Abstract
The effect of zidovudine on plasma and genital human immunodeficiency virus type 1 (HIV-1) was determined in 42 antiretroviral-naive HIV-1-seropositive women in Nairobi. After 7 days of zidovudine treatment, HIV-1 RNA levels decreased by 0.5 to 1.1 log10 in plasma and genital secretions. HIV-1 RNA half-life following zidovudine treatment was 4.7, 1.3, and 0.9 days in plasma, cervix, and vagina, respectively, and significantly shorter in genital secretions than in plasma (P < 0.001). Defining the short-term effect of zidovudine on plasma and genital HIV-1 is important for improving perinatal HIV-1 interventions.
An estimated 800,000 infants are infected with human immunodeficiency virus type 1 (HIV-1) annually, the majority in Africa (19). To counter this, short-course regimens of antiretrovirals, which are effective in reducing HIV-1 transmission to infants, are rapidly being implemented (8, 22). Concurrent with implementation, it is important to define the mechanism of action of these regimens in order to improve them. Thus, it is important to evaluate the effect of these treatments on virus levels in secretions that are a source of the transmitted virus.
Several lines of evidence indicate that exposure of infants to genital HIV-1 during delivery is associated with increased risk of infant HIV-1 infection. Previous studies have reported observations of increased mother-to-child HIV-1 transmission with prolonged duration of ruptured membranes and vaginal delivery and in first- versus second-born twins (7, 9, 12). In two perinatal HIV-1 cohorts, maternal genital HIV-1 shedding was associated with a ∼2-fold increase in the risk of infant HIV-1, independent of maternal plasma HIV-1 RNA levels (3, 10). Collectively, these studies suggest that exposure to genital HIV-1 during passage through the birth canal is an important determinant of infant HIV-1 infection.
Little is known regarding the dynamics of viral turnover in the genital tract or the timing of drug effect on genital virus. Zidovudine was the first drug shown to decrease mother-to-child transmission of HIV-1, is effective in short-course perinatal HIV-1 regimens, and is a recommended component of multidrug regimens for prevention of infant HIV-1 (4, 18, 22). Thus, we conducted a prospective study among nonpregnant antiretroviral-naive women in Nairobi to determine the effect of zidovudine on genital and plasma HIV-1 RNA during the first few days of administration. Serial evaluation of RNA levels allowed us to estimate and compare viral half-lives in these compartments following zidovudine treatment.
Nonpregnant, nonlactating, HIV-1-infected women with hemoglobin levels of >8g/dl and without laboratory evidence of genital infections (trichomonas, bacterial vaginosis, gonorrhea, or chlamydia) were enrolled after providing written informed consent. All participating women were antiretroviral naive. Zidovudine was dispensed in a previously labeled pack (300 mg twice daily for 7 days). Collection of blood and genital specimens was conducted on days 0, 1, 2, and 7 of zidovudine treatment and at 7 days after cessation of zidovudine treatment. Day 0 was 7 to 10 days after the last menses, in order to complete testing to exclude genital infections.
Between May and June 1999, 42 women were enrolled in the study and completed specimen collection. The mean age was 28 years (21 to 43 years). The majority (79%) of women had no HIV-1 signs or symptoms. The median CD4 cell count was 449/mm3 (range, 59 to 1,170/mm3). None of the women had previously received antiretrovirals. At baseline, none had abnormal cervical or vaginal discharge, ulcers, or warts, and eight (19%) showed scant blood upon cervical swabbing. Sixty-nine percent of women reported current use of hormonal contraception. Compliance with the drug regimen was 96%, as determined by either pill count or self-report. Thirty-one women (74%) reported being sexually abstinent over the study period. None reported intercourse on the day before genital specimen collection. Of 168 sampling visits, six (4%) were with women who reported douching on the day before specimen collection.
Genital specimens were collected by using Dacron swabs, and cervical specimens were collected before vaginal ones. Cervical specimens were obtained by inserting a swab into the cervical os and rotating once; vaginal specimens were obtained by rotating a swab against the lateral vaginal wall in an area without exudate or ulceration. Swabs were inserted into freezing medium in sterile cryovials, placed on ice, and transported to the laboratory within 3 h of collection. Plasma and genital HIV-1 RNA levels were quantified in multiple runs with an HIV-1 transcription-mediated assay (Gen-Probe Inc., San Diego, Calif.) by using methods previously described (6, 13, 15, 20). The lowest level of detection for this assay was 18 copies/swab for genital specimens and 7 copies/ml for plasma specimens (15). Values below the detection level of the HIV-1 RNA assay were set at the midpoint between zero and the lowest detection level.
