Abstract
Animal models of human immunodeficiency virus 1, such as feline immunodeficiency virus (FIV), provide the opportunities to dissect the mechanisms of early interactions of the virus with the central nervous system (CNS). The aims of the present study were to evaluate viral loads within CNS, cerebrospinal fluid (CSF), ocular fluid, and the plasma of cats in the first 23 weeks after intravenous inoculation with FIVGL8. Proviral loads were also determined within peripheral blood mononuclear cells (PBMCs) and brain tissue. In this acute phase of infection, virus entered the brain in the majority of animals. Virus distribution was initially in a random fashion, with more diffuse brain involvement as infection progressed. Virus in the CSF was predictive of brain parenchymal infection. While the peak of virus production in blood coincided with proliferation within brain, more sustained production appeared to continue in brain tissue. In contrast, proviral loads in the brain decreased to undetectable levels in the presence of a strengthening PBMC load. A final observation in this study was that there was no direct correlation between viral loads in regions of brain or ocular tissue and the presence of histopathology.
Infection of domestic cats with the lentivirus feline immunodeficiency virus (FIV) may result in a range of clinical signs related to an underlying state of immunodeficiency (48, 65). FIV shares many features with human immunodeficiency virus type 1 (HIV-1) (48, 49, 67), particularly in causing lymphoid system pathology (13, 14), immune dysfunction (27, 64), and high-grade B-cell lymphomas (15). Furthermore, FIV and HIV share a common mechanism of infection via the CXCR4 receptor molecule (66). The trademark five clinical stages of HIV-1 infection are mirrored in FIV-infected cats, beginning with an acute flu-like illness shortly after infection and ending with conditions associated with severe immunodeficiency (19, 39, 50).
FIV, like HIV-1, infects the central nervous system (CNS) and is associated with neuropathology in natural and experimental infections (25, 26, 38, 40, 55-58). Early after intravenous inoculation, virus can be recovered from primary cultures of the cerebral cortex, caudate nucleus, midbrain, cerebellum, caudal brain stem, and cerebrospinal fluid (CSF) (25, 56, 67). FIV-infected cells have been detected in the brain as early as 7 days following intravenous challenge (7), and in vitro studies demonstrate that FIV preferentially infects astrocytes and brain macrophages, with low affinity for brain endothelial cells (26).
HIV-1 infection is associated with neuropathology in the early and AIDS stages of infection (12). There are, however, limited opportunities to examine early stages of HIV infection (5), and the spectrum of neuropathology in HIV-1 infection is naturally biased towards the end stages of disease, which are complicated by opportunistic infections and intensive therapies (12). The time and manner in which HIV-1 gains entry into and subsequently spreads within the microglial cells is poorly understood due to limited opportunities to examine the brain in the presymptomatic stages of infection (1). It is generally accepted that the characterization of these early stages can most readily be achieved through the use of animal models (12, 52). The lentivirus infections of macaques and domestic cats are excellent models for HIV infection of humans (28, 47), with early infection of the brain documented in both animal groups (7, 8, 16, 58). The dissection of the early virus-host interactions in such models may assist the development of therapies to modulate lentivirally induced CNS infection and help to investigate to what extent CNS tissue harbors reservoirs of latently infected cells, which are an important challenge when formulating lentiviral therapeutic strategies (6, 59).
While FIV viral and proviral loads within peripheral blood have been determined by several PCR-based methods (10, 41, 43, 44, 54, 58, 62), viral loads within FIV-, simian immunodeficiency virus (SIV)-, or HIV-infected brains have been determined by visual quantification of infected cells using in situ hybridization or immunocytochemical techniques (2, 7, 8) and to a limited extent by PCR technologies (18, 58). Taqman, fluorogenic, real-time PCR has recently been applied in the determination of FIV, SIV, and HIV viral and proviral loads within peripheral blood mononuclear cells (PBMCs) and various tissues (9, 30, 35, 41, 43-45, 60, 61). This technique is an improvement on previous quantitative or semiquantitative PCR-based methods. Studies correlating plasma, CSF, and brain viral and proviral loads have been documented to a limited degree for HIV and SIV infection and in particular have focused on asymptomatic or end stages of disease (20, 63, 68). More recently, evaluations of the acute phase of FIV and SIV infections have been reported (10, 18). Plasma and CSF viremias have been monitored during the first 18 weeks following FIV infection (10), and using an accelerated model of SIV-induced encephalitis, monitoring of CNS viral DNA and RNA together with CSF and plasma RNA was achieved over an 8-week period (18). Similarly to the latter study, the present study addresses the dynamics of viral and proviral loads during the acute phase of infection. This period is particularly important, since it represents one window of time in which virus is believed to enter the brain (29).
The aims of the present study were to determine viral loads within compartments of the brain at sequential time points in early FIVGL8 infection by Taqman, fluorogenic, real-time PCR methods. Specifically, this study was designed to determine if FIV enters brain tissue following intravenous infection, over what time frame infection occurs, and whether virus distribution is in a uniform pattern. Second, the study evaluated virus dynamics in the early stages of infection by determining the viral concentrations within the brain and correlating them to plasma and CSF concentrations, to brain tissue and PBMC proviral loads, and to brain histopathology.
MATERIALS AND METHODS
Study design.
Twelve (six male and six female), 16- to 20-week-old specific-pathogen-free cats were inoculated intravenously with 2,000 infectious-unit doses of the FIVGL8 isolate (31). Eight (four male and four female) 16- to 20-week-old specific-pathogen-free cats were maintained separately as controls. Five cats (three infected and two controls) were sacrificed at 1, 4, 10, and 23 weeks after infection. Blood samples were taken at 1, 4, 8, 10, and 23 weeks after infection. Blood was stored in EDTA (1.3 mg/ml) (LIP Ltd., Galway, Ireland) at 4°C until nucleic acid was extracted. At 4 weeks after infection, all remaining infected animals were confirmed to be seropositive by immunofluorescence (32). Animal experimental procedures were performed under an approved license. FIVGL8 is a primary isolate that has been passaged minimally in feline T cells (32). In contrast to the prototypic Petaluma strain isolated (FIVPET), FIVGL8 has a limited in vivo cell host range that does not extend to the CrFK cell line (33), and it is virulent in vivo, leading to high viral loads in plasma (35).
Before necropsy, animals were anaesthetized with 10 mg of ketamine hydrochloride (Vetalar; Pharmacia & Upjohn, Corby, United Kingdom)/kg of body weight and 3 mg of Xylazine (Bayer, Bury St. Edmunds, United Kingdom)/kg, and after intracardiac exsanguination, were sacrificed by administering 150 mg of pentobarbitone sodium (Euthatal; Rhone Merieux, Harlow, United Kingdom)/kg by the intracardiac route. Plasma was stored at −80°C, and whole blood was maintained at 4°C.
