleus and cytoplasm, but with net export (48), resulting in cytoplasmic accumulation in unstimulated cells (50). In response to IFN stimulation, both STAT1 and STAT2 rapidly translocate to and accumulate within the nucleus (48, 50), as also observed here in control cells that did not express measles virus V protein (Fig. 5A). In cells expressing measles virus V protein, the basal STAT1 and STAT2 distribution did not change. However, IFN stimulation failed to induce STAT nuclear redistribution in measles virus V-expressing cells, which retained the distribution pattern of unstimulated cells. The defective STAT protein nuclear translocation was observed uniformly in cells expressing measles virus V protein in several independent experiments (Fig. 5A presents illustrative examples). These results verify that measles virus V protein does not induce STAT protein degradation. In the context of the tyrosine phosphorylation results, the results demonstrate that the measles virus V protein inhibits IFN signaling at a point between STAT activation and nuclear import.
The cytoplasmic distribution of many proteins, including the Nipah virus V protein, relies on Crm1-dependent nuclear export, which can be inhibited by a fungal metabolite, leptomycin B (LMB) (65). A role for Crm1-dependent nuclear export in the distribution of measles virus V protein and its effects on STAT redistribution were tested in LMB-treated cells (Fig. 5B). No redistribution of measles virus V protein toward nuclear accumulation was observed in LMB-treated cells. Even following LMB treatment, IFN stimulation failed to induce STAT nuclear redistribution in measles virus V-expressing cells, which retained the distribution patterns of unstimulated cells. These results demonstrate that neither the distribution of measles virus V protein nor its effects on STAT redistribution depend on Crm1-mediated nuclear shuttling.
STAT protein redistribution in measles virus-infected cells.
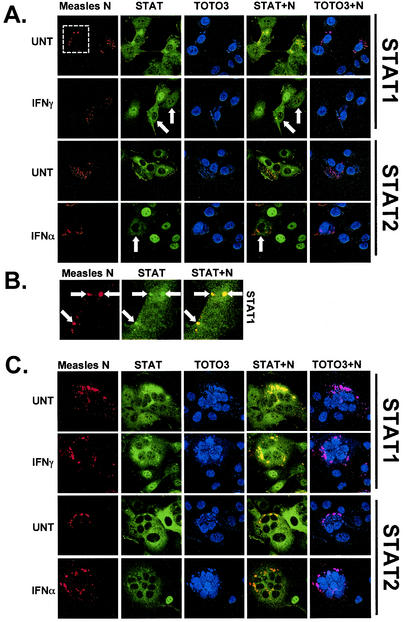
Indirect immunofluorescence was used to evaluate STAT protein distribution in the context of measles virus infections. An antibody that recognizes the measles virus nucleocapsid (N) protein was used to identify infected cells (Fig. 6). The N protein staining was localized to discrete cytoplasmic bodies, a pattern that has been observed for other paramyxoviruses (12, 13, 36, 44-46, 57, 61). All cells that stained positive for N also exhibited a significant difference in the distribution of STAT1, which was also concentrated in punctate cytoplasmic bodies that were observed to colocalize with the N protein (Fig. 6B). Notably, these cytoplasmic bodies also colocalized with nucleic acid, as detected by TOTO3 staining in the same cells (Fig. 6A and C). These results demonstrate that measles virus infection alters the subcellular localization of a portion of the host STAT1 protein. While IFN-stimulated STAT nuclear import was observed in uninfected cells, no STAT protein nuclear accumulation was observed in infected cells, in agreement with the results from isolated V protein expression.
FIG. 6.
Measles virus infection alters STAT protein distribution. (A) Human 2fTGH cells were infected with 0.1 PFU measles virus per cell. The cells were processed as for the experiment in Fig. 5 but stained for measles virus nucleocapsid protein (N; red) or STAT1 or STAT2 (green), TOTO3 was used to stain nucleic acids (blue). Arrows point to infected cells for clarity. The cells were visualized with a 40× objective; the dashedbox indicates a cell viewed at higher magnification in panel B. (B) Infected cells from panel A visualized with a 100× objective. Arrows point to regions where N and STAT1 colocalize in cytoplasmic bodies. (C) Same as in panel A, but measles virus-induced syncytia were visualized. For all panels. STAT+N indicates merged images for protein colocalization (yellow) and TOTO3+N indicates merged images for nucleic acid and N-protein colocalization (purple).