At baseline, HIV-1 RNA was detected in the plasma of all women and in cervical and vaginal secretions of 33 (79%) and 26 (62%) women, respectively. HIV-1 RNA levels in the cervix and vagina were both positively correlated with plasma viral load at baseline (for cervix, R = 0.66, P < 0.001; for vagina, R = 0.56, P < 0.001 [Spearman's correlation coefficient]). Use of hormonal contraception, cervical bleeding, or cervical friability was not associated with increased detection of cervical HIV-1 or vaginal HIV-1 or with higher levels of cervical or vaginal HIV-1 RNA (chi-square and paired t tests).
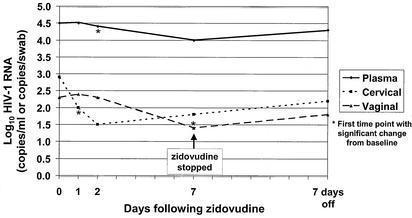
Median log10 plasma, cervical, and vaginal HIV-1 RNA levels at baseline were compared to median levels for different days of treatment by using the Wilcoxon sign rank test. Plasma HIV-1 RNA decreased in 86% of the women after 7 days of zidovudine treatment. Median plasma viral RNA levels decreased from 4.5 log10 copies/ml to 4.0 log10 copies/ml (P < 0.001) (Table 1 and Fig. 1). Following 7 days of zidovudine treatment, cervical HIV-1 RNA levels decreased from baseline in 31 (94%) of 33 women with detectable cervical HIV-1 RNA at baseline and became undetectable in six women (18%). Overall, median HIV-1 RNA in cervical secretions declined from 2.9 to 1.8 log10 copies/swab (P < 0.001) (Table 1). In the subset of 33 women with cervical HIV-1 RNA at baseline, median levels decreased from 3.2 to 2.3 log10 copies/swab (P < 0.001). Vaginal HIV-1 RNA levels decreased in 24 (92%) of 26 women with detectable vaginal HIV-1 RNA at baseline and became undetectable in nine women (35%). Among all women, median vaginal HIV-1 RNA levels decreased from 2.3 to 1.3 log10 copies/swab after 7 days of zidovudine treatment (P = 0.003). In the subset of 26 women with vaginal HIV-1 RNA at baseline, median levels decreased from 3.3 to 1.8 log10 copies/swab (P < 0.001).
TABLE 1.
Median log10 HIV-1 RNA levels in plasma and in cervical and vaginal secretions
| Time point | Median log10 HIV-1 RNA level (IQR) in:
|
||
|---|---|---|---|
| Plasma | Cervical secretions | Vaginal secretions | |
| Day 0 | 4.5 (3.7-5.3) | 2.9 (1.5-3.8) | 2.3 (1.0-3.4) |
| Day 1 | 4.5 (3.7-5.3) | 2.0a (1.0-3.1) | 2.4 (1.0-4.0) |
| Day 2 | 4.3a (3.7-4.8) | 1.5a (1.0-3.0) | 2.3 (1.5-3.9) |
| Day 7 | 4.0a (3.0-4.8) | 1.8a (1.0-2.9) | 1.4a (1.0-2.5) |
| Postcessation (7 days) | 4.3 (3.3-4.7) | 2.2 (1.0-3.4) | 1.8 (0.8-3.3) |
P ≤ 0.005 versus day 0 level.
FIG. 1.
Log10 HIV-1 RNA levels in plasma and in cervical and vaginal secretions at days 0, 1, 2, and 7 of zidovudine treatment.