At necropsy, CSF was obtained from the foramen magnum and immediately stored at −80°C. A midsagittal sectioning of the brain was performed. One portion was fixed in neutral buffered formalin, and the second portion was separated into cerebrum, cerebellum, and brain stem, which were snap-frozen in liquid nitrogen and stored at −80°C. Ocular fluid was sampled from the anterior chamber of the right eye and stored at −80°C. Both eyes were then fixed in neutral buffered formalin.
Following fixation in formalin, sections of cerebrum, cerebellum, midbrain, pons, medulla, spinal cord, and eye were paraffin embedded, cut at 4 μm, and stained with hematoxylin and eosin. Utilizing a cryostat, cross sections of frozen brain, weighing 30 mg, were obtained from similar portions of the cerebrum and cerebellum and from the pons region of the brain stem.
Nucleic acid extraction.
To determine FIV viral loads, RNA was extracted from 30 mg of brain tissue using an RNeasy mini kit (Qiagen, Hilden, Germany). Viral RNA was extracted from plasma, CSF, and ocular fluid using a QIAamp Viral RNA mini kit (Qiagen). DNA was extracted from 30-mg tissue samples from the cerebrum, cerebellum, and brain stem using a QIAamp DNA kit (Qiagen) and from 200 μl of whole blood using QIAamp blood DNA mini kits (Qiagen). The extracted DNA and RNA samples were eluted with 30 μl of water, from which 5 μl was used in the Taqman real-time PCRs.
Real-time PCR
The 25-μl real-time PCR mixtures contained 10 mM Tris (pH 8.3), 50 mM KCI, 3 mM MgCl2, 200 uM deoxynucleotide triphosphates, 300 nM (each) primer, 200 nM fluorogenic probe, 1.25 U of Taq DNA polymerase per reaction, and 5 μl of diluted template or genomic standard. After initial denaturation for 2 min at 95°C, amplification was performed with 45 cycles of 95°C for 15 s and 60°C for 1 min followed by a holding step of 25°C. Amplification, data acquisition and data analysis were carried out in an ABI Prism 7700 sequence detector (Applied Biosystems, Foster City, Calif.). Data were analyzed with Sequence Detection Software (version 1.6.3; Applied Biosystems). The primers and probes used are described in Table 1.
TABLE 1.
FIV gag-specific primers and probe used in FIV fluorogenic Taqman real-time PCR assaya
| Primer or probe | Sequence (5′ to 3′) | Fragment size (bp) |
|---|---|---|
| Forward primer, FIV 1360f | GCA GAA GCA AGA TTT GCA CCA | |
| Reverse primer, FIV1437r | TAT GGC GGC CAA TTT TCC T | 78 |
| Probe, FIV1416p | FAM-TGC CTC AAG ATA CCA TGC TCT ACA CTG CA-TAMRA | |
| Forward primer, rRNA343f | CCA TCG AAC GTC TGC CCT A | |
| Reverse primer, RRNA409r | TCA CCC GTG GTC ACC ATG | 67 |
| Probe, RRNA370p | FAM-CGA TGG TGG TCG CCG TGC CTA-TAMRA |
Cell number estimation was done using primers and probe targeting the 18S ribosomal DNA genes.
Real-time RT-PCR.
The 25-μl RT-PCR mixtures contained 10 μl of AMV/Tfl 5× reaction buffer (Access RT-PCR system; Promega, Mannheim, Germany), 3 mM MgSO4, 200 μM dATP, dCTP, dGTP, and dTTP, 300 nM (each) primer, 200 nM fluorogenic probe, 5 U of avian myeloblastosis virus reverse transcriptase, 5 U of Tfl DNA polymerase and 5 μl of the sample or RNA standard. After a reverse-transcription step of 45 min at 48°C followed by a denaturation step (2 min at 95°C), amplification was performed with 45 cycles of 15 s at 95°C and 60 s at 60°C. Reverse transcription and amplification were performed using an ABI Prism 7700 sequence detection system (Applied Biosystems). The detected fluorescence signals are analyzed using the Sequence Detection Software version 1.6.3 (Applied Biosystems). The primers and probes used are described in Table 1.
RESULTS
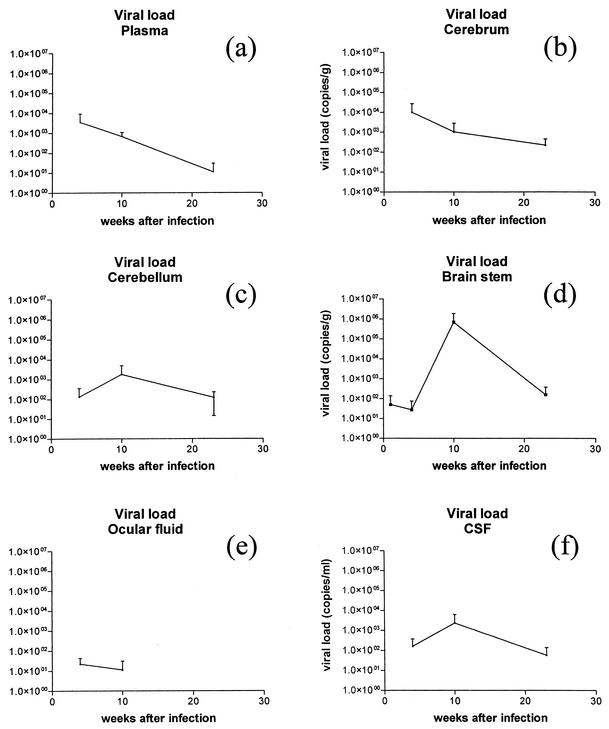
To evaluate the FIVGL8 intravenous infection model, the study had first to establish if in fact the time course of the infection did include the acute phase characterized by a transient increase in viral plasma loads coupled with a follow-on period of undetectable to minimal peripheral virus production. The purpose of the present study was not to undertake a detailed frequent sequential analysis of viral loads but rather to relate loads observed in plasma to those in the CNS at the selected times of necropsy. However, it was clear that following intravenous FIVGL8 infection, highest viral loads of the order of between 1 × 102 and 1 × 104 copies per ml of plasma were documented at 4 and 10 weeks following infection and by 23 weeks after infection loads had dramatically decreased or were at undetectable levels (Table 2 and Fig. 1a) in animals that had seroconverted. While, in contrast to previous FIV studies (22, 36, 51, 62), such peak loads in the acute phase of infection were relatively small, previous studies would support the presence of greater concentrations in between the relatively long sampling points which were undertaken in this study. Such concentrations were also comparable with those of HIV and SIV infections (23, 31, 53). A marked reduction in viral loads followed the initial viremia and was in contrast to observations in previous FIV, SIV, and HIV studies, where loads stabilized at between 2 × 103 and 7 × 105 copies/ml (18, 23, 31, 51, 53, 62).
TABLE 2.