The STAT2 staining pattern was less frequently observed to colocalize with N protein in individual infected cells. However, multinucleated syncytia, where more densely staining N protein aggregates are observed, exhibited a more pronounced presence of both STAT1 and STAT2 in the N-containing bodies (Fig. 6C). While IFN treatment resulted in efficient STAT1 and STAT2 nuclear translocation in nearby uninfected cells, STAT protein nuclear translocation was not observed in syncytia. In combination, these observations not only confirm the defective STAT nuclear translocation phenotype obtained from isolated V protein expression but also reveal a unique property of host protein redistribution in measles virus-infected cells.
DISCUSSION
Measles virus infection has severe consequences for the host organism, including suppression of innate and adaptive immunity. The results demonstrate that the measles virus V protein forms immune evasion complexes that can interrupt normal JAK-STAT signal transduction. As in other paramyxoviruses, the V protein functions to suppress the activity of IFN-α/β and IFN-γ. The measles virus V protein associates with both STAT1 and STAT2 proteins but does not interfere with receptor-mediated STAT activation by specific tyrosine phosphorylation. Instead, it prevents the nuclear translocation of these activated STATs, possibly a consequence of inappropriate oligomerization that prevents STAT nuclear import. As a consequence, IFN signaling to target genes is abrogated.
Affinity chromatography analysis of measles virus V-interacting proteins revealed STAT1, STAT2, and STAT3, but not DDB1 or Cul4A, as interaction partners. Interestingly, the DNA-binding subunit of ISGF3, IRF9, was also found in the measles virus V affinity preparation but not in that of SV5 V. The reason for STAT1, STAT2, and IRF9 in the evasion complex(es) may be rationalized from the evolutionary pressure from IFN antiviral effects, similar to the evasive activities demonstrated for other paramyxoviruses. Interaction with IRF9 is a unique feature for measles virus that is not shared with SV5 and might ensure more complete ISGF3 inactivation. The discovery of STAT3 as a component of the measles virus V affinity preparation was not as easily explained. However, IL-6 biosynthesis has been reported for measles virus infections (3, 18), and STAT3-responsive transcriptional assays reveal that the measles virus V protein can inhibit STAT3 activity induced by IL-6 or by v-Src. It is important to note that the effect on STAT3 was partial for our Edmonston-derived V protein. It is conceivable that strain-specific differences in STAT3 interference might result in different degrees of measles virus-induced immune suppression. STAT3 has not been generally considered to be a major component of the IFN-induced antiviral system, but STAT3 activation during IFN signaling has been reported (8, 18-20, 47, 59, 62, 63), implying a potential antiviral role for STAT3 that has yet to be fully elucidated. The recent finding that the mumps virus V protein functions as a ubiquitin ligase that targets STAT3 for degradation further supports the concept that STAT3 evasion is beneficial to paramyxoviruses (57). In addition to any potential benefits of STAT3 antagonism associated with IFN signaling evasion, STAT3 inhibition facilitates the evasion of innate and adaptive immune responses that occur downstream of numerous cytokines, mitogenic growth factors, tyrosine kinases, or G proteins, all of which can activate STAT3 signaling (28). Therefore, inhibition of STAT3 signaling will provide a much broader spectrum of cytokine and growth factor suppression in vivo than that illustrated here (28). STAT3 interference would allow the virus to effectively evade diverse cellular responses, a property that could provide several general or tissue-specific replication advantages to measles virus.
The subcellular distribution of measles virus V protein is distinct from that of other V proteins that either are nuclear (Rubulavirus genus) or shuttle between cytoplasm and nucleus, with cytoplasmic accumulation (Henipavirus genus). While LMB had no effect on the distribution of measles virus V, it remains possible that non-Crm1-dependent nuclear export systems can contribute to its localization. Significantly, the measles virus V protein has no effect on basal nuclear STAT1 distribution. Since basal STAT1 redistribution is apparent with Nipah virus V protein (48), it is likely that nuclear shuttling is not involved in the activity of measles virus V protein.