In the cohort overall, in linear mixed effects models with log10 HIV-1 RNA load as the outcome, the average change in viral loads over 7 days was −0.078 log10 copies/ml per day in plasma (P < 0.001), −0.066 log10 copies/swab per day in cervical secretions (P = 0.007), and −0.091 log10 copies/swab per day in vaginal secretions (P = 0.001). Following 7 days of zidovudine treatment, the median percent change from baseline log10 HIV-1 RNA levels was 14% in plasma, 30% in cervical secretions, and 38% in vaginal secretions among women with detectable virus at baseline. Among women with detectable virus in all three compartments at baseline, median percent change in log10 HIV-1 RNA levels was higher in genital secretions than in plasma (for cervical secretions versus plasma, P = 0.03; for vaginal secretions versus plasma, P = 0.004 [paired t test]) (Table 2).
TABLE 2.
Changes in viral load after zidovudine treatment
| Sample type | Baseline HIV-1 RNA level | Percent decrease after 7 days of zidovudine treatment | HIV-1 RNA half-life (days) following zidovudine treatment (IQR) | Correlate of reduction of baseline viral loadc |
|---|---|---|---|---|
| Plasma | 4.5 log10 copies/ml | 14 | 4.74 (1.45-9.38) | Baseline plasma viral load [−0.54 (−0.76 to −0.32)]d |
| Cervical secretions | 2.9 log10 copies/swab | 30a | 1.34b (0.85-2.30) | Baseline cervical viral load [−0.48 (−0.68 to −0.28)]e |
| Vaginal secretions | 2.3 log10 copies/swab | 38a | 0.93b (0.00-1.63) | Baseline vaginal viral load [−0.33 (−0.60 to −0.06)]f |
For cervical secretions versus plasma, P = 0.03; for vaginal secretions versus plasma, P = 0.004; for cervical versus vaginal secretions, P = 0.2 (among women with detectable virus in all compartments).
Significantly lower than half-life in plasma (P < 0.001).
For cervical and vaginal viral loads, values are the results of multivariate analysis with adjustment for baseline plasma viral load.
P < 0.001.
P < 0.001.
P = 0.02.
To determine viral half-life, linear regression models (with no intercept terms) were used to model each woman's individual viral load data as follows: ln(RNAti/RNA0i) = −αit, where t is time, RNAti is the viral load in the compartment of interest for woman i at time t, RNA0i is the viral load in the compartment for woman i at day 0, and αi is the parameter estimated by linear regression of the data from the compartment of woman i. With the linear regression parameter estimate for a particular compartment of woman i designated as Âi, the half-life of HIV-1 RNA for woman i in a particular compartment was estimated as ln(0.5)/−Âi and median values were determined for each compartment. Following zidovudine treatment, the median half-life of HIV-1 RNA was 4.74 days (interquartile range [IQR], 1.45 to 9.38 days) in plasma, 1.34 days (IQR, 0.85 to 1.63 days) in cervical secretions, and 0.93 days (IQR, 0.0 to 1.63 days) in vaginal secretions. Median HIV-1 RNA half-life was significantly lower for genital virus than for plasma virus (for cervical secretions versus plasma and for vaginal secretions versus plasma, P = 0.001 [Wilcoxon sign rank test]) but did not differ significantly between cervical and vaginal secretions (P = 0.6).
Correlates of reduction in genital viral load were assessed by using multivariate linear regression. For each compartment, the strongest predictor of viral load reduction was baseline viral load in that compartment. For every log increase in plasma viral load at baseline there was an additional 54% reduction in viral load after 7 days of zidovudine treatment (95% confidence interval, 16 to 72%; P < 0.003). In multivariate analysis, cervical and vaginal HIV-1 RNA levels at baseline predicted response to treatment independent of baseline plasma HIV-1 RNA (Table 2).
Seven days after cessation of zidovudine treatment, median plasma viral load increased to 4.3 log10 copies/ml, which was not significantly different from the median baseline level of 4.5 log10 copies/ml (P = 0.2). Similarly, there was an increase in median genital viral load 7 days after cessation of zidovudine treatment compared to levels on the seventh day of treatment, but levels did not exceed median baseline levels prior to therapy (Table 1).
