Abstract
Tva is the receptor for subgroup A Rous sarcoma virus, and it contains a single LDL-A module which is the site of virus interaction. In this study, we expressed the entire extracellular region of Tva (referred to as Ecto-Tva) as a GST fusion protein and characterized its refolding properties. We demonstrated that the correct folding of the Ecto-Tva protein, like that of the Tva LDL-A module, is calcium dependent. We used the IAsys system to measure the kinetics of binding between the surface (SU) subunit of the viral glycoprotein and Tva in real time. We found that the Ecto-Tva protein and the Tva LDL-A module displayed similar affinities for SU, providing direct evidence that the LDL-A module of Tva is the only viral interaction domain of the receptor. Furthermore, misfolded Tva proteins displayed lower binding affinities to SU, largely due to a decrease in their association rates, suggesting that a high association rate between SU and Tva is crucial for efficient virus-host interaction. Furthermore, we found that calcium did not influence the overall binding affinity between Tva and SU. These results indicate that, although calcium is important in facilitating correct folding of the LDL-A module of Tva, it is not essential for ligand binding. Thus, these results may have broad implications for the mechanism of protein folding and ligand recognition of the LDL receptor and other members of the LDL receptor superfamily.
The cellular receptor for subgroup A Rous sarcoma virus (RSV-A) is Tva, a small membrane-associated glycoprotein (2, 3, 34). Entry of RSV-A into host cells is mediated by interactions between Tva and its cognate viral glycoprotein, EnvA (13, 16). Like glycoproteins of most, if not all, retroviruses, such as human immunodeficiency virus and murine leukemia virus, and a large number of other enveloped viruses, such as influenza virus and filoviruses, the native form of EnvA on the virion consists of a trimeric complex of heterodimeric surface (SU [or gp85]) and transmembrane (TM [or gp37]) subunits (8, 11). The initial EnvA-Tva interaction is determined by high-affinity binding between SU and Tva (1, 13, 19, 22, 36), which subsequently induces a series of conformational changes in both SU and TM that are required for the fusion of viral and cellular membranes (7, 12, 14, 18). Expression of Tva in otherwise nonsusceptible cells from diverse species and tissues renders them infectible by RSV-A, strongly suggesting that there is no coreceptor requirement for RSV-A entry. A 40-residue cysteine-rich motif, referred to as the LDL-A module, which is highly conserved in all members of the LDL receptor (LDLR) superfamily, is located near the N terminus of the Tva extracellular domain (2). In human LDLR, seven such imperfect LDL-A modules consist of the ligand-binding domain, responsible for binding to lipoprotein apoB- and apoE-containing LDL particles (9, 24). It has been demonstrated that the LDL-A module of Tva could efficiently mediate RSV-A entry when it was anchored to the membrane by a heterologous membrane-spanning domain (20). Furthermore, this module of Tva could be functionally replaced by a modified human LDL-A4 module in mediating efficient RSV-A entry (21). These results suggested that the LDL-A module of Tva is the viral interaction site. Because receptor-triggered conformational changes on the cognate viral glycoprotein as a prerequisite for membrane fusion and viral entry appear to be a common mechanism used by many enveloped viruses, and because the elucidation of the viral entry mechanism depends on an integrated approach of biochemical, structural, and functional analyses of these components, we believe that analysis of Tva/EnvA interactions can serve as a model for dissecting the entry mechanism(s) of a large number of enveloped viruses.
Sequence alignment indicates that each LDL-A module contains six invariable cysteines and five highly conserved acidic residues. The X-ray crystal structures of the human LDL-A5 and other LDL-A modules revealed that four of the five highly conserved acidic residues are involved in calcium coordination via their side chains (10, 25). It has been shown that calcium is also required for correct folding of the Tva LDL-A module and that calcium is coordinated by the four highly conserved acidic residues via their side chains and by two nonacidic residues via their carbonyl oxygen groups (28-30). In this study, using the IAsys system to measure the kinetics between SU and Tva in real time, we found that the misfolded Tva proteins displayed lower binding affinities to SU, largely due to a decrease in their association constants, suggesting that a high association rate between SU and Tva is crucial for efficient virus-host interaction. Surprisingly, although calcium is required for correct folding of the Tva proteins, it is not essential for ligand binding. These results may also have broad implications for LDLR-ligand interactions.
MATERIALS AND METHODS
Expression and purification of the Ecto-Tva protein.
The coding region of the gene for the extracellular domain of Tva (here referred to as Ecto-Tva) was amplified by PCR using quail Tva DNA as the template (2). To facilitate the cloning procedure, a BamHI site and an XhoI site were engineered into the upstream and the downstream primers, respectively. The PCR product was cloned into a glutathione S-transferase (GST) fusion vector, pGEX-4T-1, and the construct was confirmed by DNA sequencing.
The Ecto-Tva protein was expressed in Escherichia coli strain BL21 as a GST fusion protein and purified by glutathione (GSH) affinity column, following a previous protocol (28). After thrombin cleavage, the GST portion was removed by GSH affinity column, and the Ecto-Tva protein was further purified by reverse-phase high-performance liquid chromatography (HPLC). The same protocol was used for the preparation of the wild-type Tva LDL-A module and its mutant proteins.
Characterization of the in vitro folding properties of the Ecto-Tva protein.
The folding properties of the Ecto-Tva protein were examined by reverse-phase HPLC and two-dimensional nuclear magnetic resonance (NMR) spectroscopy, and its Ca2+ binding affinity was measured with an isothermal titration calorimeter (ITC) (MicroCal MSC). The purified Ecto-Tva protein was refolded either in the presence of 10 mM CaCl2 or in the presence of 1 mM EDTA. Following the refolding reactions, the samples were analyzed by reverse-phase HPLC on a Vydac C18 column operated at a flow rate of 3.00 ml/min, using a linear gradient of 0.1% trifluoroacetic acid and 90% acetonitrile. The 15N-labeled Ecto-Tva protein was used to acquire the [1H-15N]-heteronuclear single quantum correlation (HSQC) spectra. The NMR data were collected on a Bruker DRX600 spectrometer equipped with a pulse field gradient accessory and operating at 600.13 MHz for 1H and were processed and analyzed using Triad version 6.3. The central frequencies were 4.70 and 118 ppm for 1H and 15N, respectively. To measure the Ca2+ binding affinity of the Ecto-Tva protein, 10 μM Ecto-Tva protein in buffer (20 mM Tris-HCl, 100 mM NaCl, and 0.02% NaN3, pH 7.4) was titrated with a 4-μl injection of 2 mM CaCl2 in buffer at 25°C, and the data were analyzed by Origin MicroCal.
