Abstract
While it has been established that peptides modeling the C-helical region of human immunodeficiency virus type 1 gp41 are potent in vivo inhibitors of virus replication, their mechanism of action has yet to be determined. It has been proposed, but never directly demonstrated, that these peptides block virus entry by interacting with gp41 to disrupt the formation or function of a six-helix bundle structure. Using a six-helix bundle-specific monoclonal antibody with isolate-restricted Env reactivity, we provide the first direct evidence that, in receptor-activated viral Env, C-peptide entry inhibitors bind to the gp41 N-helical coiled-coil to form a peptide/protein hybrid structure and, in doing so, disrupt native six-helix bundle formation.
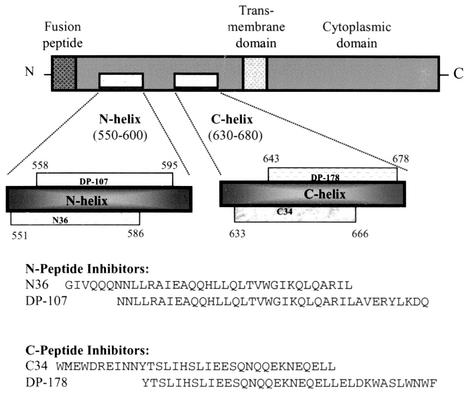
The role of the gp41 N- and C-helical domains (amino acid residues 551 to 595 and 633 to 678, respectively, of the human immunodeficiency virus type 1 LAI [HIV-1LAI] sequence) (Fig. 1) in virus replication has been the focus of considerable study. It has been established that the N helix plays a role in stabilizing the envelope oligomer (14, 15), and mutagenesis studies have determined that a coiled-coil structure formed by the association of the N-helical domains is required for membrane fusion (3, 5, 22). Also, it has been shown that determinants in the N helix form a gp120 contact site that is believed to play a role in stabilizing surface and transmembrane (TM) protein interactions (3, 13). The C-helical domain of gp41 is also critical to virus replication. Mutations in this region of the TM protein have a dramatic effect on fusion phenotype (17), while the broadly neutralizing human monoclonal antibody (MAb) 2F5 maps to a linear epitope overlapping this region of the TM (11, 12). In addition to these observations, it has been demonstrated that the N and C helices specifically interact to form a structure termed a six-helix bundle that is believed to play a critical role in virus entry (1, 19, 21).
FIG. 1.
Schematic diagram of HIV-1 gp41 showing several functional regions, including the N- and C-helical domains, and amino acid sequences of inhibitory peptides modeling the helical domains from the HIV-1LAI isolate.
Many of the studies characterizing the role of HIV envelope structure in virus entry have employed synthetic peptides. One example involves the use of peptides modeling the N- and C-helical domains of the TM protein (N36 and C34) (Fig. 1), which have been shown to self-assemble to form a six-helix bundle, to characterize structures found in the fusion-active form of gp41 (1, 19). In addition to modeling structural features found in the viral TM protein, these peptides have been observed to be virus-specific inhibitors of HIV entry (10, 20, 22; S. Jiang, K. Lin, N. Strick, and A. R. Neurath, Letter, Nature 365:113, 1993). In particular, peptides modeling the C helix (DP-178 and C34) are exceedingly potent inhibitors, with in vitro 90% inhibitory concentrations in the low nanomolar range. One of these C-peptides, DP-178 (Fig. 1), has recently completed phase III clinical trials under the trade name T20.
Several theories have been put forward to account for the antiviral activity of these C-peptide inhibitors. It has been proposed that they block virus entry by interacting with gp41 fusion intermediates, disrupting the formation and/or function of the fusion pore (9, 16). Alternatively, it has been suggested that these peptides bind to the N-terminal gp41 fusion domain and interfere with its ability to insert itself into the target cell membrane (8). A third theory, perhaps the most popular, holds that C-peptide inhibitors competitively disrupt the formation and/or function of the gp41 six-helix bundle structure (2, 18). According to this hypothesis, formation of the native six-helix bundle is critical to virus entry and C-peptides disrupt this process through a dominant-negative interaction with the N-helical region of the TM protein. Consistent with this proposal, it has been previously demonstrated that a C-peptide (DP-178) binds to a receptor-activated form of gp41 (7).