Viral loads in the brain, CSF, ocular fluid, and plasma and proviral loads in the brain and PBMCs from all 12 FIVGL8-infected cats at four time points over the 23-week course of infectiona
| Week p.i., animal, or sample type | Viral loadb | PV load (copies/106 cells) | Pathc | Week p.i., animal, or sample type | Viral loadb | PV load (copies/106 cells) | Pathc | |
|---|---|---|---|---|---|---|---|---|
| Week 1 | ||||||||
| Animal 1 | ||||||||
| Cerebrum | 0 | 0 | − | |||||
| Cerebellum | * | 0 | − | |||||
| Brain stem | 152 | 0 | − | |||||
| CSF | 0 | |||||||
| Ocular | 0 | − | ||||||
| Plasma/PBMC | 0 | 5 | ||||||
| Animal 2 | ||||||||
| Cerebrum | 0 | 0 | − | |||||
| Cerebellum | * | 0 | − | |||||
| Brain stem | 0 | 0 | − | |||||
| CSF | 0 | |||||||
| Ocular | 0 | − | ||||||
| Plasma/PBMC | 0 | 0 | ||||||
| Animal 3 | ||||||||
| Cerebrum | 0 | 0 | − | |||||
| Cerebellum | * | 0 | − | |||||
| Brain stem | 0 | 0 | − | |||||
| CSF | 0 | |||||||
| Ocular | 0 | − | ||||||
| Plasma/PBMC | 0 | 0 | ||||||
| Week 4 | ||||||||
| Animal 6 | ||||||||
| Cerebrum | 29,780 | 418 | − | |||||
| Cerebellum | 392 | 4 | − | |||||
| Brain stem | 82 | 116 | − | |||||
| CSF | 400 | |||||||
| Ocular | 42 | + | ||||||
| Plasma/PBMC | 10,292 | 425 | ||||||
| Animal 7 | ||||||||
| Cerebrum | 0 | 7 | − | |||||
| Cerebellum | 0 | 0 | + | |||||
| Brain stem | 0 | 244 | − | |||||
| CSF | 37 | |||||||
| Ocular | 24 | + | ||||||
| Plasma/PBMC | 77 | 384 | ||||||
| Animal 8 | ||||||||
| Cerebrum | 0 | 24 | − | |||||
| Cerebellum | 0 | 0 | − | |||||
| Brain stem | 0 | 0 | − | |||||
| CSF | 40 | |||||||
| Ocular | 0 | + | ||||||
| Plasma/PBMC | 187 | 344 | ||||||
| Week 10 | ||||||||
| Animal 11 | ||||||||
| Cerebrum | 3,074 | 476 | ++ | |||||
| Cerebellum | 5,336 | 130 | + | |||||
| Brain stem | 2,012,080 | 586 | + | |||||
| CSF | 6,837 | |||||||
| Ocular | 34 | + | ||||||
| Plasma/PBMC | 1,151 | 87,169 | ||||||
| Animal 12 | ||||||||
| Cerebrum | 0 | 0 | + | |||||
| Cerebellum | 0 | 0 | + | |||||
| Brain stem | 4,630 | 0 | − | |||||
| CSF | 105 | |||||||
| Ocular | 0 | + | ||||||
| Plasma/PBMC | 496 | 1,774 | ||||||
| Animal 13 | ||||||||
| Cerebrum | 0 | 0 | + | |||||
| Cerebellum | 0 | 0 | − | |||||
| Brain stem | 51,210 | 109 | − | |||||
| CSF | 61 | |||||||
| Ocular | 0 | + | ||||||
| Plasma/PBMC | 374 | 1,895 | ||||||
| Week 23 | ||||||||
| Animal 16 | ||||||||
| Cerebrum | 202 | 0 | ++ | |||||
| Cerebellum | 200 | 0 | + | |||||
| Brain stem | 316 | 0 | − | |||||
| CSF | 151 | |||||||
| Ocular | 0 | ++ | ||||||
| Plasma/PBMC | 0 | 3,350 | ||||||
| Animal 17 | ||||||||
| Cerebrum | 0 | 0 | + | |||||
| Cerebellum | 0 | 0 | − | |||||
| Brain stem | 0 | 0 | − | |||||
| CSF | 0 | |||||||
| Ocular | 0 | + | ||||||
| Plasma/PBMC | 33 | 1,636 | ||||||
| Animal 18 | ||||||||
| Cerebrum | 456 | 0 | + | |||||
| Cerebellum | 162 | 0 | − | |||||
| Brain stem | 70 | 0 | − | |||||
| CSF | 22 | |||||||
| Ocular | 0 | + | ||||||
| Plasma/PBMC | 0 | 5,668 |
Asterisk, not done; −, absent; +, mild; ++, moderate.
Viral load is expressed as number of copies/gram for cerebrum, cerebellum, and brain stem and as number of copies/milliliter for CSF, ocular, and plasma/PBMC.
Path, presence of CNS or ocular histopathology.
FIG. 1.
Mean viral loads in cats challenged with FIVGL8 (copies per gram of tissue or copies per milliliter of fluid) detected by Taqman real-time RT-PCR during the 23-week postinfection period.
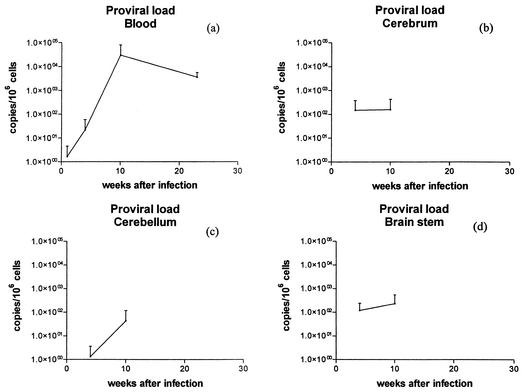
In the same time frame in the present study, PBMC proviral loads concentrations, estimated at 1, 4, 8, 10, and 23 weeks after infection, increased (Table 3, Fig. 2a). For this calculation, two different real-time PCR assays were used: the FIV proviral copy number was calculated targeting the FIV gag gene (43), and the cell number was estimated targeting the 18s ribosomal DNA genes (42). Low proviral copy numbers (5 to 65 copies/106 cells) were initially detected in 2 of 12 infected animals at week 1 after infection. Proviral loads between 107 and 1,254 copies/106 cells were observed in all nine infected animals sampled at 4 weeks after infection. The greatest concentrations were of the order of between 1,500 copies/106 cells and 87,169 copies/106 cells, noted from 8 weeks postinfection onwards until the completion of the study at 23 weeks after infection. The magnitude of and individual variation between proviral loads were in agreement with results in other FIV, SIV, and HIV studies (4, 21, 31, 35, 36, 58).
TABLE 3.