Several novel observations were made with respect to STAT protein localization in measles virus-infected cells. The measles virus nucleocapsid protein was found to accumulate in punctate cytoplasmic bodies, a feature of measles infections (4) that is observed with other paramyxoviruses. Similar granular structures are produced by paramyxovirus infections and, in some cases, by simple expression of the nucleocapsid protein along with the P, V, or C protein. These structures may be universal for the paramyxoviruses and have been observed for SV5 (12, 45, 46), type II human parainfluenza virus (36, 61), respiratory syncytial virus (13), Sendai virus (44), and mumps virus (57). The exact function of these structures is still unknown, but it has been proposed previously that in murine fibroblasts which are persistently infected with SV5, these “cytoplasmic inclusion bodies” represent an inactive reservoir from which virus may occasionally be reactivated (12). It has been more recently appreciated that STAT1 targeting by SV5 is defective in murine cell systems (10, 39, 66). Further, it has been observed that cellular IRF3 protein can localize to similar bodies induced during SV5 infection (15) and that STAT2 localizes to NP-containing cytoplasmic bodies induced by mumps virus infection (57). Our present results provide new information that STAT proteins and nucleic acids are components of cytoplasmic bodies along with the measles virus N protein. These observations suggest an alternate interpretation, i.e., that these cytoplasmic bodies may represent active subcellular sites for paramyxovirus replication. We further hypothesize that these dots may represent macromolecular assemblies of viral and cellular components required for a range of enzymatic activities contributing to host immune evasion and virus replication.
The mechanisms described here for measles virus-induced STAT inhibition are unprecedented among the Paramyxoviridae genera, adding to the catalog of STAT inhibition by V proteins alongside the Rubulavirus-encoded E3 ubiquitin ligase activities (56) and the Henipavirus-encoded STAT cytoplasmic sequestration mechanisms (48). Together, these suppression activities contribute to the severity of measles virus pathogenicity.
Acknowledgments
We are grateful to Huabao Xiong, Sergio Lira, Adrian Ting, Patricia Cortes, and Horvath laboratory members for helpful discussions and comments about the manuscript.
This work was supported by research grants to C.M.H. from the American Cancer Society (Research Scholar Grant GMC-103079) and the NIH (AI-48722 and AI-50707). J.J.R. is a trainee in the integrated training program in pharmacological sciences (GM-62754). Confocal microscopy at Mt. Sinai is partially supported by NIH Shared Instrumentation grant 1S10 RR0 9145 and NSF Major Research Instrumentation grant DBI-9724504.
REFERENCES
- 1.Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 296:1653-1655. [DOI] [PubMed] [Google Scholar]
- 2.Andrejeva, J., E. Poole, D. F. Young, S. Goodbourn, and R. E. Randall. 2002. The p127 subunit (DDB1) of the UV-DNA damage repair binding protein is essential for the targeted degradation of STAT1 by the V protein of the paramyxovirus simian virus 5. J. Virol. 76:11379-11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. Ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohn, W., F. Ciampor, R. Rutter, and K. Mannweiler. 1990. Localization of nucleocapsid associated polypeptides in measles virus-infected cells by immunogold labelling after resin embedding. Arch. Virol. 114:53-64. [DOI] [PubMed] [Google Scholar]
- 5.Bolt, G., K. Berg, and M. Blixenkrone-Moller. 2002. Measles virus-induced modulation of host-cell gene expression. J. Gen. Virol. 83:1157-1165. [DOI] [PubMed] [Google Scholar]
- 6.Bowman, T., R. Garcia, J. Turkson, and R. Jove. 2000. STATs in oncogenesis. Oncogene 19:2474-2488. [DOI] [PubMed] [Google Scholar]
- 7.Bromberg, J., and J. E. Darnell, Jr. 2000. The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19:2468-2473. [DOI] [PubMed] [Google Scholar]
- 8.Caldenhoven, E., M. Buitenhuis, T. B. van Dijk, J. A. Raaijmakers, J. W. Lammers, L. Koenderman, and R. P. de Groot. 1999. Lineage-specific activation of STAT3 by interferon-gamma in human neutrophils. J. Leukoc. Biol. 65:391-396. [DOI] [PubMed] [Google Scholar]