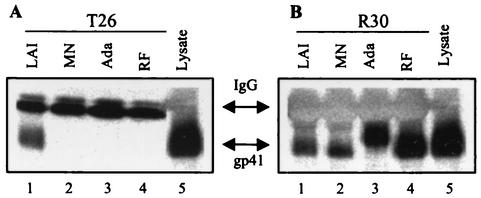
The goal of the present study was to extend earlier work by defining the specific molecular target for these antiviral peptides. To accomplish this, we carried out experiments to determine the effect of C-peptides on native six-helix bundle formation. Using a six-helix bundle-specific MAb with restricted envelope reactivity, we were able to demonstrate that C-peptide entry inhibitors bind to the N-helical coiled-coil in receptor-activated gp41 to form a peptide/protein hybrid structure and, in doing so, disrupt native six-helix bundle formation. Preparation and characterization of polyclonal sera specific for the gp41 six-helix bundle (R30) have been previously described (4). A previously reported gp41-specific murine MAb (T26, generously provided by Patricia Earl, National Institute of Allergy and Infectious Diseases) (6) was further characterized for six-helix bundle reactivity. Binding specificities of the antibody to gp41 from different HIV-1 isolates were established by using immunoprecipitation assays. Cell lysate was prepared by treating HIV-infected cells with a buffer consisting of 1% Igepal CA-630 (Sigma) in 50 mM NaCl-50 mM Tris (pH 8.0). Approximately 106 HIV-infected cells (either U87 CD4 CXCR4 or CCR5 cells at 3 days postinfection or chronically infected H9 cells) were used to generate the volume of lysate required for each sample. Lysate was clarified by centrifugation and stored at −20°C until use. In these experiments, 20 μl of envelope lysate was diluted into 180 μl of lysis buffer, followed by the addition of 3 μl of concentrated hybridoma supernatant or immune serum. The mixture was allowed to incubate overnight at 4°C, followed by the addition of 100 μl of protein A/G beads (Invitrogen) and a second incubation for 1 h at 4°C. At the end of this time, the samples were centrifuged at 13,000 rpm in a Heraeus Biofuge, the supernatant was removed, and the protein A/G pellets were washed twice with lysis buffer. Samples were stored at 4°C until they were analyzed by Western blotting as described previously (4). In these experiments, the restricted reactivity of T26 was established. Notably, this MAb exhibited binding only to gp41 from the LAI isolate (Fig. 2A). This behavior contrasts with that of the rabbit polyclonal sera (R30) generated against a mixture of peptides modeling the N- and C-helical domains (N36 and C34), which exhibited high levels of binding to gp41 from all isolates tested (Fig. 2B). Sequence analysis indicates that the limited envelope reactivity of T26 is most likely due to amino acid residues located in the N-terminal half of the C helix of the LAI envelope (Fig. 3). The observation that the conservative D-to-E change and the nonconservative N-to-D or -E and H-to-Y changes at positions corresponding to LAI residues 637, 641, and 648, respectively, occur in each of the three nonreactive isolates suggests that amino acid residues at one or more of these positions serve as determinants of antibody specificity.
FIG. 2.
(A) The T26 MAb is restricted in its binding to the LAI isolate, (B) while the R30 sera is broadly reactive. Western blot analysis of samples from lysate immunoprecipitation assays was used to characterize the binding of the T26 MAb and polyclonal sera R30 to HIV-1 gp41 from four virus isolates. In these experiments, antibody was added to an infected-cell lysate and precipitated with protein A/G beads. The precipitated samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose, and probed with the gp41-specific MAb Chessie 8 (contributed to the National Institutes of Health AIDS Research and Reference Reagent Program by George Lewis). Lanes 5 of panels A and B represent lysate from LAI-infected cells loaded directly onto the gel without immunoprecipitation. IgG, immunoglobulin G.
FIG. 3.