Proviral loads in PBMCs (copies of FIV/106 cells) from cats challenged with FIVGL8 during the 23-week postinfection period
| Cat no. | Proviral load (no. of copies/106 cells) at no. of weeks postinfectiona
|
||||
|---|---|---|---|---|---|
| 1 | 4 | 8 | 10 | 23 | |
| 1 | 5 | ||||
| 2 | 0 | ||||
| 3 | 0 | ||||
| 6 | 65 | 425 | |||
| 7 | 0 | 384 | |||
| 8 | 0 | 344 | |||
| 11 | 0 | 1,209 | 13,908 | 87,169 | |
| 12 | 0 | 506 | 1,574 | 1,774 | |
| 13 | 0 | 107 | 863 | 1,895 | |
| 16 | 0 | 542 | 2,554 | * | 3,350 |
| 17 | 0 | 295 | 2,280 | * | 1,636 |
| 18 | 0 | 1,254 | 2,466 | * | 5,668 |
Asterisk, no data was obtained.
FIG. 2.
Mean proviral loads (viral copies per 106 cells) in cats in the 23-week period following intravenous infection with FIVGL8. Results are shown for PBMCs (a), cerebrum (b), cerebellum (c) and brain stem (d).
Viral and proviral loads in the cerebrum, cerebellum, and brain stem were estimated at the time of necropsy (Table 2; Fig. 1b, c, and d; Fig. 2b, c, and d). At each time, loads were quite variable between individual animals and between regions of the brain. By week 1 after infection, virus was detected in the brain stem region of one animal (animal no. 1; 152 copies/g). At this time point no provirus was detected. By week 4, while only the animal with the highest serum viral load (no. 6) had detectable viral RNA in all three brain regions examined (80 copies/g to nearly 3 × 104 copies/g), all three animals had proviral loads (between 4 and 418 copies/106 cells). By week 10, all three animals had detectable viral RNA in brain tissue. The brain stem consistently contained high viral RNA loads, but the concentrations were quite variable (4 × 103, 5 × 104, and 2 × 106 copies/g). Proviral concentrations of between 109 and 586 copies/106 cells were observed in two animals only. By week 23, two of the three infected animals had detectable virus in all compartments of the brain examined. However, no provirus was detected in the brain tissue of any of the three animals examined. There was no clear pattern in either the regional distribution or in the magnitude of the concentrations of virus or provirus within the brain. Of the three regions examined for 12 animals, 14 regions had detectable virus and 10 regions had detectable provirus in quantities that varied between 80 and 2 × 106 copies/g and between 4 and 586 copies/106 cells, respectively.
To date, there has been very little information on viral loads within the CNS in FIV infection (58). The present study strengthened the observations that for the majority of animals the acute phase of infection is a significant time in which virus invades the CNS (7, 8, 37, 58). In fact, at the last time point of the study, only one animal had no detectable virus within the CNS or CSF, which would be more suggestive of a lack of CNS infection than of a transient infection which had been cleared or reduced to undetectable concentrations. While studies using similar quantification techniques have been undertaken using the SIV model, such studies have been primarily concerned with viral loads as they related to the end stages of disease, when the animal is likely to have encephalitis (20, 68). A recent study on an accelerated model of SIV-induced encephalitis revealed CNS viral loads in all animals at 10 weeks after infection, but in contrast to the present study, loads rapidly reduced to undetectable concentrations by 3 weeks, with a resurgence of loads in selected animals by 7 weeks (18).
Of concern in the present study, as in other studies, has been the fact that viral and proviral loads in blood may contribute to tissue values (18, 63, 68). Some studies have overcome this problem through tissue perfusion with saline (18, 68), while others have calculated the influence based on the assumptions that between approximately 5 and 10% of tissue volume may be blood (63). To reduce, but not eliminate, the effects of blood volume, our animals were exsanguinated during the euthanasia procedure. In addition, our results in many instances would not correlate with blood contamination, particularly when brain concentrations exceed 1/10 that of plasma. Thirdly, frequently in the present study regions of the brain were devoid of detectable virus or provirus even when a viral or proviral load was detectable within the plasma or PBMCs, respectively.
The magnitude of the concentrations of FIV RNA in 14 of 33 regions of brain parenchyma examined varied between approximately 1 × 102 and 2 × 106 copies per g of brain. Since similar studies focusing on the acute phase of infection have not been undertaken with HIV, it was not possible to truly compare viral loads between lentiviral infections. However, studies by Wiley and colleagues (63) documented viral loads between 1 × 103 and 4.5 × 107 copies of RNA per gram of brain tissue in HIV-infected AIDS patients with minimal to severe neuropathological changes. In a study by Zink and colleagues (68) to induce SIV AIDS and encephalitis, greater viral concentrations of between 1.4 × 109 and 3.8 × 1010 copies per g of brain tissue were calculated for terminally ill animals. However, studies on the acute stages of infection by this group revealed that viral RNA within the CNS is rapidly downregulated, to resurge with the onset of encephalitis (18). In agreement with the work of Wiley and colleagues (63), in the present study it was clear that viral RNA was not uniformly distributed throughout the brain during initial infection. However, in animals sacrificed towards the end of the study, a more uniform distribution of virus among all brain compartments was noted. It is difficult to draw conclusions as to why FIV, like HIV, appears to proliferate in many regions of the brain independent of their connectivity. However, while the limited sampling from each region may in part contribute to such findings, Wiley and colleagues (63) observed such changes with extensive sampling and suggested that local factors and monocyte trafficking may promote viral expression. In SIV infection, regional variations in viral loads have also been documented, with cerebellar viral loads lower than cortical, midbrain, and brain stem loads but in general less variable than those documented in HIV-1 infection and in the present study (68).
As with viral loads, proviral loads also appeared to vary between compartments. It was somewhat surprising that by 23 weeks of infection provirus was not detected in the brain, although this features was also noted by Pederson and colleagues (51) following a 20-week study with both FIV-Apetaluma and FIV-Cpgammar. Such findings were in contrast those of to another FIV study, which documented proviral loads over 2-year period, although loads did decrease following acute infection and prolonged sampling times were in operation (58).
In comparing viral RNA concentrations in plasma to those of the CNS, the transient virus proliferation in plasma coincided with the onset of CNS virus proliferation, although CNS viral loads remain elevated throughout the duration of the study. Animals euthanatized between weeks 4 and 10 displayed both the greatest concentrations of viral RNA within plasma and in the brain (Fig. 1). In addition, at these time points animals 6 and 7, with the greatest concentrations of plasma viral RNA, had detectable viral RNA in all compartments of the brain examined. In contrast, by week 23, animals with undetectable plasma viral RNA continued to have detectable viral RNA in all brain compartments examined. A second contrasting feature was the trend for proviral PBMC loads to increase with progression of infection while proviral loads within the CNS were detected only in weeks 4 and 10 following infection and were absent by week 23 (Fig. 2d).