We observed significant decreases of 0.5, 1.1, and 1.0 log in levels of HIV-1 RNA in plasma and in cervical and vaginal secretions, respectively, within a week of initiating zidovudine treatment. In addition, we observed significant decreases in cervical and plasma HIV-1 RNA levels within 1 and 2 days, respectively, of treatment. Thus, the response to zidovudine was rapid both in the blood and in the genital tract. These data complement and extend the results of a study conducted in Thailand, in which median HIV-1 RNA levels in cervicovaginal lavage samples decreased significantly within a week of the start of zidovudine treatment (3). In contrast to the Thai study, we sampled cervical and vaginal secretions separately. We observed an earlier significant decrease in cervical HIV-1 (2 days) than in vaginal HIV-1 (7 days). Thus, the viral decay curve following zidovudine treatment appears to have a more acute rapid initial decrease for cervical HIV-1 than for vaginal HIV-1. This difference in decay rates may reflect differences in the sources of virus, in drug penetration levels, or in distinct target cells in the cervix and vagina. While clearance of plasma virus was not seen following 7 days of zidovudine treatment, 18 and 35% of women had clearance of cervical and vaginal virus, respectively, perhaps suggesting that short-course zidovudine treatment decreases transmission to infants in part due to its effect on genital HIV-1.
Reduction in viral load in response to monotherapy in an antiretroviral-naive patient is rapid because of the high turnover of HIV-1-infected cells (every 1 to 2 days) and circulating free virus (every ∼6 h) (2, 17). With prolonged monotherapy, drug-resistant viral species multiply, leading to increases in plasma viral load over the course of weeks, eventually to pretreatment levels. This lack of sustained effect makes antiretroviral monotherapy suboptimal for treatment but adequate for prevention of transmission during a time-limited period, as is evident with effective short-course regimens. While short-course zidovudine treatment (∼4 weeks in late pregnancy) is effective in preventing transmission to infants, zidovudine administered only intrapartum does not protect infants from transmission (8). The significant reduction in plasma and cervical viral loads seen in our study within 2 days of the initiation of treatment suggests that, although intrapartum-alone zidovudine regimens do not have an effect, there may be some benefit from the initiation of zidovudine treatment at least 2 days before labor.
We did not observe rebound viremia following drug cessation. Our study extends findings from that of Ekpini et al., in which 2 weeks of zidovudine treatment was not associated with rebound at 2, 4, and 12 weeks following cessation (5). We observed a level of decrease in plasma HIV-1 RNA (0.48 log10) similar to that observed by Ekpini et al., without evidence of rebound in plasma or genital secretions at 1 week following drug cessation. However, it is possible that in both studies the sampling frequency was not adequate to detect rebound, particularly if it occurred within days of drug cessation.
We observed that viral half-lives in cervical and vaginal secretions were significantly shorter than that in plasma (1.3, 0.9, and 4.7 days, respectively). Clearance of virus from genital secretions was more rapid than that from plasma, perhaps in part because baseline levels were lower than those in plasma. In addition, the more rapid clearance of virus from the genital tract could be due to increased sensitivity to drug effect in genital secretions or to relative accumulation of drug in secretions combined with a lag in trafficking of virus from plasma to genital secretions. To our knowledge, this is the first study estimating viral half-life in the female genital tract.
In our study, the half-life of HIV-1 RNA in plasma following zidovudine treatment was estimated to be 4.7 days, higher than other studies' estimates of plasma HIV-1 RNA half-life, which range from a few hours to 3 days (1, 11, 14, 21). Factoring time for drug distribution into our half-life estimates would shorten the estimates by 2 to 8 h (17). A more important reason for our longer estimate of HIV-1 RNA half-life in plasma is uneven responses to zidovudine. Most studies estimating viral half-life have evaluated fewer than 10 individuals. In one study of 11 antiretroviral-naive HIV-1-infected men, plasma RNA levels fell within 1 to 2 days of the initiation of zidovudine treatment, reaching nadir by 7 days, and HIV-1 half-life was estimated to be 2.7 days (11, 14). However, if data for one patient with an HIV-1 half-life of 11 days were excluded from the calculations, median half-life was 1.9 days. The latter half-life estimate was similar to viral half-life following treatment with nevirapine or a protease inhibitor in other studies (16, 21). Thus, an antiretroviral-naive individual receiving zidovudine may initially respond as rapidly as someone receiving more potent suppressive antiretrovirals. However, if there is limited drug response in some individuals in a population, median half-life within the cohort increases. In addition, it is not known whether viral subtype affects drug response; previous estimates of plasma viral half-life have been limited to subtype B infections. Viral half-life estimates for cohorts with varied individual responses to therapy are necessary for recommendations at a population level regarding the duration of therapy, particularly to optimize short-term clearance of virus. Our study involved 42 women with five evaluations of HIV-1 RNA at identical time points following zidovudine treatment, which enabled comprehensive assessment of plasma and genital viral half-lives in response to zidovudine.