Amino acid sequences for peptides modeling the C-helical regions of the T26-reactive LAI isolate and the nonreactive RF, MN, and Ada isolates. Three positions (underlined) in the LAI sequence are sites of either conservative or nonconservative changes in all of the nonreactive isolate envelopes (underlined and bolded). We believe that one or some combination of these consensus changes is responsible for the isolate-restricted binding of the T26 MAb. Nonconsensus sequence changes in the nonreactive isolate envelopes are bolded.
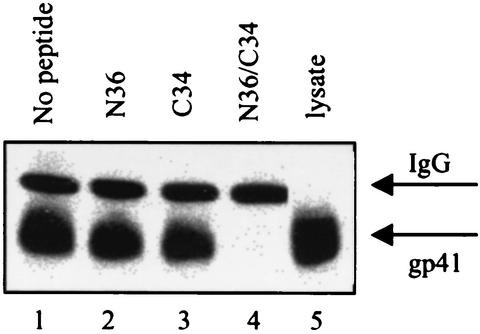
In order to establish that T26 was specifically targeting the gp41 six-helix bundle, we carried out peptide adsorption studies. These experiments consisted of immunoprecipitation assays modified to include pretreatment of the antibody samples (1/2 h at room temperature) with N36, C34, or a mixture of N36 and C34 peptides (N36-C34) modeled after the LAI envelope sequence at a total concentration of 10 μg/ml. Peptides were prepared by using 9-fluorenylmethoxy carbonyl (FMOC) chemistry and purified by reversed-phase high-performance liquid chromatography. N36 is 94.3% pure, and C34 is >85% pure. Mass spectrometry results were consistent with the molecular weights of the peptides. Pretreatment of T26 with either the N- or C-peptide alone resulted in no detectable loss of binding; however, pretreatment of this same antibody sample with a 1:1 mixture of the N- and C-peptides, which self-assemble to form a six-helix bundle, resulted in a complete loss of gp41-binding activity (Fig. 4). Complex formation between the N36 and C34 peptides used in this study to form a six-helix bundle was confirmed by circular dichroism experiments (data not shown). This result indicated that the MAb was specifically targeting a conformational epitope within this higher-order protein structure.
FIG. 4.
Lysate immunoprecipitation assays were used to establish the binding specificity of the T26 MAb for the six-helix bundle. Western blot results from experiments in which T26-containing samples were adsorbed with N36, C34, and N36-C34 peptide mixtures are shown. In these assays, antibody-containing samples were treated with peptide at a concentration of 10 μg/ml prior to the addition of infected-cell lysate. As shown, neither the N-peptide nor the C-peptide alone adsorbed the gp41-binding activity of T26 (lanes 2 and 3), while the N-peptide-C-peptide mixture, which self-assembles to form a six-helix bundle, completely adsorbed all antibody binding (lane 4). Lane 5 represents lysate from LAI-infected cells loaded directly onto the gel without immunoprecipitation. IgG, immunoglobulin G.
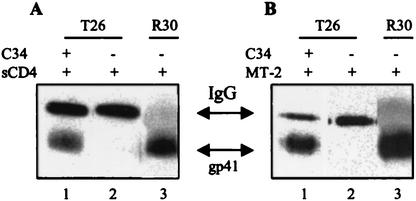
Having established the specificity of T26 for the six-helix bundle found in the HIV-1LAI envelope, we carried out a series of cell-surface immunoprecipitation experiments that utilized this restricted binding behavior to characterize the effect of C-peptide entry inhibitors on formation of the native six-helix bundle. We reasoned that if the C-peptide inhibitors did in fact bind to the gp41 N-helical domain to form a hybrid six-helix bundle structure, this binding could be established by using the appropriate combination of C-peptide, viral envelope, and T26. Specifically, we believed that we should be able to rescue T26 binding to gp41 in a nonreactive virus isolate by using peptide modeled after the C-helical domain of a reactive isolate. In the first set of experiments, chronically infected cells (7.5 × 105 cells in a volume of 500 μl of tissue culture media) expressing the HIV-1 RF envelope (T26 nonreactive) were incubated with a C-peptide inhibitor (C34; 5 μg/ml for 30 min at 37°C) modeled after the HIV-1LAI sequence (T26 reactive). This incubation (5 μg/ml for 30 min at 37°C) was followed by the addition of soluble CD4 (sCD4) at 1 μg/ml, which has been previously demonstrated to trigger six-helix bundle formation in surface-expressed viral envelope (4). Following incubation with sCD4 for 1 h at 37°C, samples were washed to remove free peptide, cells were lysed, and envelope was immunoprecipitated with the isolate-restricted T26 MAb as described earlier. As shown in Fig. 5A, triggering in the presence of the LAI-derived C-peptide allows a significant amount of gp41 from the MAb-nonreactive RF isolate to be immunoprecipitated by our LAI-specific MAb (lane 1). In contrast, T26 was unable to precipitate gp41 from RF envelope-expressing cells triggered in the absence of C-peptide (lane 2). From these results, we conclude that the LAI-based C-peptide inhibitor was binding to the N-helical coiled-coil in receptor-activated gp41 of the RF envelope to generate a hybrid peptide or protein six-helix bundle structure recognized by the LAI envelope-specific MAb.