- 9.Decker, T., D. J. Lew, J. Mirkovitch, and J. E. Darnell, Jr. 1991. Cytoplasmic activation of GAF, an IFN-γ regulated DNA binding factor. EMBO J. 10:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. Sendai virus and simian virus 5 block activation of interferon- responsive genes: importance for virus pathogenesis. J. Virol. 73:3125-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didcock, L., D. F. Young, S. Goodbourn, and R. E. Randall. 1999. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 73:9928-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fearns, R., D. F. Young, and R. E. Randall. 1994. Evidence that the paramyxovirus simian virus 5 can establish quiescent infections by remaining inactive in cytoplasmic inclusion bodies. J. Gen. Virol. 75:3525-3539. [DOI] [PubMed] [Google Scholar]
- 13.Garcia, J., B. Garcia-Barreno, A. Vivo, and J. A. Melero. 1993. Cytoplasmic inclusions of respiratory syncytial virus-infected cells: formation of inclusion bodies in transfected cells that coexpress the nucleoprotein, the phosphoprotein, and the 22K protein. Virology 195:243-247. [DOI] [PubMed] [Google Scholar]
- 14.Guschin, D., N. Rogers, J. Briscoe, B. Witthuhn, D. Watling, F. Horn, S. Pellegrini, K. Yasukawa, P. Heinrich, G. R. Stark, J. N. Ihle, and I. M. Kerr. 1995. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT pathway in response to interleukin-6. EMBO J. 14:1421-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, B., R. G. Paterson, N. Stock, J. E. Durbin, R. K. Durbin, S. Goodbourn, R. E. Randall, and R. A. Lamb. 2002. Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology 303:15-32. [DOI] [PubMed] [Google Scholar]
- 16.Heinrich, P. C., I. Behrmann, G. Muller-Newen, F. Schaper, and L. Graeve. 1998. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334:297-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helin, E., S. Matikainen, I. Julkunen, J. Heino, T. Hyypia, and R. Vainionpaa. 2002. Measles virus enhances the expression of cellular immediate-early genes and DNA-binding of transcription factor AP-1 in lung epithelial A549 cells. Arch. Virol. 147:1721-1732. [DOI] [PubMed] [Google Scholar]
- 18.Helin, E., R. Vainionpaa, T. Hyypia, I. Julkunen, and S. Matikainen. 2001. Measles virus activates NF-kappa B and STAT transcription factors and production of IFN-alpha/beta and IL-6 in the human lung epithelial cell line A549. Virology 290:1-10. [DOI] [PubMed] [Google Scholar]
- 19.Horvath, C. M., and J. E. Darnell, Jr. 1996. The antiviral state induced by alpha interferon and gamma interferon requires transcriptionally active Stat1 protein. J. Virol. 70:647-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horvath, C. M., Z. Wen, and J. E. Darnell, Jr. 1995. A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 9:984-994. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda, H., L. J. Old, and R. D. Schreiber. 2002. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 13:95-109. [DOI] [PubMed] [Google Scholar]
- 22.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 23.Kubota, T., N. Yokosawa, S. Yokota, and N. Fujii. 2002. Association of mumps virus V protein with RACK1 results in dissociation of STAT-1 from the alpha interferon receptor complex. J. Virol. 76:12676-12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubota, T., N. Yokosawa, S. Yokota, and N. Fujii. 2001. C terminal CYS-RICH region of mumps virus structural V protein correlates with block of interferon alpha and gamma signal transduction pathway through decrease of STAT 1-alpha. Biochem. Biophys. Res. Commun. 283:255-259. [DOI] [PubMed] [Google Scholar]
- 25.Lamb, R. A., and D. Kolakofsky. 1996. Paramyxoviridae: the viruses and their replication, p. 1177-1204. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 26.Levy, D. E., and J. E. Darnell, Jr. 2002. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3:651-662. [DOI] [PubMed] [Google Scholar]
- 27.Levy, D. E., D. S. Kessler, R. I. Pine, and J. E. Darnell, Jr. 1989. Cytoplasmic activation of ISGF3, the positive regulator of interferon-α stimulated transcription, reconstituted in vitro. Genes Dev. 3:1362-1372. [DOI] [PubMed] [Google Scholar]
- 28.Levy, D. E., and C. K. Lee. 2002. What does Stat3 do? J. Clin. Investig. 109:1143-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, G. Y., and R. A. Lamb. 2000. The paramyxovirus simian virus 5 V protein slows progression of the cell cycle. J. Virol. 74:9152-9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, G. Y., R. G. Paterson, C. D. Richardson, and R. A. Lamb. 1998. The V protein of the paramyxovirus SV5 interacts with damage-specific DNA binding protein. Virology 249:189-200. [DOI] [PubMed] [Google Scholar]