Viral loads in the CSF were determined at the time of necropsy (Table 2; Fig. 1f). Viral RNA concentrations of between 40 and 400 copies/ml were first detected at 4 weeks following infection for all three animals (Table 2; Fig. 1f). At 10 weeks, all three animals also had detectable viral RNA (60 to 6,837 copies/ml) in CSF, and the two animals at 23 weeks after infection with detectable virus in the CNS also had CSF virus at concentrations between 22 and 151 copies/ml. While portions of brain tissue varied in viral and proviral loads and frequently compartments sampled had no detectable viral RNA or provirus, CSF consistently predicted that virus or provirus would be present in at least one portion of the brain. This would suggest that within this model, CSF viral load determinations would be the best predictor of early brain infection and could be a useful tool in CNS antiviral therapy studies which may focus on the acute stage of infection. The only exception was animal 1, euthanatized within 1 week of infection, in which virus was detected in the brain stem only and not in the CSF, suggesting that in the initial weeks of infection there is a potential short lag period before virus is observed in the CSF. The viral loads observed in the present study varied between 20 and 6 × 103 copies per ml and were obtained at four time points. Thus, they were unlikely to reflect peak concentrations. Evaluations of SIV CSF viral loads in acute infection revealed peak values of the order of 1 × 104 to 1 × 106 copies per ml between 10 and 14 days after infection, followed by a slight decrease or a stabilization at these values (68). Similar concentration were noted for FIV (10). No time course studies to evaluate CSF viral loads in the acute stages of HIV infection have been undertaken due to inabilities to capture this phase; however, many studies, biased towards the latter stages of infection, have positively correlated CSF viral loads with encephalopathy and dementia (11, 17, 24, 46).
A final observation of the study was that the magnitude and distribution of histopathological changes in brain and ocular tissue did not correlate with either tissue viral or proviral loads. Histopathological findings are summarized in Table 2 and form part of a more detailed publication on the neuropathology of FIVGL8 (G. Ryan, M. Mabruk, T. Grimes, B. Brankin, M. Hosie, O. Jarrett, and J. Callanan, unpublished data). In brief, microscopic examination of the cerebrum, cerebellum, and brain stem (midbrain, medulla pons) revealed perivascular lymphocyte cuffing of blood vessels within the meninges covering the cerebrum, cerebellum, and brain stem within the cerebral white matter and within the choroid plexus. While lesions were initially noticed 4 weeks after infection, they were more widespread and of greatest severity at weeks 10 and 23 after infection. At these time points there was clear variation between individuals in the severity of the microscopic changes. Ocular lesions consisted of perivascular lymphocyte accumulations within the scleral limbus and within the iris, ciliary body, and choroid. Lesions were initially noticed at 4 weeks after infection, but again they were more consistently observed, and of greatest severity, at weeks 10 and 23 postinfection.
In general the wave of viral replication within tissues preceded histopathological changes, since lesions were more prominent by weeks 10 and 23 after infection. However, a clear correlation between the concentration of virus within regions of brain and the magnitude of histopathological changes was not found. Similarly, while histopathological changes in the eye were noted in all nine animals sacrificed from week 4 onwards, virus was only detected in the ocular fluid of two animals at 4 weeks after infection and in one animal at 10 weeks (range, 24 to 42 copies per ml) (Table 2; Fig. 1e).
Both animals 11 and 16 had marked cerebral and ocular pathology. For animal 11, this coincided with markedly elevated viral loads. However, for animal 16, viral loads were considerably lower and were like those of animal 18, which had minimal pathology. Similarly, animal 6, at 4 weeks after infection, had prominent viral and proviral brain tissue loads but did not display any detectable histopathological changes in the brain. This animal did have limited ocular pathology, and virus was detected in ocular fluid. In contrast, at week 23, animal 17, while not having detectable virus in brain or ocular fluid, did have CNS and ocular histopathology changes.
Relating viral and proviral concentrations to CNS and ocular histopathology highlighted that while there was an overall trend for histopathological lesions to follow the onset of peripheral and CNS virus production, the magnitude of lesions within individual compartments of tissue did not correlate with the concentrations of virus and provirus observed in these regions. This factor, together with the observation that proviral DNA was detectable only transiently within the CNS, raises two interesting questions. Is the histopathology observed a response to viral presence in the CNS? Is viral CNS infection in the acute stages of disease a transient feature?
While many studies positively correlated viral loads with end-stage neuropathology in HIV, SIV, and FIV infections (7, 20, 29, 63, 68), the present study focused on the pathology associated with the acute phase of infection, and thus, there are few comparable studies (10, 18, 58). The lesions of meningeal, choroid plexus, and parenchymal mononuclear cell perivascular cuffing observed in the acute phase of infection are essentially features of the trafficking of mononuclear cells through the CNS, and as documented previously (7, 8, 20, 37), some of these cells are likely to be infected with virus. It is believed, however, that in HIV infection such cell accumulations are not due to localized virus production but are a reflection of a generalized systemic immune stimulation (29). This hypothesis is supported in the present study, since generalized reactive hyperplasia has been observed in association with early FIVGL8 infection commencing in a time frame similar to that of the onset of neuropathological lesions and the presence of perivascular mononuclear cell lesions in other organs, such as ocular tissue (13, 14). A second supporting feature was the observed trend for lesions to develop following the systemic proliferation of virus, and it would explain why animals such as animal 17, while showing no evidence of viral infection of the CNS, did show neuropathology. However, in the latter case, although unlikely, one cannot discount the possibility that virus had been in the brain earlier and had been eliminated or reduced to undetectable concentrations. Previous recent studies on the early stages of FIV infection, while not concentrating on histopathology, have documented an increased toxic activity of CSF and upregulation of CD18 in association with a decreased CSF and plasma viremia (10), while a positive correlation was noted between proviral loads and tumor necrosis factor alpha expression (58). In a comparable SIV study, histopathology was not observed in 17 of 18 macaques sequentially examined in an 8-week period following infection, although subtle immune and inflammatory changes, such as macrophage activation and the infiltration of cytotoxic lymphocytes, were noted (18).
While it is clear that FIV does ultimately cause neuropathology, it is not clear if the early observation of viral RNA in CSF and brain tissue is sustained and if this leads to the development of reservoirs of infection within the brain or this initial CNS infection is truly transient. It is well known that CSF viral loads may not necessarily reflect virus replication in the brain parenchyma but can develop as a result of virus replication in the meninges, from infected cells trafficking through the CSF, or from plasma-derived virus entering through a compromised blood-brain barrier (20, 63, 68). Similarly, it has been suggested that SIV loads in brain parenchyma may also be a reflection of transient infection by trafficking mononuclear cells (20). Studies of asymptomatic HIV-1-infected patients who die some years after seroconversion either show exceptionally low proviral loads within brain tissue, consistent with levels expected from the presence of blood vessel viral loads, or may show evidence of true low-grade infections (3, 5). With the increasing sensitivity of virus detection methods, it has been suggested that viral loads become dramatically reduced or regionalized and that the CNS functions as a reservoir for latent infection during the asymptomatic phase of infection (6, 18, 59). This obviously has major implications in the design of therapies to prevent or modify lentiviral CNS infections, since it would suggest that the acute stages of infection may be the most significant period in viral infection of the brain. Such possibilities highlight the need for an extension of the present study which would examine FIV-infected animals at frequent intervals over a longer period from the acute phase of infection and into an established asymptomatic phase. In particular, it would be desirable to perform extensive brain tissue sampling corroborated by in situ hybridization techniques in this period to further track the fate of CNS viral loads in an environment of reduced or undetectable peripheral blood loads and to monitor proviral loads in brain tissue in the presence of a strengthening PBMC proviral load.