In order to frequently evaluate genital HIV-1, we conducted this study with a nonpregnant cohort. Our results may therefore not apply completely to pregnant women. However, although zidovudine pharmacokinetics may be modified by pregnancy, it is likely that the drug effects on plasma and genital HIV-1 that we observed would be similar in pregnant and nonpregnant women. Hormonal contraception or timing of assessment within the menstrual cycle may have influenced viral levels in the genital tract and perhaps response to treatment. However, given the high use of hormonal contraceptives in this cohort, normal changes in viral levels that may occur during the menstrual cycle are less likely to confound this data.
Currently, Africa is the main region in which short-course antiretroviral drugs are being widely implemented for prevention of infant HIV-1. Thus, our findings are specifically relevant to understanding the mechanism of antiretroviral effect in this setting. With increased use of antiretrovirals, there will be a need to modify approaches for prevention of mother-to-child transmission of HIV-1. Further studies that compare effects of short-course antiretroviral regimens on genital and breast milk shedding of HIV-1 will be useful to inform the design of new regimens.
Acknowledgments
This work was supported by a grant from the Pediatric AIDS Foundation (grant #50809 to G.J.-S.). D. Mbori-Ngacha, R. Nduati, and G. John-Stewart were scholars in the International AIDS Research and Training Program, supported by the Fogarty International Center, National Institutes of Health (D43-TW00007 and T22-TW00001). J.O. is a recipient of the Elizabeth Glaser Scientist Award from the Pediatric AIDS Foundation.
REFERENCES
- 1.Blankson, J. N., D. Persaud, and R. Siciliano. 2002. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 53:557-593. [DOI] [PubMed] [Google Scholar]
- 2.Cavert, W., D. W. Notermans, K. Staskus, S. W. Wietgrefe, M. Zupancic, K. Gebhard, K. Henry, Z. Q. Zhang, R. Mills, H. McDade, C. M. Schuwirth, J. Goudsmit, S. A. Danner, and A. T. Haase. 1997. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science 276:960-964. [DOI] [PubMed] [Google Scholar]
- 3.Chuachoowong, R., N. Shaffer, W. Siriwasin, P. Chaisilwattana, N. L. Young, P. A. Mock, S. Chearskul, N. Waranawat, T. Chaowanachan, J. Karon, R. J. Simonds, T. D. Mastro, et al. 2000. Short-course antenatal zidovudine reduces both cervicovaginal human immunodeficiency virus type 1 RNA levels and risk of perinatal transmission. J. Infect. Dis. 181:99-106. [DOI] [PubMed] [Google Scholar]
- 4.Connor, E. M., R. S. Sperling, R. Gelber, P. Kiselev, G. Scott, M. J. O'Sullivan, R. VanDyke, M. Bey, W. Shearer, R. L. Jacobson, et al. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type-1 with zidovudine treatment. N. Engl. J. Med. 331:1173-1180. [DOI] [PubMed] [Google Scholar]
- 5.Ekpini, R. A., J. Nkengasong, T. Sibailly, C. Maurice, C. Adje, B. Monga, T. Roels, A. Greenberg, and S. Wiktor. 2002. Changes in plasma HIV-1 RNA viral load and CD4 counts, and lack of zidovudine resistance among pregnant women receiving short-course zidovudine. AIDS 16:625-630. [DOI] [PubMed] [Google Scholar]
- 6.Emery, S., S. Bodrug, B. A. Richardson, C. Giachetti, M. A. Bott, D. D. Panteleeff, L. L. Jagodzinski, N. L. Michael, R. Nduati, J. Bwayo, J. K. Kreiss, and J. Overbaugh. 2000. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J. Clin. Microbiol. 38:2688-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goedert, J. J., A. M. Duliege, C. I. Amos, S. Felton, and R. J. Biggar. 1991. High risk of HIV-1 infection for first-born twins. The international registry of HIV-exposed twins. Lancet 338:1471-1475. [DOI] [PubMed] [Google Scholar]