Purification of SUA-rIgG and SUB-rIgG proteins.
Expression and purification of the SUA-rabbit immunoglobulin G (rIgG) and SUB-rIgG proteins were performed according to the method of Zingler and Young (36) and Mothes et al. (18) with minor modifications. Briefly, each 15-cm-diameter plate of 293T cells in selective Dulbecco's modified Eagle's medium (supplemented with 10% fetal calf serum and 300 μg of G418/ml) was transfected with 45 μg of the plasmid DNA by a CaPO4 method. After incubation for 18 h, the cells were washed with 1× Dulbecco's phosphate-buffered saline (DPBS) and then incubated with precleared selective Dulbecco's modified Eagle's medium. The medium was harvested 48 h later and filtered through a 0.8-μm-pore-size membrane to remove cell debris and then loaded twice onto a 1.5-ml protein A column (Pierce) at a flow rate of 1 to 2 ml/min, followed by washing with calcium and magnesium-free DPBS at 4 ml/min. The SUA-rIgG and SUB-rIgG proteins were eluted with 0.1 M citric acid (pH 3) and immediately neutralized in 1 M Tris (pH 9.5). The purified SUA-rIgG and SUB-rIgG proteins were concentrated in a Centricon-30 column (Amicon) after dialysis against calcium- and magnesium-free DPBS and stored at −80°C for further analysis. The SUA-rIgG and SUB-rIgG proteins were detected by blotting them with horseradish peroxidase-conjugated goat antibody specific for rabbit immunoglobulins. The concentrations of the purified proteins were determined by Bradford assay, and the protein purity was assessed by Coomassie blue staining.
Ligand-blocking assay.
The abilities of the E. coli-expressed Tva proteins to interact with their ligand, SUA, were examined by a ligand-blocking assay. Briefly, the E. coli-expressed Tva proteins, following the in vitro refolding reactions either in the presence or absence of calcium and purification by reverse-phase HPLC, were incubated with 150 nM purified SUA-rIgG protein for 1.5 h at 4°C. Equal amounts (25 μl) of the 293T cell lysate containing the myc-Tva protein were then added to each incubation mixture, and the mixtures were incubated for an additional 1.5 h. Finally, 15 μl of a 50% slurry of protein A-Sepharose beads (Pharmacia) was added, and the mixture was incubated for 1 h. After centrifugation, the supernatants were saved, and the beads were washed three times with 1× DPBS buffer and then resuspended in 1% Triton lysis buffer (100 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100). Both the supernatant and the beads were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis using a monoclonal antibody, 9E10, to detect the myc-tagged Tva protein. The purified SUB-rIgG protein was used as a negative control. To examine the role of calcium in ligand binding, 5 mM EDTA was added to the ligand-blocking assay in some experiments.
Determination of the binding kinetics by IAsys.
A resonant mirror biosensor (IAsys Auto+; Affinity Sensors) with a carboxylate cuvette was used to determine the kinetic constants (association constant [kass] and dissociation constant [kdiss]) and binding affinities (KD) of SUA-rIgG to the Tva proteins. All the experiments were performed at 25°C. Briefly, the Tva proteins were first refolded with or without calcium and then purified by reverse-phase HPLC. The lyophilized proteins were dissolved in 10 mM acetate buffer either with or without 10 mM CaCl2, and these proteins were immobilized to the EDC/NHS [1-ethyl-3-(3-dimethylaminopropyl) carbodiimide HCl/N-hydroxysuccinimide] activated carboxylate cuvettes following the manufacturer's protocol. The unreacted NHS esters and uncovered surface area were blocked by 2 mg of β-casein/ml. The tightly associated but noncovalently bound protein was removed with 10 mM HCl. The immobilization levels for the Tva proteins ranged from 400 to 700 arc seconds (600 arc seconds = 1 ng of protein/mm2). Binding of the SUA-rIgG protein to the immobilized Tva proteins was performed in PBS with additional 100 mM NaCl (to minimize the nonspecific binding) with or without 250 μM CaCl2. In some experiments, 5 mM EDTA was added. The binding kinetics were examined by monitoring the association phase for 5 min, followed by monitoring the dissociation phase for 4 min. After each cycle, the cuvettes were regenerated with 100 mM HCl for 2 to 5 min. The binding cycle was repeated using six to eight different concentrations of the SUA-rIgG protein dissolved in PBS per 100 mM NaCl with or without 250 μM CaCl2. The purified SUB-rIgG protein was used to measure nonspecific binding. The experimental data were processed using FASTfit software (Affinity Sensor). The kass was calculated from the gradient of the plot of the apparent rate constant (kon) versus the SUA-rIgG protein concentration, which was obtained by a linear fit. The kdiss was determined from the intercept of the kon-versus-[ligate] plot. When data were poorly fitted to a single exponential phase, a double exponential phase was used to process the association and dissociation data. KD was obtained from the equation KD = kdiss/kass.
RESULTS
Expression and purification of the Ecto-Tva protein.
The quail Ecto-Tva construct was expressed as a GST fusion protein in E. coli BL21 cells. High levels of expression of this fusion protein were achieved by induction with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). The fusion protein was purified by one-step chromatography with a GSH-Sepharose column. Following cleavage by thrombin, the GST portion of the fusion protein was removed by GSH-Sepharose chromatography. The cleaved Ecto-Tva protein was further purified by reverse-phase HPLC (Fig. 1A). The predicted Ecto-Tva protein contains 92 amino acids: the 83-residue extracellular domain of Tva, two additional residues (GS) at the N terminus, and seven additional residues derived from the vector at the C terminus (Fig. 1B). We could routinely obtain ∼30 mg of the purified Ecto-Tva protein after thrombin cleavage from 1 liter of bacterial culture. The purified Ecto-Tva protein ran as an ∼22-kDa protein species by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis instead of the predicted 10-kDa size as calculated (data not shown).