Acknowledgments
We acknowledge the technical assistance of colleagues in the Department of Veterinary Pathology, University College Dublin (S. Worrall, C. King, B. Cloak, and J. Brady) and in the University of Veterinary Medicine, Vienna, Austria.
This work was funded by a Faculty of Veterinary Medicine, University College Dublin, Research Stimulus Grant. Collaborations with the University of Veterinary Medicine, Vienna, Austria, were funded through the FAVEUR Concerted Action of the European Commission.
REFERENCES
- 1.Achim, C. L., R. D. Schrier, and C. A. Wiley. 1991. Immunopathogenesis of HIV encephalitis. Brain Pathol. 1:177-184. [DOI] [PubMed] [Google Scholar]
- 2.Achim, C. L., R. Wang, D. K. Miners, and C. Wiley. 1994. Brain viral burden in HIV infection. J. Neuropathol. Exp. Neurol. 53:284-294. [DOI] [PubMed] [Google Scholar]
- 3.An, S. F., and F. Scaravilli. 1997. Early HIV-1 infection of the central nervous system. Arch. Anat. Cytol. Pathol. 45:94-105. [PubMed] [Google Scholar]
- 4.Bagnarelli, P., S. Menzo, A. Manzin, M. Giacca, P. E. Varaldo, and M. Clementi. 1991. Detection of human immunodeficiency virus type 1 genomic RNA in plasma samples by reverse-transcription polymerase chain reaction. J. Med. Virol. 34:89-95. [DOI] [PubMed] [Google Scholar]
- 5.Bell, J. E., A. Busuttil, J. W. Iornside, S. Rebus, Y. K. Donaldson, P. Simmonds, and J. F. Peutherer. 1993. Human immunodeficiency virus and the brain: an investigation of virus load and neuropathologic change in pre-AIDS subjects. J. Infect. Dis. 168:818-824. [DOI] [PubMed] [Google Scholar]
- 6.Blankson, J. N., D. Persuad, and R. F. Siliciano. 2002. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 53:557-593. [DOI] [PubMed] [Google Scholar]
- 7.Boche, D., M. Hurtel, F. Gray, M-A. Claessens-Marie, J.-P. Ganière, L. Montagnier, and B. Hurtrel. 1996. Virus load and neuropathology in the FIV model. J. Neurovirol. 2:377-387. [DOI] [PubMed] [Google Scholar]
- 8.Boche, D., E. Khatissian, F. Gray, P. Falanga, L. Montagnier, and B. Hurtrel. 1999. Viral load and neuropathology in the SIV model. J. Neurovirol. 5:232-240. [DOI] [PubMed] [Google Scholar]
- 9.Boretti, F. S., C. M. Leutenegger, C. Mislin, R. Hofmann-Lehmann, S. Konig, M. Schroff, C. Junghans, D. Fehr, S. W. Huettner, A. Habel, J. N. Flynn, A. Aubert, N. C. Pedersen, B. Wittig, and H. Lutz. 2000. Protection against FIV challenge infection by genetic vaccination using minimalistic DNA constructs for FIV env gene and feline IL-12 expression. AIDS 14:1749-1757. [DOI] [PubMed] [Google Scholar]
- 10.Bragg, D. C., L. C. Hudson, Y. H. Liang, M. B. Tompkins, A. Fernandes, and R. B. Meeker. 2002. Choroid plexus macrophages proliferate and release toxic factors in response to feline immunodeficiency virus. J. Neurovirol. 8:225-239. [DOI] [PubMed] [Google Scholar]
- 11.Brew, B. J., L. Pemberton, P. Cunningham, and M. G. Law. 1997. Levels of human immunodeficiency virus type 1 RNA in cerebrospinal fluid correlate with AIDS dementia stage. J. Infect. Dis. 175:963-966. [DOI] [PubMed] [Google Scholar]
- 12.Budka, H. 1991. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1:163-175. [DOI] [PubMed] [Google Scholar]
- 13.Callanan, J. J., H. Thompson, S. R. Toth, B. O'Neill, C. E. Lawrence, B. Willett, and O. Jarrett. 1992. Clinical and pathological findings in feline immunodeficiency virus experimental infection. Vet. Immunol. Immunopathol. 35:3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callanan, J. J., P. Racz, H. Thompson, and O. Jarrett. 1993. Morphological characterisation of lymph node changes in feline immunodeficiency virus infection as an animal model of AIDS, p. 115-136. In P. Racz, N. L. Letvin, and J. C. Gluckmann (ed.), Animal models of HIV infection. Karger, Basel, Switzerland.