- 31.Meyer, T., A. Begitt, I. Lodige, M. van Rossum, and U. Vinkemeier. 2002. Constitutive and IFN-gamma-induced nuclear import of STAT1 proceed through independent pathways. EMBO J. 21:344-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray, C. J., and A. D. Lopez. 1997. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 349:1269-1276. [DOI] [PubMed] [Google Scholar]
- 34.Naniche, D., A. Yeh, D. Eto, M. Manchester, R. M. Friedman, and M. B. Oldstone. 2000. Evasion of host defenses by measles virus: wild-type measles virus infection interferes with induction of alpha/beta interferon production. J. Virol. 74:7478-7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishio, M., D. Garcin, V. Simonet, and D. Kolakofsky. 2002. The carboxyl segment of the mumps virus V protein associates with Stat proteins in vitro via a tryptophan-rich motif. Virology 300:92-99. [DOI] [PubMed] [Google Scholar]
- 36.Nishio, M., M. Tsurudome, M. Kawano, N. Watanabe, S. Ohgimoto, M. Ito, H. Komada, and Y. Ito. 1996. Interaction between nucleocapsid protein (NP) and phosphoprotein (P) of human parainfluenza virus type 2: one of the two NP binding sites on P is essential for granule formation. J. Gen. Virol. 77:2457-2463. [DOI] [PubMed] [Google Scholar]
- 37.Parisien, J.-P., J. F. Lau, J. J. Rodriguez, B. M. Sullivan, A. Moscona, G. D. Parks, R. A. Lamb, and C. M. Horvath. 2001. The V protein of human parainfluenza virus 2 antagonizes type I interferon responses by destabilizing signal transducer and activator of transcription 2. Virology 283:230-239. [DOI] [PubMed] [Google Scholar]
- 38.Parisien, J.-P., J. F. Lau, J. J. Rodriguez, C. M. Ulane, and C. M. Horvath. 2002. Selective STAT protein degradation induced by paramyxoviruses requires both STAT1 and STAT2, but is independent of alpha/beta interferon signal transduction. J. Virol. 76:4190-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parisien, J. P., J. F. Lau, and C. M. Horvath. 2002. STAT2 acts as a host range determinant for species-specific paramyxovirus interferon antagonism and simian virus 5 replication. J. Virol. 76:6435-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park, M. S., M. L. Shaw, J. Munoz-Jordan, J. F. Cros, T. Nakaya, N. Bouvier, P. Palese, A. Garcia-Sastre, and C. F. Basler. 2003. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 77:1501-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paterson, R. G., G. P. Leser, M. A. Shaughnessy, and R. A. Lamb. 1995. The paramyxovirus SV5 V protein binds two atoms of zinc and is a structural component of virions. Virology 208:121-131. [DOI] [PubMed] [Google Scholar]
- 42.Patterson, J. B., D. Thomas, H. Lewicki, M. A. Billeter, and M. B. Oldstone. 2000. V and C proteins of measles virus function as virulence factors in vivo. Virology 267:80-89. [DOI] [PubMed] [Google Scholar]
- 43.Poole, E., B. He, R. A. Lamb, R. E. Randall, and S. Goodbourn. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-beta. Virology 303:33-46. [DOI] [PubMed] [Google Scholar]
- 44.Portner, A., K. C. Gupta, J. M. Seyer, E. H. Beachey, and D. W. Kingsbury. 1986. Localization and characterization of Sendai virus nonstructural C and C′ proteins by antibodies against synthetic peptides. Virus Res. 6:109-121. [DOI] [PubMed] [Google Scholar]
- 45.Precious, B., D. F. Young, A. Bermingham, R. Fearns, M. Ryan, and R. E. Randall. 1995. Inducible expression of the P, V, and NP genes of the paramyxovirus simian virus 5 in cell lines and an examination of NP-P and NP-V interactions. J. Virol. 69:8001-8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randall, R. E., and A. Bermingham. 1996. NP:P and NP:V interactions of the paramyxovirus simian virus 5 examined using a novel protein:protein capture assay. Virology 224:121-129. [DOI] [PubMed] [Google Scholar]
- 47.Rani, M. R., D. W. Leaman, Y. Han, S. Leung, E. Croze, E. N. Fish, A. Wolfman, and R. M. Ransohoff. 1999. Catalytically active TYK2 is essential for interferon-beta-mediated phosphorylation of STAT3 and interferon-alpha receptor-1 (IFNAR-1) but not for activation of phosphoinositol 3-kinase. J. Biol. Chem. 274:32507-32511. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez, J. J., J. P. Parisien, and C. M. Horvath. 2002. Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. J. Virol. 76:11476-11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schindler, C., K. Shuai, V. R. Prezioso, and J. E. Darnell, Jr. 1992. Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science 257:809-813. [DOI] [PubMed] [Google Scholar]