FIG. 1.
(A) Purification of the Ecto-Tva protein after thrombin cleavage. The GST/Ecto-Tva fusion protein was expressed in and purified from E. coli. After thrombin cleavage, the majority of the GST was removed by GST-Sepharose column, and the cleaved Ecto-Tva protein was further purified by reverse-phase HPLC. The Ecto-Tva protein after cleavage was eluted at about 27 min, and the GST portion was eluted at 45 to 50 min as indicated in the HPLC profile. (B) Predicted amino acid sequence of the Ecto-Tva protein after thrombin cleavage. The residues of the extracellular region of Tva (83 amino acids) are shown in boldface. The N-terminal two residues and C-terminal six residues of the Ecto-Tva protein are derived from the vector.
In vitro folding properties of the Ecto-Tva protein.
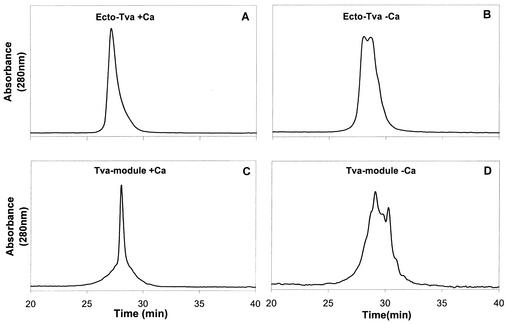
It has been demonstrated that Ca2+ is required for correct in vitro folding of the Tva LDL-A module (28). We predicted that the Ecto-Tva protein also requires calcium for correct folding under the same in vitro conditions. To test this, the purified Ecto-Tva protein, after refolding either in the presence or absence of calcium, was eluted in a reverse-phase HPLC column. As expected, the Ecto-Tva protein was eluted as a single sharp peak when it was refolded in the presence of 10 mM CaCl2 (Fig. 2A). In contrast, the protein was eluted as broad, multiple peaks when calcium was absent in the refolding reaction (Fig. 2B). As controls, the Tva LDL-A module was shown to elute as a single sharp peak in the presence of calcium (Fig. 2C), and it was eluted as multiple peaks in the absence of calcium (Fig. 2D), as previously demonstrated (28). These results suggest that the Ecto-Tva protein, like the Tva LDL-A module, requires calcium for correct in vitro folding.
FIG. 2.
HPLC elution profiles of Ecto-Tva and Tva LDL-A proteins after in vitro refolding. The purified Ecto-Tva and Tva LDL-A proteins were refolded in the presence of either 10 mM CaCl2 (A and C) or 1 mM EDTA (B and D), and the samples were eluted by reverse-phase HPLC on a Vydac column.
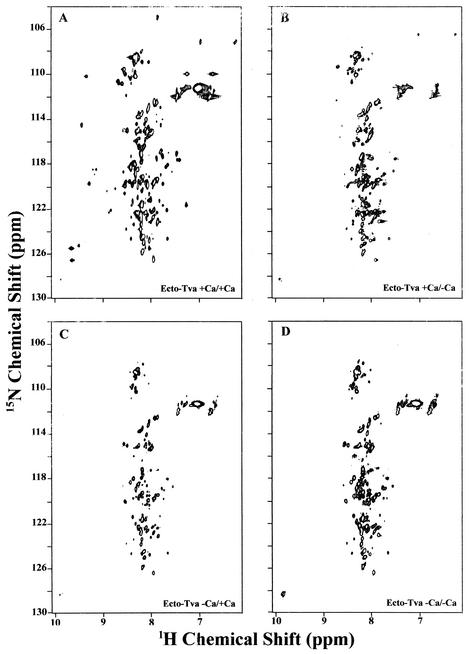
Two-dimensional [1H-15N]-HSQC NMR spectroscopy was previously used to demonstrate that Ca2+ is required not only for correct folding but also for maintaining the structural integrity of the Tva LDL-A module (28). Here, we used the same technique to examine the Ca2+-dependent in vitro folding properties of the Ecto-Tva protein. It is known that well-folded proteins give well-dispersed spectra while misfolded proteins give highly clustered spectra. It was found that, like the Tva LDL-A module, the Ecto-Tva protein gave a reasonably well-dispersed spectrum in both the 1H and 15N dimensions when calcium was present during the refolding and spectral-acquisition steps (Fig. 3A). In contrast, when calcium was absent in the refolding reactions, the Ecto-Tva protein displayed clustered spectra, especially in the 1H dimension, regardless of whether calcium was added (Fig. 3C) or omitted (Fig. 3D) in the NMR spectral-acquisition step. These results suggest that, as for the Tva LDL-A module, correct folding of the Ecto-Tva protein is Ca2+ dependent. Furthermore, the Ecto-Tva protein, like the Tva LDL-A module, requires calcium to maintain the correct conformation (compare Fig. 3A and B). However, examination of the NMR HSQC spectra of the Ecto-Tva protein with those of the Tva LDL-A module indicates that most of the cross-peaks of the Ecto-Tva protein which were well-dispersed and clearly identified corresponded to the residues of the Tva LDL-A module (Fig. 4). Here, the HSQC spectra of the Ecto-Tva protein and the Tva LDL-A module, when calcium was present in the refolding step and in the NMR spectral-acquisition step, were superimposed for comparison. These results suggest that the in vitro folding and the conformation of the Tva LDL-A module are not greatly influenced by the N- or C-terminal sequences. In addition, these results appear to suggest that the N-terminal 10 residues and the C-terminal 33 residues of the Ecto-Tva protein, in contrast to the LDL-A module region, are not well structured under these conditions.
FIG. 3.
Two-dimensional NMR [1H-15N] HSQC spectra of the Ecto-Tva protein. The 15N-labeled Ecto-Tva protein was refolded either in the presence (+Ca/) or in the absence (−Ca/) of Ca2+, and the NMR [1H-15N] HSQC spectra were acquired with (/+Ca) or without (/−Ca) Ca2+ during the NMR spectral-acquisition step.
FIG. 4.