- 15.Callanan, J. J., B. A. Jones, J. Irvine, B. J. Willett, I. A. P. McCandlish, and O. Jarrett. 1996. Histological classification and immunophenotype of lymphosarcomas in cats with naturally and experimentally acquired feline immunodeficiency virus infections. Vet. Pathol. 33:264-272. [DOI] [PubMed] [Google Scholar]
- 16.Chakrabarti, L., M. Hurtrel, M.-A. Marie, R. Vazeux, D. Dormont, L. Montagnier, and B. Hurtrel. 1991. Early viral replication in the brain of SIV-infected rhesus monkeys. Am. J. Pathol. 139:1273-1280. [PMC free article] [PubMed] [Google Scholar]
- 17.Cinque, P., L. Vago, D. Ceresa, F. Mainini, M. R. Terreni, A. Vagani, W. Torri, S. Bossolasco, and A. Lazzarin. 1998. Cerebrospinal fluid HIV-1 RNA levels: correlation with HIV encephalitis. AIDS 12:389-394. [DOI] [PubMed] [Google Scholar]
- 18.Clements, J. E., T. Babas, J. L. Mankowski, K. Suryanarayana, M. Piatak, Jr., P. M. Tarwater, J. D. Lifson and M. C. Zink. 2002. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J. Infect. Dis. 186:905-913. [DOI] [PubMed] [Google Scholar]
- 19.Cooper, D. A., J. Gold, P. Maclean, B. Donovan, R. Finlayson, T. G. Barnes, H. M. Michelmore, P. Brook, and R. Penny. 1985. Definition of a clinical illness associated with seroconversion. Lancet i:537-540. [DOI] [PubMed]
- 20.Demuth, M., S. Czub, U. Sauer, E. Koutsilieri, P. ten Haaft, J. Heeney, C. Stahl-Hennig, V. ter Meulen, and S. Sopper. 2000. Relationship between viral load in blood, cerebrospinal fluid, brain tissue and isolated microglia with neurological disease in macaques infected with different strains of SIV. J. Neurovirol. 6:187-210. [DOI] [PubMed] [Google Scholar]
- 21.Désiré, N., A. Dehée, V. Schneider, C. Jacomet, C. Goujon, P.-M. Girard, W. Rozenbaum, and J.-C. Nicolas. 2001. Quantification of human immunodeficiency virus type 1 proviral load by a taqman real-time PCR assay. J. Clin. Microbiol. 39:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diehl, L. J., C. K. Mathiason-DuBard, L. L. O'Neil, and E. Hoover. 1995. Longitudinal assessment of feline immunodeficiency virus kinetics in plasma by use of a quantitative competitive reverse transcriptase PCR. J. Virol. 69:2328-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diop, O. M., A. Gueye, M. Dias-Tavares, C. Kornfeld, A. Faye, P. Ave, M. Huerre, S. Corbet, F. Barre-Sinoussi, and M. C. Müller-Trutwin. 2002. High levels of viral replication during the primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys. J. Virol. 74:7538-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Stefano, M., L. Monno, J. R. Fiore, G. Buccoliero, A. Appice, L. M. Perulli, G. Pastore, and G. Angarano. 1998. Neurological disorders during HIV-1 infection correlate with viral load in cerebrospinal fluid but not with virus phenotype. AIDS 12:737-743. [DOI] [PubMed] [Google Scholar]
- 25.Dow, S. W., M. L. Poss, and E. A. Hoover. 1990. Feline immunodeficiency virus: a neurotropic lentivirus. J. Acquir. Immune Defic. Syndr. 3:658-668. [PubMed] [Google Scholar]
- 26.Dow, S. W., M. J. Dreitz, and E. A. Hoover. 1992. Feline immunodeficiency virus neurotropism. Evidence that astrocytes and microglial cells are the primary target cells. Vet. Immunol. Immunopathol. 35:23-35. [DOI] [PubMed] [Google Scholar]
- 27.Flynn, J. N., C. A. Cannon, C. E. Lawrence, and O. Jarrett. 1994. Polyclonal B-cell activation in cats infected with feline immunodeficiency virus. Immunology 81:626-630. [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner, M. B., and P. A. Luciw. 1989. Animal models of AIDS. FASEB J. 3:2593-2606. [DOI] [PubMed] [Google Scholar]
- 29.Gray, F., F. Scaravilli, I. Everall, F. Chretien, S. An, D. Boche, H. Adle-Biassette, L. Wingertsmann, M. Durigon, B. Hurtrel, F. Chiodi, J. Bell, and P. Lantos. 1996. Neuropathology of early HIV-1 infection. Brain Pathol. 6:1-15. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann-Lehmann, R., R. K. Swenerton, V. Liska, C. M. Leutenegger, H. Lutz, H. M. McClure, and R. M. Ruprecht. 2000. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one-versus two-enzyme systems. AIDS Res. Hum. Retrovir. 16:1247-1257. [DOI] [PubMed] [Google Scholar]
- 31.Holzammar, S., E. Holznagel, A. Kaul, R. Kurth, and S. Norley. 2001. High virus loads in naturally and experimentally SIVagm-infected African green monkeys. Virology 283:324-331. [DOI] [PubMed] [Google Scholar]
- 32.Hosie, M. J., and O. Jarrett. 1990. Serological responses of cats to feline immunodeficiency virus. AIDS 4:215-220. [DOI] [PubMed] [Google Scholar]
- 33.Hosie, M. J., B. Willett, T. Dunsford, O. Jarrett, and J. C. Neil. 1993. A monoclonal antibody which blocks infection with feline immunodeficiency virus identifies a possible non-CD4 receptor. J. Virol. 67:1667-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosie, M. J., R. Osborne, J. K. Yamamoto, J. C. Neil, and O. Jarrett. 1995. Protection against homologous but not heterologous challenge induced by inactivated feline immunodeficiency virus vaccines. J. Virol. 69:1253-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosie, M. J., T. Dunsford, D. Klein, B. J. Willett, C. Cannon, R. Osborne, J. MacDonald, N. Spibey, N. Mackay, O. Jarrett, and J. C. Neil. 2000. Vaccination with inactivated virus but not viral DNA reduces virus load following challenge with a heterologous and virulent isolated of feline immunodeficiency virus. J. Virol. 74:9403-9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosie, M. J., B. J. Willett, D. Klein, T. H. Dunsford, C. Cannon, M. Shimojima, J. C. Neil, and O. Jarrett. 2002. Evolution of replication efficiency following infection with molecularly cloned feline immunodeficiency virus of low virulence. J. Virol. 76:6062-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurtrel, B., L. Chakrabarti, M. Hurtrel, M.-A. Maire, D. Dormont, and L. Montagnier. 1991. Early SIV encephalopathy. J. Med. Primatol. 20:159-166. [PubMed] [Google Scholar]
- 38.Hurtrel, M., J.-P. Ganière, J.-F. Guelfi, L. Chakrabarti, M.-A. Maire, F. Gray, L. Montagnier, and B. Hurtrel. 1992. Comparison of early and late feline immunodeficiency virus encephalopathies. AIDS 6:399-406. [DOI] [PubMed] [Google Scholar]
- 39.Ishida, T., and I. Tomoda. 1990. Clinical staging of feline immunodeficiency virus infection. Jpn. J. Vet. Sci. 52:645-648. [DOI] [PubMed] [Google Scholar]
- 40.Johnson, R. T., J. C. McArthur, and O. Narayan. 1988. The neurobiology of human immunodeficiency virus infections. FASEB J. 2:2970-2981. [DOI] [PubMed] [Google Scholar]
- 41.Klein, D., P. Janda, R. Steinborn, M. Muller, B. Salmons, and W. H. Günzburg. 1999. Proviral load determination of different feline immunodeficiency virus isolates using real-time polymerase chain reaction: influence of mismatches on quantification. Electrophoresis 20:291-299. [DOI] [PubMed] [Google Scholar]