FIG. 5.
The C34 peptide modeling the HIV-1LAI sequence can rescue the binding activity of the T26 MAb to gp41 from the antibody-nonreactive RF isolate. (A) Western blot analysis of samples from cell-surface immunoprecipitation experiments shows that the binding activity of MAb T26 to cell-surface-expressed RF envelope triggered with sCD4 can be rescued by the addition of C34. Compare binding activities in the presence and absence of peptide (lanes 1 and 2). (B) Results from a similar experiment are shown (lanes 1 and 2); however, in this assay envelope activation was accomplished by using cell-expressed receptors. Lanes 3 in panels A and B show control samples in which the RF-reactive polyclonal sera R30 recognized the native six-helix bundle structure in the absence of the LAI-derived C-peptide. IgG, immunoglobulin G.
To extend this result, a second assay was carried out using a modified form of this surface immunoprecipitation assay employing cell-surface-expressed receptors rather than sCD4 for triggering. In these experiments, the LAI-based C34 peptide was added to RF envelope-expressing cells in the presence of uninfected receptor-expressing MT2 target cells (7.5 × 105 cells). The mixture of cells was cocultivated for 6 h at 37°C. At the end of that time, the mixture was washed, cells were lysed, and envelope was immunoprecipitated with T26 as described earlier. The results from this experiment were identical to those observed in the assays employing sCD4 (Fig. 5B). Our LAI-specific MAb precipitated RF envelope from chronically infected cells incubated with the LAI-derived C-peptide in the presence of receptor-expressing cells (Fig. 5B, lane 1), while envelope triggered in the absence of peptide was not precipitated (Fig. 5B, lane 2).
The present study extends previous work characterizing the mechanism of action of C-peptide entry inhibitors (7). In a prior report, coimmunoprecipitation experiments were used to show that C-peptide inhibitors interact with the HIV-1 TM protein following receptor activation. However, that approach did not allow for the identification of the specific binding site within gp41. In the present work, we utilize an envelope-specific six-helix bundle-binding antibody to provide what we believe is the first direct evidence for C-peptide binding to the N-helical domain of HIV-1 gp41. Specifically, we show that the interaction of a C-peptide modeled after the envelope of an antibody-reactive viral isolate can rescue binding to the TM protein of a nonreactive viral envelope. Due to the conformational specificity of the six-helix bundle-binding MAb, this process could occur only through the interaction of the C-peptide with the N-helical region of gp41 from the nonreactive envelope to form a peptide/protein hybrid six-helix bundle. Although the specific interaction of C-peptide inhibitors with the N-helical domain of gp41 has been one of several mechanisms proposed to account for the antiviral activity of C-peptides, this report provides the first direct proof that this specific binding actually occurs.
The results from this study are important for several reasons. By establishing the molecular target for this potent class of HIV therapeutics, we have validated the gp41 N-helical domain as a viable target in HIV drug development. This information should expedite the discovery of new families of entry inhibitors and aid in the development of new members of the current classes with improved development characteristics. Additionally, knowledge of the specific molecular target will add to our understanding of the determinants associated with the development of resistance to the current generation of peptide-based inhibitors and could aid in our attempts to identify compounds that overcome this resistance. Finally, these results provide new information regarding the role of HIV-1 envelope proteins in viral entry and extend our understanding of this fundamental process.