Comparison of NMR spectra of Ecto-Tva protein and Tva LDL-A protein. The NMR HSQC cross-peaks of the Ecto-Tva protein (blue) (Fig. 3A) and the Tva LDL-A protein (red) (Wang et al. [28]) are superimposed for comparison. Calcium was present in both the refolding step and the NMR spectral-acquisition step.
The Ca2+ binding affinity of the Ecto-Tva protein was directly measured by ITC. The Ecto-Tva protein gave an apparent KD of 28 μM, which is similar to that of the Tva LDL-A module (KD = 37 μM). Furthermore, the binding process of Ecto-Tva, like that of the Tva LDL-A module protein, is also endothermic (ΔH = 1.27 kcal mol−1) and entropically favored, with −TΔS° being −7.48 kcal mol−1 (out of a ΔG° of −6.21 kcal mol−1). These results indicate that the Ecto-Tva protein has a binding affinity to calcium similar to that of the Tva LDL-A module.
Examination of SUA-rIgG/Tva interaction by a ligand-blocking assay.
A simple ligand-blocking assay was developed to examine whether the E. coli-expressed Tva proteins displayed SUA-binding activity. In this semiquantitative assay, a purified subgroup A SU-immunoglobulin fusion protein (SUA-rIgG [36]) was first incubated with the E. coli-expressed Tva proteins, either Ecto-Tva or the wild-type or mutant Tva LDL-A module. If an E. coli-expressed Tva protein binds SUA-rIgG, it will block the association between the immunoadhesin and an epitope-typed myc-Tva protein, expressed in human 293T cells.
First, we examined how correct folding of the E. coli-expressed Tva proteins affected their ligand-binding activities. As demonstrated above, the Ecto-Tva protein requires calcium for correct folding. Thus, it was expected that only the Ecto-Tva protein refolded in the presence of calcium could efficiently block SUA-rIgG/myc-Tva association. Various amounts of the Ecto-Tva protein, refolded either in the presence or absence of calcium, were incubated with the purified SUA-rIgG protein (150 nM in each incubation) prior to the addition of the same amount of the 293T cell lysate containing the myc-Tva protein. As shown in Fig. 5A, at low concentrations (6 and 60 nM), the Ecto-Tva protein could not efficiently block SUA-rIgG/myc-Tva association even when the Ecto-Tva protein was refolded in the presence of calcium (lanes 1 and 3). As the concentrations increased (300 and 600 nM), the Ecto-Tva protein, after refolding in the presence of calcium, could completely block myc-Tva binding to the SUA-rIgG-bound beads (lanes 5 and 7). In contrast, when the Ecto-Tva protein was folded in the absence of calcium, it could not efficiently block SUA-rIgG/myc-Tva association at all the concentrations tested (lanes 9, 11, 13, and 15). Similar results were obtained when the Tva LDL-A module protein was examined using this blocking assay. The Tva LDL-A module protein, following refolding with calcium, could completely block SUA-rIgG/myc-Tva association at the highest concentration (600 nM) tested (Fig. 5B, lane 7), while it could not do so at the lower concentrations (Fig. 5B, lanes 1, 3, and 5). Furthermore, the Tva LDL-A module protein refolded without calcium could not efficiently block myc-Tva binding to SUA (lanes 9, 11, 13, and 15). SUA-rIgG/myc-Tva association is specific, as expected, since the myc-Tva protein could not be precipitated with subgroup B SU-immunoglobulin fusion protein (SUB-rIgG)-coated protein A-Sepharose beads (data not shown). The subgroup B SU protein binds to a distinct type of cellular receptor, Tvb (4). These results demonstrated that the Ecto-Tva and the Tva LDL-A module proteins, following refolding in the presence of calcium, can block SUA-rIgG/myc-Tva association in a dose-dependent manner, suggesting that the biological activity of Tva depends on correct protein folding.
FIG. 5.
Binding analysis of Ecto-Tva, Tva LDL-A, and its mutants to SUA-rIgG by a ligand-blocking assay. (A to C) Different amounts of the Ecto-Tva protein (A) or the Tva LDL-A protein (B) or 600 nM LDL-A mutant proteins (C) was used to block myc-Tva binding to SUA-rIgG in a ligand-blocking assay as described in Materials and Methods. (D) 600 nM Ecto-Tva or Tva LDL-A protein was used in the presence or absence of 5 mM EDTA in the binding assay. The Western blots were probed with a monoclonal anti-myc antibody. The Tva proteins were refolded either in the presence (+Ca/) or absence (−Ca/) of Ca2+, and Ca2+ was either added (/+Ca) or omitted (/−Ca) during the binding assay. Molecular mass markers are given in kilodaltons. S, supernatant; B, beads.
We also examined the binding properties of the four Tva mutant proteins which have been previously characterized by reverse-phase HPLC, two-dimensional NMR, and ITC (28). These LDL-A mutants represent three classes. D46A and E47A are calcium binding defective mutations, because both D46 and E47 were shown to be directly involved in calcium coordination. The L34A amino acid substitution also affected protein folding, since L34 was shown to be involved in the formation of a small hydrophobic core in Tva with residues F16 and P21. Therefore, all these mutant proteins displayed folding defects. In contrast, the W48A mutant protein was shown to be essentially correctly folded, and the mutation likely disrupts specific contacts between Tva and SU, since W48 is a viral interaction determinant (28, 29). These mutant proteins were first refolded with calcium, and the refolded samples were used in the ligand-blocking assay. The mutant proteins W48A, D46A, and E47A, when added at 600 nM final concentration, could not effectively block SUA-rIgG/myc-Tva association when calcium was either added or omitted (Fig. 5C, lanes 9, 11, 13, 15, 17, and 19), while the L34A mutant protein appeared to partially block myc-Tva binding to SUA (Fig. 5C, lanes 5 and 7). These results are consistent with the previous studies, which demonstrated that these mutant proteins are defective either in proper protein folding or function. Furthermore, they are in agreement with the kinetics reported in this study (see below). As pointed out previously, since calcium was released from the Tva proteins during the purification by reverse-phase HPLC after in vitro folding (28), the role of calcium in ligand binding could be assessed by either addition or omission of calcium in the blocking assay. It was surprising to us that both the Ecto-Tva and Tva LDL-A proteins could completely block SUA/myc-Tva association without calcium once the Tva proteins were refolded with calcium (Fig. 5C, lanes 1 and 3). These results seem to suggest that maintaining the structural integrity of the Tva LDL-A module with calcium is not crucial for ligand binding once the module is properly folded. This notion is fully supported by the binding kinetics described below.