- 51.Schneider, H., K. Kaelin, and M. A. Billeter. 1997. Recombinant measles viruses defective for RNA editing and V protein synthesis are viable in cultured cells. Virology 227:314-322. [DOI] [PubMed] [Google Scholar]
- 52.Shuai, K., C. M. Horvath, L. H. Tsai-Huang, S. Qureshi, D. Cowburn, and J. E. Darnell, Jr. 1994. Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell 76:821-828. [DOI] [PubMed] [Google Scholar]
- 53.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 54.tenOever, B. R., M. J. Servant, N. Grandvaux, R. Lin, and J. Hiscott. 2002. Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation. J. Virol. 76:3659-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas, S. M., R. A. Lamb, and R. G. Paterson. 1988. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell 54:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulane, C. M., and C. M. Horvath. 2002. Paramyxoviruses SV5 and HPIV2 assemble STAT protein ubiquitin ligase complexes from cellular components. Virology 304:160-166. [DOI] [PubMed] [Google Scholar]
- 57.Ulane, C. M., J. J. Rodriguez, J.-P. Parisien, and C. M. Horvath. 2003. STAT3 ubiquitylation and degradation by mumps virus suppresses cytokine and oncogene signaling. J. Virol. 77:6385-6393. [DOI] [PMC free article] [PubMed]
- 58.Valsamakis, A., H. Schneider, P. G. Auwaerter, H. Kaneshima, M. A. Billeter, and D. E. Griffin. 1998. Recombinant measles viruses with mutations in the C, V, or F gene have altered growth phenotypes in vivo. J. Virol. 72:7754-7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Velichko, S., T. C. Wagner, J. Turkson, R. Jove, and E. Croze. 2002. STAT3 Activation by type I interferons is dependent on specific tyrosines located in the cytoplasmic domain of interferon receptor chain 2c. Activation of multiple STATs proceeds through the redundant usage of two tyrosine residues. J. Biol. Chem. 277:35635-35641. [DOI] [PubMed] [Google Scholar]
- 60.Wansley, E. K., and G. D. Parks. 2002. Naturally occurring substitutions in the P/V gene convert the noncytopathic paramyxovirus simian virus 5 into a virus that induces alpha/beta interferon synthesis and cell death. J. Virol. 76:10109-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watanabe, N., M. Kawano, M. Tsurudome, M. Nishio, M. Ito, S. Ohgimoto, S. Suga, H. Komada, and Y. Ito. 1996. Binding of the V proteins to the nucleocapsid proteins of human parainfluenza type 2 virus. Med. Microbiol. Immunol. 185:89-94. [DOI] [PubMed] [Google Scholar]
- 62.Yang, C. H., A. Murti, and L. M. Pfeffer. 1998. STAT3 complements defects in an interferon-resistant cell line: evidence for an essential role for STAT3 in interferon signaling and biological activities. Proc. Natl. Acad. Sci. USA 95:5568-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang, C. H., W. Shi, L. Basu, A. Murti, S. N. Constantinescu, L. Blatt, E. Croze, J. E. Mullersman, and L. M. Pfeffer. 1996. Direct association of STAT3 with the IFNAR-1 chain of the human type I interferon receptor. J. Biol. Chem. 271:8057-8061. [DOI] [PubMed] [Google Scholar]
- 64.Yokosawa, N., S. Yokota, T. Kubota, and N. Fujii. 2002. C-terminal region of STAT-1alpha is not necessary for its ubiquitination and degradation caused by mumps virus V protein. J. Virol. 76:12683-12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshida, M., and S. Horinouchi. 1999. Trichostatin and leptomycin. Inhibition of histone deacetylation and signal-dependent nuclear export. Ann. N. Y. Acad. Sci. 886:23-36. [DOI] [PubMed] [Google Scholar]
- 66.Young, D. F., N. Chatziandreou, B. He, S. Goodbourn, R. A. Lamb, and R. E. Randall. 2001. Single amino acid substitution in the V protein of simian virus 5 differentiates its ability to block interferon signaling in human and murine cells. J. Virol. 75:3363-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young, D. F., L. Didcock, S. Goodbourn, and R. E. Randall. 2000. Paramyxoviridae use distinct virus-specific mechanisms to circumvent the interferon response. Virology 269:383-390. [DOI] [PubMed] [Google Scholar]
- 68.Yu, C. L., D. J. Meyer, G. S. Campbell, A. C. Larner, C. Carter-Su, J. Schwartz, and R. Jove. 1995. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269:81-83. [DOI] [PubMed] [Google Scholar]
- 69.Zhong, Z., Z. Wen, and J. E. Darnell, Jr. 1994. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264:95-98. [DOI] [PubMed] [Google Scholar]