REFERENCES
- 1.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 2.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 3.Chen, S. S. 1994. Functional role of the zipper motif region of human immunodeficiency virus type 1 transmembrane protein gp41. J. Virol. 68:2002-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Rosny, E., R. Vassell, P. T. Wingfield, C. T. Wild, and C. D. Weiss. 2001. Peptides corresponding to the heptad repeat motifs in the transmembrane protein (gp41) of human immunodeficiency virus type 1 elicit antibodies to receptor-activated conformations of the envelope glycoproteins. J. Virol. 75:8859-8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubay, J. W., S. J. Roberts, B. Brody, and E. Hunter. 1992. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J. Virol. 66:4748-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earl, P. L., C. C. Broder, R. W. Doms, and B. Moss. 1997. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 71:2674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuta, R. A., C. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276-279. [DOI] [PubMed] [Google Scholar]
- 8.Jiang, S., K. Lin, N. Strick, and A. R. Neurath. 1993. Inhibition of HIV-1 infection by a fusion domain binding peptide from the HIV-1 envelope glycoprotein GP41. Biochem. Biophys. Res. Commun. 195:533-538. [DOI] [PubMed] [Google Scholar]
- 9.Kliger, Y., S. A. Gallo, S. G. Peisajovich, I. Munoz-Barroso, S. Avkin, R. Blumenthal, and Y. Shai. 2001. Mode of action of an antiviral peptide from HIV-1. Inhibition at a post-lipid mixing stage. J. Biol. Chem. 276:1391-1397. [DOI] [PubMed] [Google Scholar]
- 10.Lu, M., and P. S. Kim. 1997. A trimeric structural subdomain of the HIV-1 transmembrane glycoprotein. J. Biomol. Struct. Dyn. 15:465-471. [DOI] [PubMed] [Google Scholar]
- 11.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muster, T., R. Guinea, A. Trkola, M. Purtscher, A. Klima, F. Steindl, P. Palese, and H. Katinger. 1994. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J. Virol. 68:4031-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park, E. J., and G. V. Quinnan, Jr. 1999. Both neutralization resistance and high infectivity phenotypes are caused by mutations of interacting residues in the human immunodeficiency virus type 1 gp41 leucine zipper and the gp120 receptor- and coreceptor-binding domains. J. Virol. 73:5707-5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poumbourios, P., K. A. Wilson, R. J. Center, W. El Ahmar, and B. E. Kemp. 1997. Human immunodeficiency virus type 1 envelope glycoprotein oligomerization requires the gp41 amphipathic alpha-helical/leucine zipper-like sequence. J. Virol. 71:2041-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poumbourios, P., W. el Ahmar, D. A. McPhee, and B. E. Kemp. 1995. Determinants of human immunodeficiency virus type 1 envelope glycoprotein oligomeric structure. J. Virol. 69:1209-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu, J. R., B. S. Jin, M. J. Suh, Y. S. Yoo, S. H. Yoon, E. R. Woo, and Y. G. Yu. 1999. Two interaction modes of the gp41-derived peptides with gp41 and their correlation with antimembrane fusion activity. Biochem. Biophys. Res. Commun. 265:625-629. [DOI] [PubMed] [Google Scholar]
- 17.Salzwedel, K., J. T. West, and E. Hunter. 1999. A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J. Virol. 73:2469-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weissenhorn, W., A. Dessen, L. J. Calder, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1999. Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 6:3-9. [DOI] [PubMed] [Google Scholar]
- 19.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 20.Wild, C., T. Greenwell, and T. Matthews. 1993. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res. Hum. Retrovir. 9:1051-1053. [DOI] [PubMed] [Google Scholar]
- 21.Wild, C., T. Greenwell, D. Shugars, L. Rimsky-Clark, and T. Matthews. 1995. The inhibitory activity of a HIV-1 peptide correlates with its ability to interact with a leucine zipper structure. AIDS Res. Hum. Retrovir. 11:323-325. [DOI] [PubMed] [Google Scholar]
- 22.Wild, C. T., D. C. Shugars, T. Greenwell, C. McDanal, and T. Matthews. 1994. Peptides corresponding to a predictive α-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 91:9770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]