To further examine the role of calcium in ligand binding, a calcium chelator, EDTA, was included in the ligand-blocking assay. As shown in Fig. 5D, both the Ecto-Tva and Tva LDL-A module proteins which were refolded in the presence of calcium could still efficiently block SUA-rIgG/myc-Tva binding with or without the addition of 5 mM EDTA in the binding steps (lanes 3, 5, 9 and 11). The Tva proteins refolded without calcium were used as controls (lanes 1 and 7). These results further demonstrated that, although calcium is required for the correct folding and structural integrity of the Tva protein, it is not essential for SU binding.
Examination of the binding kinetics of SU/Tva proteins by IAsys.
To characterize the binding kinetics between SUA and Tva in real time, an optical biosensor, the IAsys system, was used. This system allowed us to measure association rates (kass), dissociation rates (kdiss), and KD between SUA and Tva, including the Tva mutant proteins.
Initially, the purified SUA-rIgG protein was immobilized onto carboxylate cuvettes via the primary amino groups, and the purified Tva proteins, following thrombin cleavage and in vitro refolding with or without calcium, were added to the cuvettes. However, it was found that the immobilized SUA-rIgG protein did not display Tva-binding activity (data not shown). The most likely explanation of this failure is that immobilization of the SUA-rIgG protein onto the carboxylate cuvettes via the primary amines of SU hinders the access of the receptor-binding site on SU. Thus, all the binding kinetics reported in this study were conducted using the Tva proteins immobilized onto the carboxylate cuvettes via amine coupling.
Figure 6A shows the dose curves of SUA binding to the immobilized Ecto-Tva protein. In this experiment, the Ecto-Tva protein was first refolded in the presence of 10 mM CaCl2, and the correctly folded Ecto-Tva protein was eluted by reverse-phase HPLC and immobilized onto the carboxylate cuvette. The binding of the SUA-rIgG protein to the immobilized Ecto-Tva protein was determined by adding ramping concentrations of the purified SUA-rIgG protein ranging from 9.375 to 300 nM, and the amounts of the SUA-rIgG protein bound to the Ecto-Tva were measured as arc seconds. These raw sensorgram data were then processed using FASTfit software to obtain kass, kdiss, and KD values (Fig. 6B), which are 27.6 (±2) × 104 M−1 s−1, 4.5 (±3.2) × 10−3 s−1, and 16.4 × 10−9 M, respectively (Table 1). The dose curves of SUA binding to the immobilized Tva LDL-A module protein were determined by following the same procedure (Fig. 6C and D), which gave the following values: kass = 53.0 (±2) × 104 M−1 s−1, kdiss = 5.1 (±2.5) × 10−3 s−1, and KD = 9.6 × 10−9 M (Table 1). Thus, SUA-rIgG had similar binding affinities to the Ecto-Tva protein (KD = 16.4 nM) and the Tva LDL-A module protein (KD = 9.6 nM) under these conditions.
FIG. 6.
Binding curves and association plots of Ecto-Tva protein and Tva LDL-A protein to SU by IAsys system. The SUA-rIgG binding curves and association plots to the immobilized Ecto-Tva protein (A and B) or the Tva LDL-A protein (C and D) are shown as examples for analysis of the binding kinetics between Tva and SU proteins. Here, the Tva proteins were refolded with Ca2+, but the binding was performed without Ca2+ (see Materials and Methods for details).
TABLE 1.
Kinetic constants of SUA-rIgG binding to immobilized Tva proteins
| Immobilized ligand | Caa | kass (M−1 s−1) (104) | kdiss (s−1) (10−3) | KD (M) (10−9) |
|---|---|---|---|---|
| Tva protein | ||||
| Ecto-Tva | + | 95.2 ± 12.9 | 11.2 ± 0.8 | 11.8 |
| − | 27.6 ± 2.2 | 4.5 ± 3.2 | 16.4 | |
| +EDTAc | 26.8 ± 4.0 | 4.6 ± 6.8 | 17.2 | |
| Unfolded Ecto-Tvab | − | 5.6 ± 0.1 | 8.4 ± 0.9 | 149.3 |
| LDL-A module | + | 160.0 ± 10.4 | 19.6 ± 8.8 | 12.3 |
| − | 53.0 ± 2.0 | 5.1 ± 2.5 | 9.6 | |
| +EDTAc | 24.3 ± 2.0 | 2.4 ± 2.6 | 9.8 | |
| Unfolded LDL-A moduleb | − | 9.7 ± 2.0 | 13.7 ± 2.9 | 140.6 |
| Tva mutant protein | ||||
| L34A | + | 12.2 ± 1.2 | 9.8 ± 0.8 | 80.7 |
| − | 7.9 ± 1.4 | 7.6 ± 0.9 | 94.8 | |
| W48A | + | 7.8 ± 2.4 | 9.2 ± 1.7 | 118.6 |
| − | 7.2 ± 0.9 | 9.1 ± 0.6 | 126.3 | |
| D46A | + | 14.6 ± 3.2 | 18.3 ± 2.2 | 123.1 |
| − | 28.4 ± 6.8 | 47.2 ± 10.5 | 166.0 | |
| E47A | + | 14.3 ± 3.5 | 17.9 ± 2.5 | 125.0 |
| − | 29.8 ± 8.1 | 29.9 ± 1.9 | 100.4 |
Ca2+ is either present (+) or absent (−) during the immobilization and binding steps.
The protein was refolded in the absence of Ca2+.
5 mM EDTA was present during the binding steps.