- 42.Klein, D., B. Bugl, W. H. Günzburg, and B. Salmons. 2000. Accurate estimation of transduction efficiency necessitates a multiplex real-time PCR. Gene Ther. 7:458-463. [DOI] [PubMed] [Google Scholar]
- 43.Klein, D., C. M. Leutenegger, C. Bahula, P. Gold, R. Hofmann-Lehmann, B. Salmons, H. Lutz, and W. H. Günzburg. 2001. Influence of preassay and sequence variations on viral load determination by a multiplex real-time reverse transcriptase-polymerase chain reaction for feline immunodeficiency virus. J. Acquir. Immune. Defic. Syndr. 26:8-20. [DOI] [PubMed] [Google Scholar]
- 44.Leuteneggar, C., D. Klein, R. Hofmann-Lehmann, C. Mislin, U. Hummel, J. Boni, F. Boretti, W. Günzberg, and H. Lutz. 1999. Rapid feline immunodeficiency virus provirus quantification by polymerase chain reaction using Taqman fluorogenic real-time detection system. J. Virol. Methods 78:105-116. [DOI] [PubMed] [Google Scholar]
- 45.Leutenegger, C. M., J. Higgins, T. B. Matthews, A. F. Tarantal, P. A. Luciw, N. C. Pederseon, and T. W. North. 2001. Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantification of simian immunodeficiency virus RNA. AIDS Res. Hum. Retrovir. 17:243-251. [DOI] [PubMed] [Google Scholar]
- 46.McArthur, J. C., D. R. McClernon, M. F. Cronin, T. E. Nance-Sproson, A. J. Saa, M. St. Clair, and E. R. Lanier. 1997. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann. Neurol 42:675-678. [DOI] [PubMed] [Google Scholar]
- 47.Overbaugh, J., P. A. Luciw, and E. A. Hoover. 1997. Models for AIDS pathogenesis: simian immunodeficiency virus, simian-human immunodeficiency virus and feline immunodeficiency virus infections. AIDS 11:S47-S54. [PubMed] [Google Scholar]
- 48.Pedersen, N. C., E. W. Ho, M. L. Brown, and J. K. Yamamoto. 1987. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency like syndrome. Science 235:790-793. [DOI] [PubMed] [Google Scholar]
- 49.Pedersen, N. C., J. K. Yamamoto, T. Ishida, and H. Hansen. 1989. Feline immunodeficiency virus infection. Vet Immunol. Immunopathol. 21:229-243. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen, N. C., and J. E. Barlough. 1991. Clinical overview of feline immunodeficiency virus. J. Am. Vet. Med. Assoc. 199:1298-1305. [PubMed] [Google Scholar]
- 51.Pedersen, N. C., C. M. Leuteneggar, J. Woo, and J. Higgins. 2001. Virulence difference between two field isolates of feline immunodeficiency virus (FIV-Apetaluma and FIV-Cpgammar) in young adult specific pathogen free cats. Vet. Immunol. Immunopathol. 79:53-67. [DOI] [PubMed] [Google Scholar]
- 52.Persidsky, Y., H. S. Nottet, V. G. Sasserville, L. G. Epstein, and H. E. Gendelman. 1995. The development of animal model systems for HIV-1 encephalitis and its associated dementia. J. Neurovirol. 1:229-243. [DOI] [PubMed] [Google Scholar]
- 53.Piatak, M., Jr., M. S. Saag, L. C. Yang, S. J. Clark, J. C. Kappes, K.-C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259:1749-1754. [DOI] [PubMed] [Google Scholar]
- 54.Pistello, M., S. Menzo, M. Giorgi, L. Da Prato, G. Cammarota, M. Clementi, and M. Bendinelli. 1994. Competitive polymerase chain reaction for quantitating feline immunodeficiency virus load in infected cat tissues. Mol. Cell. Probes 8:229-234. [DOI] [PubMed] [Google Scholar]
- 55.Podell, M., M. Oglesbee, L. Mathes, S. Krakowka, R. Olmstead, and L. Lafrado. 1993. AIDS-associated encephalopathy with experimental feline immunodeficiency virus infection. J. Acquir. Immune. Defic. Syndr. 6:758-771. [PubMed] [Google Scholar]
- 56.Podell, M., K. Hayes, M. Oglesbee, and L. Mathes. 1997. Progressive encephalopathy associated with CD4/CD8 inversion in adult FIV-infected cats. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 15:332-340. [DOI] [PubMed] [Google Scholar]
- 57.Podell, M., P. A. March, W. R. Buck, and L. E. Mathes. 2000. The feline model of neuroAIDS: understanding the progression towards AIDS dementia. J. Psychopharmacol. 14:205-213. [DOI] [PubMed] [Google Scholar]
- 58.Poli, A., M. Pistello, M. A. Carli, F. Abramo, G. Mancuso, E. Nicoletti, and M. Bendinelli. 1999. Tumour necrosis factor-α and virus expression in the central nervous system of cats infected with feline immunodeficiency virus. J. Neurovirol. 5:465-473. [DOI] [PubMed] [Google Scholar]
- 59.Pomerantz, R. J. 2002. Reservoirs of human immunodeficiency virus type-1: the main obstacles to viral eradication. Clin. Infect. Dis. 34:91-97. [DOI] [PubMed] [Google Scholar]
- 60.Schutten, M., B. van den Hoogan, M. E. van der Ende, R. A. Gruters, A. D. Osterhaus, and H. G. Niesters. 2000. Development of a real-time quantitative RT-PCR for the detection of HIV-2 RNA in plasma. J. Virol. Methods 88:81-87. [DOI] [PubMed] [Google Scholar]
- 61.Suryanarayana, K., T. A. Wiltrout, G. M. Vasquez, V. M. Hirsch, and J. D. Lifson. 1998. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res. Hum. Retrovir. 14:183-189. [DOI] [PubMed] [Google Scholar]
- 62.Vahlenkamp, T. W., H. F. Egberink, M. J. T. van Eijl, A. M. E. Slotboom-Kamphorst, E. J. Verschoor, M. C. Horzinek, and A. de Ronde. 1995. Competitive reverse transcription-polymerase chain reaction for quantification of feline immunodeficiency virus. J. Virol. Methods 52:335-346. [DOI] [PubMed] [Google Scholar]
- 63.Wiley, C. A., V. Sootornniyomkij, L. Radhakrishnan, E. Masliah, J. Mellors, S. A. Hermann, P. Dailey, and C. L. Achim. 1998. Distribution of brain HIV load in AIDS. Brain Pathol. 8:227-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willett, B. J., M. J. Hosie, J. J. Callanan, J. C. Neil, and O. Jarrett. 1993. Infection with feline immunodeficiency virus is followed by the rapid expansion of a CD8+ lymphocyte subset. Immunology 78:1-6. [PMC free article] [PubMed] [Google Scholar]
- 65.Willett, B. J., J. N. Flynn, and M. J. Hosie. 1997. FIV infection of the domestic cat: an animal model for AIDS. Immunol. Today 18:182-189. [DOI] [PubMed] [Google Scholar]
- 66.Willett, B. J., L. Picard, M. J. Hosie, L. D. Turner, K. Adema, and P. R. Claphman. 1997. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J. Virol. 71:6407-6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto, J. K., E. Sparger, E. W. Ho, P. R. Andersen, T. P. O'Connor, C. P. Mandell, L. Lowenstine, R. Munn, and N. C. Pedersen. 1988. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am. J. Vet. Res. 8:1246-1258. [PubMed] [Google Scholar]
- 68.Zink, M. C., K. Suryanarayana, J. Mankowski, A. Shen, M. Piatak, Jr., J. P. Spelman, D. L. Carter, R. J. Adams, J. D. Lifson, and J. E. Clements. 1999. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J. Virol. 73:10480-10488. [DOI] [PMC free article] [PubMed] [Google Scholar]