- 8.Guay, L. A., P. Musoke, T. Fleming, D. Bagenda, M. Allen, C. Nakabiito, J. Sherman, P. Bakaki, C. Ducar, M. Deseyve, L. Emel, M. Mirochnik, M. G. Fowler, L. Mofenson, P. Miotti, K. Dransfield, D. Bray, F. Mmiro, and J. B. Jackson. 1999. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 354:795-802. [DOI] [PubMed] [Google Scholar]
- 9.International Perinatal HIV Group. 1999. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N. Engl. J. Med. 340:977-987. [DOI] [PubMed] [Google Scholar]
- 10.John, G. C., R. W. Nduati, D. A. Mbori-Ngacha, B. A. Richardson, D. Panteleeff, A. Mwatha, J. Overbaugh, J. Bwayo, J. O. Ndinya-Achola, and J. K. Kreiss. 2001. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J. Infect. Dis. 183:206-212. [DOI] [PubMed] [Google Scholar]
- 11.Loveday, C., S. Kaye, M. Tenant-Flowers, M. Semple, U. Ayliffe, I. V. Weller, and R. S. Tedder. 1995. HIV-1 RNA serum-load and resistant viral genotypes during early zidovudine therapy. Lancet 345:820-824. [DOI] [PubMed] [Google Scholar]
- 12.Minkoff, H., D. N. Burn, S. Landesman, J. Youchah, J. J. Goedert, R. P. Nugent, L. R. Muenz, and A. D. Willoughby. 1995. The relationship of the duration of ruptured membranes to vertical transmission of human immunodeficiency virus. Am. J. Obstet. Gynecol. 173:585-589. [DOI] [PubMed] [Google Scholar]
- 13.Nielson, J., G. C. John, J. Carr, P. Lewis, J. Kreiss, S. Jackson, R. Nduati, D. A. Mbori-Ngacha, D. Panteleeff, S. Bodrug, C. Giachetti, M. Bott, B. Richardson, J. Bwayo, J. Ndinya-Achola, and J. Overbaugh. 1999. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J. Virol. 73:4393-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowak, M., S. Bonhoeffer, C. Loveday, P. Balfe, M. Semple, S. Kaye, M. Tenant-Flowers, and R. Tedder. 1995. HIV results in the frame. Nature 375:193. [DOI] [PubMed] [Google Scholar]
- 15.Panteleeff, D. D., S. Emery, B. Richardson, C. Rousseau, S. Benki, S. Bodrug, J. Kreiss, and J. Overbaugh. 2002. Validation of the performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay for genital swabs and breast milk samples. J. Clin. Microbiol. 40:3929-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 17.Perelson, A. S., A. Neuman, M. Markowitz, J. Leonard, and D. Ho. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271:1582-1586. [DOI] [PubMed] [Google Scholar]
- 18.Shaffer, N., R. Chuachoowong, P. A. Mock, C. Bhadrakom, W. Siriwasin, N. L. Young, T. Chotpitayasunondh, S. Chearskul, A. Roongpisuthipog, and P. Chinayon. 1999. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Lancet 353:773-780. [DOI] [PubMed] [Google Scholar]
- 19.UNAIDS. 2002. Report on the global HIV/AIDS epidemic. UNAIDS, Geneva, Switzerland.
- 20.Wang, C. C., S. McClelland, M. Reilly, J. Overbaugh, S. Emery, K. Mandaliya, B. Chohan, J. Ndinya-Achola, J. Bwayo, and J. Kreiss. 2001. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J. Infect. Dis. 183:1017-1022. [DOI] [PubMed] [Google Scholar]
- 21.Wei, X., S. Ghosh, M. Taylor, V. Johnson, E. Emini, P. Deutsch, J. Lifson, S. Bonhoeffer, M. Nowak, B. Hahn, M. Saag, and G. Shaw. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 22.Wiktor, S. Z., E. Ekpini, J. M. Karon, J. Nkengasong, C. Maurice, S. T. Severin, T. H. Roels, M. K. Kouassi, E. M. Lackritz, and I. M. Coulibaly. 1999. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire: a randomised trial. Lancet 353:781-785. [DOI] [PubMed] [Google Scholar]