Previously, it was demonstrated that calcium is not only required for correct in vitro folding of the Tva LDL-A module, it is also essential for maintaining the structural integrity of the protein (28). However, the ligand-blocking assay suggested that calcium is not essential in ligand binding (Fig. 5). Thus, IAsys experiments were performed to investigate whether calcium played any role in SUA binding for the Ecto-Tva protein or the Tva LDL-A module protein. When calcium was absent or when EDTA was included in the binding step, both Ecto-Tva and the Tva LDL-A module displayed ∼3- to 6-fold lower rates of association to SUA (Table 1). However, since the dissociation rates for these proteins were also decreased 2.5- to 8-fold for the Ecto-Tva protein and the Tva LDL-A module protein, respectively, the dissociation constants were very similar when calcium was either present or absent (11.8 versus 16.4 or 17.2 nM for the Ecto-Tva protein and 12.3 versus 9.6 nM for the Tva LDL-A module protein). Therefore, although calcium is crucial for correct folding of Tva, it is not required for ligand binding. These results suggest that the overall conformation of Tva may be altered upon ligand binding.
As shown above by the ligand-blocking assay, neither the Ecto-Tva protein nor the Tva LDL-A module displayed binding activity to SUA when they were folded in the absence of calcium (Fig. 5), indicating that calcium plays an important role in Tva folding and thus in Tva function. This conclusion is fully supported by the IAsys binding analysis. The Ecto-Tva protein and the Tva LDL-A module protein, refolded in the absence of calcium (referred to as unfolded in Table 1), gave KD values of 149.3 and 140.6 nM, respectively, ∼10-fold higher than those of the same proteins refolded in the presence of calcium. Furthermore, the lower binding affinities of the unfolded Tva proteins to SUA were largely due to a decrease in the association rate (5- to 17-fold for the Ecto-Tva protein and 5.5- to 16.5-fold for the Tva LDL-A module protein). In contrast, the dissociation rates were not greatly affected (Table 1).
The binding affinities of the four Tva LDL-A mutants, D46A, E47A, L34A, and W48A, to SUA were also determined by IAsys. As expected, all of the mutants displayed decreased affinities to the SUA protein, with KD values ∼7- to 16-fold less than that of the wild-type Tva LDL-A module protein (Table 1). The L34A mutant protein appeared to have a slightly higher binding affinity to SUA (KD = 80 nM with Ca2+; KD = 94.8 nM without Ca2+) than the other three Tva mutant proteins (W48A, D46A, and E47A), consistent with the results of the ligand-blocking assay (Fig. 5). Further, the D46A and E47A mutant proteins appeared to have slightly higher association rates and dissociation rates than the L34A and W48A mutant proteins (Table 1).
DISCUSSION
In this study, we showed that correct in vitro folding of the Ecto-Tva protein is calcium dependent and that this protein has the same calcium binding affinity as the Tva LDL-A module. We measured the kass, kdiss, and KD between SU and the E. coli-expressed Ecto-Tva protein, the Tva LDL-A module, and its mutant proteins, using the IAsys system, an optical biosensor. We found that the Ecto-Tva and the Tva LDL-A module proteins displayed similar binding affinities to SU, providing quantitative and direct evidence for the first time that the LDL-A module of Tva is indeed necessary and sufficient as the viral interaction domain. Furthermore, the unfolded Ecto-Tva protein and the Tva LDL-A module and mutants thereof displayed lower binding affinities to the SU protein, largely due to a decrease in their association rates. In contrast, the dissociation rates were less affected. These results may provide clues in elucidating the entry mechanism of this enveloped virus. Surprisingly, we found that calcium is not essential for ligand binding. In addition, since we can routinely obtain ∼30 mg of the purified and biologically active Ecto-Tva protein from 1 liter of bacterial culture, this system provides an alternative, and more efficient, method to the baculovirus expression system reported by others (1) for the preparation of large amounts of the Tva protein required for the biochemical and structural analysis of Tva/EnvA interaction.
We observed that the majority of the well-dispersed cross-peaks in the HSQC spectral profile of the Ecto-Tva protein corresponded to that of the Tva LDL-A module (Fig. 4). This may reflect the influence of the N-terminal and C-terminal sequences flanking the LDL-A module of Tva on the overall structure of the Ecto-Tva protein. One striking feature of this protein is that glycine and proline residues (12 and 13, respectively [Fig. 1B]) account for ∼30% of the extracellular region of Tva (total, 83 residues). Thus, we speculate that the Ecto-Tva protein, except for the LDL-A module, may not adopt a well-defined structure with such a high percentage of glycine and proline residues, consistent with the structural analysis of the Ecto-Tva protein by protein secondary-structure prediction (23), which suggests that neither the N-terminal nor the C-terminal region can fold as an α-helix or β-sheet (data not shown).
Binding kinetics between viral glycoproteins and their cognate receptors have been reported in other virus-host interactions, using an optical-biosensor technique. For example, it was shown that the affinities of several CD4 mutant proteins to gp120 of human immunodeficiency virus were reduced, mostly by changes in association rates, and it was suggested that this reduction in association rates is likely due to the involvement of the conformational changes in the CD4-gp120 interaction (33). Furthermore, it was recently shown that soluble CD4 could enhance the binding affinity of gp120 to 17b, a neutralizing human antibody, by ∼28-fold, largely due to a 22-fold increase in the association rate, consistent with the notion that CD4 binding induces the conformational changes in gp120 leading to higher-affinity binding to a coreceptor (35). Similarly, it was reported that herpes simplex virus glycoprotein D and its variants bound to the herpesvirus entry mediator (HveA), a cellular receptor for herpes simplex virus, with different affinities, largely due to an increase or decrease in the association rates (31). Since one common characteristic of these receptor-virus glycoprotein interactions is that conformational changes on the viral glycoproteins are triggered by binding of their cognate receptors, it is reasonable to assume that a high association rate between a viral glycoprotein and its cognate receptor may be crucial for the receptor-triggered conformational changes on the viral glycoprotein for the formation of a stable complex between the glycoprotein and its receptor. However, kinetic analysis of other virus-receptor interactions is needed to determine if this is a common mechanism for enveloped viruses.
The binding affinity between Tva and EnvA has been analyzed by several groups, and the KD was estimated to be ∼0.3 to 5 nM, using the enzyme-linked immunosorbent assay (1, 19), flow cytometry (36), or surface plasmon resonance (27) method. In this study, we demonstrated that both the Ecto-Tva protein and the Tva LDL-A module had a KD of ∼10 nM to the SU protein, which is generally consistent with other reports. However, the binding kinetics of Tva/SU reported in this study is not in agreement with another report in one important aspect: we showed that calcium was not required for ligand binding, while another group reported that calcium could greatly (27-fold) increase the binding affinity of SU to Tva (27). Although the reason for this discrepancy is not clear, we point out that in the present study we have examined the role of calcium in ligand (SU) binding for the Ecto-Tva protein, the LDL-A module of Tva, and its four mutant proteins and found that without exception calcium did not affect the KD values of these proteins to SU, using a ligand-blocking assay (Fig. 5) and IAsys (Table 1). Thus, we conclude that calcium is not required in ligand binding. We did observe that both the Ecto-Tva protein and the LDL-A module of Tva had higher rates of association to SU when calcium was present than when it was absent. This was expected, since the Tva proteins with the correct conformation (with calcium) would recognize and bind SU with higher association rates than the proteins without the correct conformation (without calcium). However, the rates of dissociation of these proteins (in the presence of calcium) from SU were also larger than those of these proteins without calcium, thus giving similar KD values either with or without calcium (Table 1).
Previously, it was found that the N-terminal region of the Tva LDL-A module (cysteine 1 to 3) is more flexible than the rest of the module, and it was proposed that the flexible N-terminal region of Tva may adopt a different conformation upon EnvA binding to promote high-affinity binding (induced fit [29]). The results reported in this study may suggest that the C-terminal calcium-coordinating region of the LDL-A module may adopt a conformation different from the calcium-binding conformation of the module when ligand binding occurs. Thus, it is possible that Tva/EnvA binding can induce conformational changes on Tva, and these conformational changes may be essential for high-affinity binding between Tva and EnvA and for efficient RSV-A entry. It has been well established that Tva binding can trigger conformational changes on the both SU and TM subunits of EnvA essential for RSV-A entry (7, 12, 14, 18). However, direct evidence will be needed to demonstrate that Tva conformation is altered upon EnvA binding.
Our results showing that calcium is not required for ligand binding may have broad implications in elucidating the mechanism of LDLR-ligand interactions. It has been well established that calcium is required for LDLR-ligand binding (6). Sequence alignment indicates that each LDL-A module contains five highly conserved acidic residues, and mutations of these conserved acidic residues reduced or abolished ligand binding, causing familial hypercholesterolemia, illustrating the critical role of these residues in ligand interaction (15). It has been proposed that apolipoprotein binding to the LDLR involves ionic interactions between the basic residues of the apolipoproteins and the acidic residues of LDLR (5, 17, 26, 32). Previously, it was shown that mutations of the basic residues in one of the receptor-binding regions (hr2) of SU impaired receptor binding, and it was proposed that these basic residues of SU are directly involved in receptor binding via ionic interactions with the acidic residues of the Tva LDL-A module (19). However, the X-ray crystal structure of human LDL-A5 showed that the side chains of the four highly conserved acidic residues in the module are involved in calcium coordination (10). Thus, it seems that these residues are not available for ligand binding via ionic interactions. Several possible mechanisms have been proposed by Brown et al. to explain this paradox (6). Our findings that calcium is required for the correct folding and structural integrity of the Tva LDL-A module but that it is not necessary for ligand binding may be used as a model to explain this seeming paradox. We speculate that the conserved acidic residues, at least some of them, may play a dual role, involved in both calcium coordination and ligand interaction. Thus, one role of these residues is to facilitate the calcium-dependent folding of an LDL-A module by permitting the formation of the correct disulfide bonds. However, upon ligand binding, either EnvA (in this study) or apoB- or apoE-containing LDL particles, the conformation of the LDL-A module of Tva, or the LDL-A modules of the LDLR, is altered in such a way that the side chains of these conserved acidic residues become exposed and thus able to interact with the basic residues on the ligand, either EnvA or LDL particles, via ionic interactions. This hypothesis, although highly speculative at present, may be directly tested using the Tva/EnvA interaction as a model.
Acknowledgments
We thank Katharina Spiegel for her help and for useful discussions on the analysis of kinetics using IAsys and for use of the IAsys system at the Keck Biophysics Facility at Northwestern University. We thank Fatima Shaikh for technical assistance.
This work was supported by National Institutes of Health grants CA092459 and AI48056 (L.R.), GM54414 (P.G.W.G.), and CA70810 (J.A.T.Y). The Bruker DRX600 was purchased with a grant from the National Science Foundation (DIR9601705). L.R. was the recipient of a Schweppe Foundation Career Development Award. Q.-Y.W. was the recipient of an American Heart Association Midwest Affiliate Predoctoral fellowship.
REFERENCES
- 1.Balliet, J. W., J. Berson, C. M. D'Cruz, J. Huang, J. Crane, J. M. Gilbert, and P. Bates. 1999. Production and characterization of a soluble, active form of Tva, the subgroup A avian sarcoma and leukosis virus receptor. J. Virol. 73:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates, P., J. A. T. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. [DOI] [PubMed] [Google Scholar]
- 3.Bates, P., L. Rong, H. E. Varmus, J. A. T. Young, and L. B. Crittenden. 1998. Genetic mapping of the cloned subgroup A avian sarcoma and leukosis virus receptor gene to the TVA locus. J. Virol. 72:2505-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brojatsch, J., J. Naughton, M. M. Rolls, K. Zingler, and J. A. Young. 1996. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 87:845-855. [DOI] [PubMed] [Google Scholar]
- 5.Brown, M. S., and J. L. Goldstein. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science 232:34-47. [DOI] [PubMed] [Google Scholar]
- 6.Brown, M. S., J. Herz, and J. L. Goldstein. 1997. Calcium cages, acid baths and recycling receptors. Nature 388:629-630. [DOI] [PubMed] [Google Scholar]
- 7.Damico, R. L., J. Crane, and P. Bates. 1998. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc. Natl. Acad. Sci. USA 95:2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Einfeld, D., and E. Hunter. 1988. Oligomeric structure of a prototype retrovirus glycoprotein. Proc. Natl. Acad. Sci. USA 85:8688-8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esser, V., L. E. Limbird, M. S. Brown, J. L. Goldstein, and D. W. Russell. 1988. Mutational analysis of the ligand binding domain of the low density lipoprotein receptor. J. Biol. Chem. 263:13282-13290. [PubMed] [Google Scholar]
- 10.Fass, D., S. Blacklow, P. S. Kim, and J. M. Berger. 1997. Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature 388:691-693. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert, J. M., L. D. Hernandez, T. Chernoy-Rogan, and J. White. 1993. Generation of a water-soluble oligomeric ectodomain of the Rous sarcoma virus envelope glycoprotein. J. Virol. 67:6889-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert, J. M., L. D. Hernandez, J. W. Balliet, P. Bates, and J. M. White. 1995. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J. Virol. 69:7410-7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert, J. M., P. Bates, H. E. Varmus, and J. M. White. 1994. The receptor for the subgroup A avian leukosis-sarcoma viruses binds to subgroup A but not to subgroup C envelope protein. J. Virol. 68:5623-5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez, L. D., R. J. Peters, S. E. Delos, J. A. T. Young, D. A. Agard, and J. M. White. 1997. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J. Cell Biol. 139:1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobbs. H. H., M. S. Brown, and J. L. Goldstein. 1992. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum. Mutat. 1:445-466. [DOI] [PubMed] [Google Scholar]
- 16.Hunter, E., and R. Swanstrom. 1990. Retrovirus envelope glycoproteins. Curr. Top. Microbiol. Immunol. 157:187-253. [DOI] [PubMed] [Google Scholar]
- 17.Mahley, R. W. 1988. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240:622-630. [DOI] [PubMed] [Google Scholar]
- 18.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. T. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 19.Rong, L., A. Edinger, and P. Bates. 1997. Role of basic residues in the subgroup-determining region of the subgroup A avian sarcoma and leukosis virus envelope in receptor binding and infection. J. Virol. 71:3458-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rong, L., and P. Bates. 1995. Analysis of the subgroup A avian sarcoma and leukosis virus receptor: the 40-residue, cysteine-rich, low-density lipoprotein receptor repeat motif of Tva is sufficient to mediate viral entry. J. Virol. 69:4847-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rong, L., K. Gendron, and P. Bates. 1998. Conversion of a human low-density lipoprotein receptor ligand-binding repeat to a virus receptor: identification of residues important for ligand specificity. Proc. Natl. Acad. Sci. USA 95:8467-8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rong, L., K. Gendron, B. Strohl, R. Shenoy, R. J. Wool-Lewis, and P. Bates. 1998. Characterization of determinants for envelope binding and infection in Tva, the subgroup A avian sarcoma and leukosis virus receptor. J. Virol. 72:4552-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rost, B. 1996. PHD: predicting one-dimensional protein structure by profile-based neural networks. Methods Enzymol. 266:525-539. [DOI] [PubMed] [Google Scholar]
- 24.Russell, D. W., M. S. Brown, and J. L. Goldstein. 1989. Different combinations of cysteine-rich repeats mediate binding of low density lipoprotein receptor to two different proteins. J. Biol. Chem. 264:21682-21688. [PubMed] [Google Scholar]
- 25.Simonovic, M., K. Dolmer, W. Huang, D. K. Strickland, K. Volz, and P. G. Gettins. 2001. Calcium coordination and pH dependence of the calcium affinity of ligand-binding repeat CR7 from the LRP. Comparison with related domains from the LRP and the LDL receptor. Biochemistry 40:15127-15134. [DOI] [PubMed] [Google Scholar]
- 26.Sudhof, T. C., J. L. Goldstein, M. S. Brown, and D. W. Russell. 1985. The LDL receptor gene: a mosaic of exons shared with different proteins. Science 228:815-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonelli, M., R. J. Peters, T. L. James, and D. A. Agard. 2001. The solution structure of the viral binding domain of Tva, the cellular receptor for subgroup A avian leukosis and sarcoma virus. FEBS Lett. 509:161-168. [DOI] [PubMed] [Google Scholar]
- 28.Wang, Q. Y., K. Dolmer, W. Huang, P. G. Gettins, and L. Rong. 2001. Role of calcium in protein folding and function of Tva, the receptor of subgroup A avian sarcoma and leukosis virus. J. Virol. 75:2051-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, Q. Y., W. Huang, K. Dolmer, P. G. Gettins, and L. Rong. 2002. Solution structure of the viral receptor domain of Tva and its implications in viral entry. J. Virol. 76:2848-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, Q.-Y., B. Manicassamy, X. Yu, K. Dolmer, P. G. W. Gettins, and L. Rong. 2002. Characterization of the LDL-A module mutants of Tva, the subgroup A Rous sarcoma virus receptor, and the implications in protein folding. Protein Sci. 11:2596-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willis, S. H., A. H. Rux, C. Peng, J. C. Whitbeck, A. V. Nicola, H. Lou, W. Hou, L. Salvador, R. J. Eisenberg, and G. H. Cohen. 1998. Examination of the kinetics of herpes simplex virus glycoprotein D binding to the herpesvirus entry mediator, using surface plasmon resonance. J. Virol. 72:5937-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson, C., M. R. Wardell, K. H. Weisgraber, R. W. Mahley, and D. A. Agard. 1991. Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science 252:1817-1822. [DOI] [PubMed] [Google Scholar]
- 33.Wu, H., D. G. Myszka, S. W. Tendian, C. G. Brouillette, R. W. Sweet, I. M. Chaiken, and W. A. Hendrickson. 1996. Kinetic and structural analysis of mutant CD4 receptors that are defective in HIV gp120 binding. Proc. Natl. Acad. Sci. USA 93:15030-15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young, J. A. T., P. Bates, and H. E. Varmus. 1993. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J. Virol. 67:1811-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, W., G. Canziani, C. Plugariu, R. Wyatt, J. Sodroski, R. Sweet, P. Kwong, W. Hendrickson, and I. Chaiken. 1999. Conformational changes of gp120 in epitopes near the CCR5 binding site are induced by CD4 and a CD4 miniprotein mimetic. Biochemistry 38:9405-9416. [DOI] [PubMed] [Google Scholar]
- 36.Zingler, K., and J. A. T. Young. 1996. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J. Virol. 70:7510-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]